Abstract
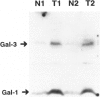
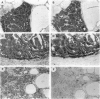
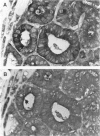
Carcinoma of the thyroid gland, the most frequently diagnosed endocrine malignancy, is often associated with early regional metastases. With the exception of papillary carcinoma, distinguishing benign from malignant thyroid neoplasms in the absence of metastatic disease is difficult. Recently, the vertebrate lectins galectin-1 and galectin-3 have been implicated in the regulation of cellular growth, differentiation, and malignant transformation of a variety of tissues. To determine whether these galectins have a role in thyroid neoplasia, we analyzed 32 specimens from thyroid malignancies (16 papillary, 7 follicular, 8 medullary carcinomas, and 1 metastasis to lymph node), 10 benign thyroid adenomas, 1 nodular goiter, and 33 specimens from adjacent normal thyroid tissue for the expression of galectin-1 and galectin-3 with immunohistochemical and immunoblotting techniques utilizing anti-galectin antibodies. All thyroid malignancies of epithelial origin (ie, papillary and follicular carcinomas) and a metastatic lymph node from a papillary carcinoma expressed high levels of both galectin-1 and galectin-3. The medullary thyroid carcinomas, which are of parafollicular C cell origin, showed a weaker and variable expression of these galectins. In contrast, neither benign thyroid adenomas nor adjacent normal thyroid tissue expressed galectin-1 or galectin-3. These results suggest that galectin-1 and galectin-3 may be associated with malignant transformation of thyroid epithelium and may potentially serve as markers for distinguishing benign thyroid adenomas from differentiated thyroid carcinomas.
Full text
PDF


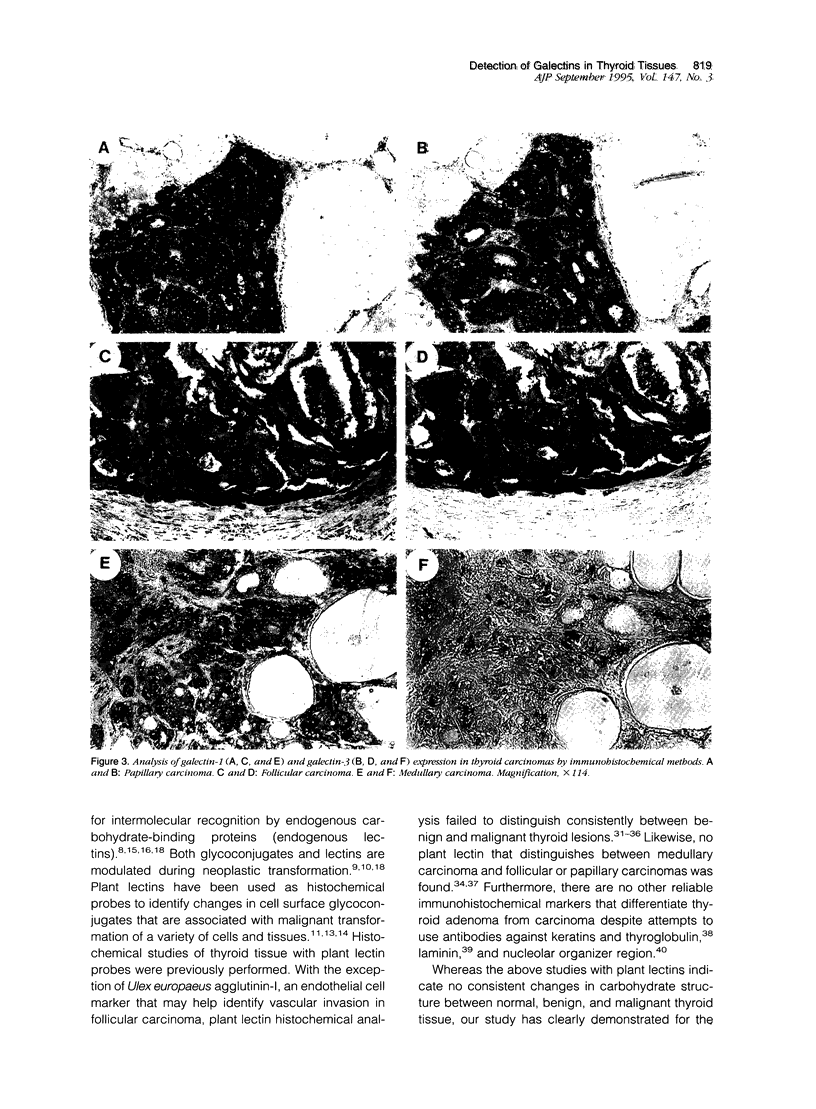



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrwal N., Wang J. L., Voss P. G. Carbohydrate-binding protein 35. Levels of transcription and mRNA accumulation in quiescent and proliferating cells. J Biol Chem. 1989 Oct 15;264(29):17236–17242. [PubMed] [Google Scholar]
- Allison R. T. Lectins in diagnostic histopathology: a review. Med Lab Sci. 1986 Oct;43(4):369–376. [PubMed] [Google Scholar]
- Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994 Feb 25;76(4):597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807–20810. [PubMed] [Google Scholar]
- Castronovo V., Campo E., van den Brûle F. A., Claysmith A. P., Cioce V., Liu F. T., Fernandez P. L., Sobel M. E. Inverse modulation of steady-state messenger RNA levels of two non-integrin laminin-binding proteins in human colon carcinoma. J Natl Cancer Inst. 1992 Aug 5;84(15):1161–1169. doi: 10.1093/jnci/84.15.1161. [DOI] [PubMed] [Google Scholar]
- Chiariotti L., Benvenuto G., Salvatore P., Veneziani B. M., Villone G., Fusco A., Russo T., Bruni C. B. Expression of the soluble lectin L-14 gene is induced by TSH in thyroid cells and suppressed by retinoic acid in transformed neural cells. Biochem Biophys Res Commun. 1994 Mar 15;199(2):540–546. doi: 10.1006/bbrc.1994.1262. [DOI] [PubMed] [Google Scholar]
- Chiariotti L., Berlingieri M. T., De Rosa P., Battaglia C., Berger N., Bruni C. B., Fusco A. Increased expression of the negative growth factor, galactoside-binding protein, gene in transformed thyroid cells and in human thyroid carcinomas. Oncogene. 1992 Dec;7(12):2507–2511. [PubMed] [Google Scholar]
- Cooper H. S. Lectins as probes in histochemistry and immunohistochemistry: the peanut (Arachis hypogaea) lectin. Hum Pathol. 1984 Oct;15(10):904–906. doi: 10.1016/s0046-8177(84)80117-3. [DOI] [PubMed] [Google Scholar]
- Crittenden S. L., Roff C. F., Wang J. L. Carbohydrate-binding protein 35: identification of the galactose-specific lectin in various tissues of mice. Mol Cell Biol. 1984 Jul;4(7):1252–1259. doi: 10.1128/mcb.4.7.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanov I. Lectin cytochemistry and histochemistry. Lab Invest. 1987 Jul;57(1):5–20. [PubMed] [Google Scholar]
- Dennis J. W. N-linked oligosaccharide processing and tumor cell biology. Semin Cancer Biol. 1991 Dec;2(6):411–420. [PubMed] [Google Scholar]
- Feizi T. Carbohydrate differentiation antigens: probable ligands for cell adhesion molecules. Trends Biochem Sci. 1991 Mar;16(3):84–86. doi: 10.1016/0968-0004(91)90038-w. [DOI] [PubMed] [Google Scholar]
- Gabius H. J. Detection and functions of mammalian lectins--with emphasis on membrane lectins. Biochim Biophys Acta. 1991 Mar 7;1071(1):1–18. doi: 10.1016/0304-4157(91)90010-t. [DOI] [PubMed] [Google Scholar]
- González-Cámpora R., Sanchez Gallego F., Martin Lacave I., Mora Marin J., Montero Linares C., Galera-Davidson H. Lectin histochemistry of the thyroid gland. Cancer. 1988 Dec 1;62(11):2354–2362. doi: 10.1002/1097-0142(19881201)62:11<2354::aid-cncr2820621117>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Griffiths D. W., Stephenson T. J. Lectin agglutinins: a comparative study of Lotus tetragonolobus and Ulex europaeus 1 as markers of vascular endothelium in follicular thyroid carcinoma. Med Lab Sci. 1988 Jan;45(1):45–51. [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Hughes R. C. Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994 Feb;4(1):5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Matsumoto A., Ito K., Kure Y., Suzuki A., Sugino K., Ozaki O., Noh J. Prediction of distant metastasis in follicular adenocarcinoma of the thyroid. World J Surg. 1990 May-Jun;14(3):425–430. doi: 10.1007/BF01658546. [DOI] [PubMed] [Google Scholar]
- Kimber S. J. Glycoconjugates and cell surface interactions in pre- and peri-implantation mammalian embryonic development. Int Rev Cytol. 1990;120:53–167. doi: 10.1016/s0074-7696(08)61599-5. [DOI] [PubMed] [Google Scholar]
- Klonoff D. C., Greenspan F. S. The thyroid nodule. Adv Intern Med. 1982;27:101–126. [PubMed] [Google Scholar]
- Lee K. Y., Loré J. M., Jr The treatment of metastatic thyroid disease. Otolaryngol Clin North Am. 1990 Jun;23(3):475–493. [PubMed] [Google Scholar]
- Lotan R., Kramer R. H., Neumann G., Lotan D., Nicolson G. L. Retinoic acid-induced modifications in the growth and cell surface components of a human carcinoma (HeLa) cell line. Exp Cell Res. 1980 Dec;130(2):401–414. doi: 10.1016/0014-4827(80)90018-x. [DOI] [PubMed] [Google Scholar]
- Lotan R., Matsushita Y., Ohannesian D., Carralero D., Ota D. M., Cleary K. R., Nicolson G. L., Irimura T. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr Res. 1991 Jun 25;213:47–57. doi: 10.1016/s0008-6215(00)90597-4. [DOI] [PubMed] [Google Scholar]
- Lotz M. M., Andrews C. W., Jr, Korzelius C. A., Lee E. C., Steele G. D., Jr, Clarke A., Mercurio A. M. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3466–3470. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanthappa N. K., Cooper D. N., Barondes S. H., Schwarting G. A. Rat olfactory neurons can utilize the endogenous lectin, L-14, in a novel adhesion mechanism. Development. 1994 Jun;120(6):1373–1384. doi: 10.1242/dev.120.6.1373. [DOI] [PubMed] [Google Scholar]
- Martin-Lacave I., Gonzalez-Campora R., Moreno Fernandez A., Sachez Gallego F., Montero C., Galera-Davidson H. Mucosubstances in medullary carcinoma of the thyroid. Histopathology. 1988 Jul;13(1):55–66. doi: 10.1111/j.1365-2559.1988.tb02003.x. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Virtanen I. Expression of laminin in thyroid gland and thyroid tumors: an immunohistologic study. Int J Cancer. 1984 Jul 15;34(1):27–30. doi: 10.1002/ijc.2910340106. [DOI] [PubMed] [Google Scholar]
- Permanetter W., Nathrath W. B., Löhrs U. Immunohistochemical analysis of thyroglobulin and keratin in benign and malignant thyroid tumours. Virchows Arch A Pathol Anat Histopathol. 1982;398(2):221–228. doi: 10.1007/BF00618871. [DOI] [PubMed] [Google Scholar]
- Raz A., Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 1987;6(3):433–452. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- Raz A., Meromsky L., Zvibel I., Lotan R. Transformation-related changes in the expression of endogenous cell lectins. Int J Cancer. 1987 Mar 15;39(3):353–360. doi: 10.1002/ijc.2910390314. [DOI] [PubMed] [Google Scholar]
- Raz A., Zhu D. G., Hogan V., Shah N., Raz T., Karkash R., Pazerini G., Carmi P. Evidence for the role of 34-kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer. 1990 Nov 15;46(5):871–877. doi: 10.1002/ijc.2910460520. [DOI] [PubMed] [Google Scholar]
- Sanford G. L., Harris-Hooker S. Stimulation of vascular cell proliferation by beta-galactoside specific lectins. FASEB J. 1990 Aug;4(11):2912–2918. doi: 10.1096/fasebj.4.11.2379767. [DOI] [PubMed] [Google Scholar]
- Sasano H., Rojas M., Silverberg S. G. Analysis of lectin binding in benign and malignant thyroid nodules. Arch Pathol Lab Med. 1989 Feb;113(2):186–189. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Shem-Tov Y., Straus M., Talmi Y. P., Rath-Wolfsom L., Zohar Y., Gal R. Nucleolar organizer regions in follicular tumors of the thyroid. Head Neck. 1994 Sep-Oct;16(5):420–423. doi: 10.1002/hed.2880160505. [DOI] [PubMed] [Google Scholar]
- Sobrinho-Simoes M., Sambade C., Nesland J. M., Holm R., Damjanov I. Lectin histochemistry and ultrastructure of medullary carcinoma of the thyroid gland. Arch Pathol Lab Med. 1990 Apr;114(4):369–375. [PubMed] [Google Scholar]
- Vijayakumar T., Augustine J., Mathew L., Aleykutty M. A., Nair M. B., Remani P., Nair M. K. Tissue binding pattern of plant lectins in benign and malignant lesions of thyroid. J Exp Pathol. 1992;6(1-2):11–23. [PubMed] [Google Scholar]
- Walker R. A. The use of lectins in histopathology. Pathol Res Pract. 1989 Dec;185(6):826–835. doi: 10.1016/s0344-0338(89)80282-1. [DOI] [PubMed] [Google Scholar]
- Wang R. L., Tan Y. B. [Immunohistochemical study and lectin distribution of thyroid carcinoma originated from follicular epithelium]. Zhonghua Bing Li Xue Za Zhi. 1990 Jun;19(2):90–93. [PubMed] [Google Scholar]
- Wingo P. A., Tong T., Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995 Jan-Feb;45(1):8–30. doi: 10.3322/canjclin.45.1.8. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Ohno S., Kawasaki H., Suzuki K. Overexpression of a beta-galactoside binding protein causes transformation of BALB3T3 fibroblast cells. Biochem Biophys Res Commun. 1991 Aug 30;179(1):272–279. doi: 10.1016/0006-291x(91)91365-j. [DOI] [PubMed] [Google Scholar]