Abstract
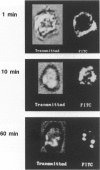
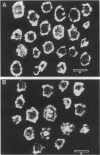
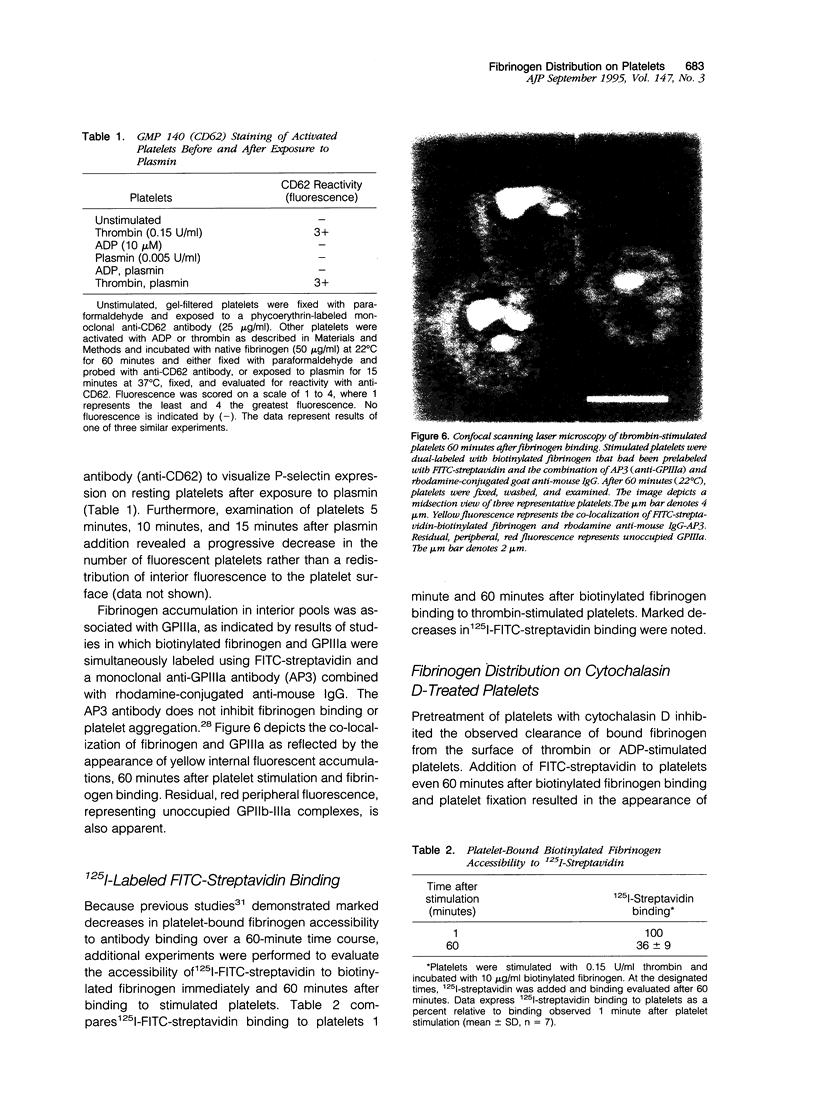
Previous studies have suggested that qualitative changes in platelet bound fibrinogen modulate platelet aggregation. The present study used confocal scanning laser microscopy to further evaluate post-ligand binding events over a 60-minute time course. When fluorescein isothiocyanate (FITC)-streptavidin was added to ADP-stimulated platelets 1 minute after biotinylated fibrinogen binding at 22 degrees C, bound fibrinogen was found in variously sized patches on the cell surface. When streptavidin was added 60 minutes later, bound fibrinogen had been cleared from the platelet surface and was observed in clusters penetrating into platelets to various extents. ADP-activated platelets did not stain with a monoclonal antibody against CD62 suggesting that platelets were not permeabilized during the experiment and had not released alpha-granules. Additional studies using either biotinylated fibrinogen that had been prelabeled with FITC-streptavidin or FITC-labeled fibrinogen revealed similar patterns of platelet-associated fibrinogen clearance and redistribution. Pretreatment of platelets with cytochalasin D prevented this redistribution. Dual labeling experiments using biotinylated fibrinogen and FITC-streptavidin as well as a monoclonal anti-GPIIIa antibody labeled with rhodamine-conjugated anti-mouse IgG demonstrated the co-localization of fibrinogen and GPIIIa. Similar observations were made with fibrinogen bound to thrombin-stimulated platelets. In contrast, fibronectin bound to thrombin-activated platelets retained a predominantly surface membrane distribution under identical experimental conditions. Since surface-cleared fibrinogen was accessible to exogenous FITC-streptavidin under conditions that did not lead to platelet permeabilization, the data suggest fibrinogen deposition in compartments that are accessible to the extracellular milieu. This is consistent with the ability of exogenous plasmin to completely remove cleared fibrinogen pools without detectable fibrinogen reexpression on the platelet surface or alpha-granule secretion. The data provide morphological evidence for the selective, GPIIb-IIIa mediated, actin-dependent clearance of bound fibrinogen from the activated platelet surface, suggesting a mechanism for preventing and limiting thrombus development.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourguignon L. Y. Receptor capping in platelet membranes. Cell Biol Int Rep. 1984 Jan;8(1):19–26. doi: 10.1016/0309-1651(84)90177-2. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E. Transmembrane signaling across the platelet integrin glycoprotein IIb-IIIa. Ann N Y Acad Sci. 1994 Apr 18;714:75–87. doi: 10.1111/j.1749-6632.1994.tb12032.x. [DOI] [PubMed] [Google Scholar]
- Frelinger A. L., 3rd, Cohen I., Plow E. F., Smith M. A., Roberts J., Lam S. C., Ginsberg M. H. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem. 1990 Apr 15;265(11):6346–6352. [PubMed] [Google Scholar]
- Frelinger A. L., 3rd, Du X. P., Plow E. F., Ginsberg M. H. Monoclonal antibodies to ligand-occupied conformers of integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991 Sep 15;266(26):17106–17111. [PubMed] [Google Scholar]
- Ghebrehiwet B., Bossone S., Erdei A., Reid K. B. Reversible biotinylation of C1q with a cleavable biotinyl derivative. Application in C1q receptor (C1qR) purification. J Immunol Methods. 1988 Jun 13;110(2):251–260. doi: 10.1016/0022-1759(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Albrecht R. M. Correlative light and electron microscopy of platelet adhesion and fibrinogen receptor expression using colloidal-gold labeling. Scanning Microsc. 1987 Jun;1(2):727–734. [PubMed] [Google Scholar]
- Harrison P., Wilbourn B., Debili N., Vainchenker W., Breton-Gorius J., Lawrie A. S., Masse J. M., Savidge G. F., Cramer E. M. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J Clin Invest. 1989 Oct;84(4):1320–1324. doi: 10.1172/JCI114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme S., Sixma J. J., Wester J., Holmsen H. ADP-induced refractory state of platelets in vitro. II. Functional and ultra studies on gel filtered platelets. Scand J Haematol. 1977 Apr;18(4):267–278. doi: 10.1111/j.1600-0609.1977.tb01196.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. The complexity of platelet adhesion to extracellular matrices. Thromb Haemost. 1991 Jul 12;66(1):40–43. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Phillips D. R., Shuman M. A., Bainton D. F. The platelet fibrinogen receptor: an immunogold-surface replica study of agonist-induced ligand binding and receptor clustering. J Cell Biol. 1987 Jun;104(6):1655–1663. doi: 10.1083/jcb.104.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. I., Bliss G. A., Newman P. J., McEver R. P. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem. 1990 Dec 5;265(34):21381–21385. [PubMed] [Google Scholar]
- Kakaiya R. M., Kiraly T. L., Cable R. G. Concanavalin A induces patching/capping of the platelet membrane glycoprotein IIb/IIIa complex. Thromb Haemost. 1988 Apr 8;59(2):281–283. [PubMed] [Google Scholar]
- Larsen E., Celi A., Gilbert G. E., Furie B. C., Erban J. K., Bonfanti R., Wagner D. D., Furie B. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989 Oct 20;59(2):305–312. doi: 10.1016/0092-8674(89)90292-4. [DOI] [PubMed] [Google Scholar]
- Leistikow E. A., Barnhart M. I., Escolar G., White J. G. Receptor-ligand complexes are cleared to the open canalicular system of surface-activated platelets. Br J Haematol. 1990 Jan;74(1):93–100. doi: 10.1111/j.1365-2141.1990.tb02544.x. [DOI] [PubMed] [Google Scholar]
- Loftus J. C., Albrecht R. M. Redistribution of the fibrinogen receptor of human platelets after surface activation. J Cell Biol. 1984 Sep;99(3):822–829. doi: 10.1083/jcb.99.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Soria C., Cramer E. M., Soria J., Maclouf J., Perrot J. Y., Li H., Commin P. L., Schumann F., Regnier O. Temperature dependence of plasmin-induced activation or inhibition of human platelets. Blood. 1991 Mar 1;77(5):996–1005. [PubMed] [Google Scholar]
- Lüscher E. F., Weber S. The formation of the haemostatic plug--a special case of platelet aggregation. An experiment and a survey of the literature. Thromb Haemost. 1993 Aug 2;70(2):234–237. [PubMed] [Google Scholar]
- Newman P. J., McEver R. P., Doers M. P., Kunicki T. J. Synergistic action of two murine monoclonal antibodies that inhibit ADP-induced platelet aggregation without blocking fibrinogen binding. Blood. 1987 Feb;69(2):668–676. [PubMed] [Google Scholar]
- Oliver J. A., Albrecht R. M. Colloidal gold labelling of fibrinogen receptors in epinephrine- and ADP-activated platelet suspensions. Scanning Microsc. 1987 Jun;1(2):745–756. [PubMed] [Google Scholar]
- Olorundare O. E., Goodman S. L., Albrecht R. M. Trifluoperazine inhibition of fibrinogen receptor redistribution in surface activated platelets: correlative video-enhanced differential interference contrast light microscopic, high voltage electron microscopic and scanning electron microscopic studies. Scanning Microsc. 1987 Jun;1(2):735–743. [PubMed] [Google Scholar]
- Peerschke E. I. Adhesive protein expression on thrombin-stimulated platelets: time-dependent modulation of anti-fibrinogen, -fibronectin, and -von Willebrand factor antibody binding. Blood. 1992 Feb 15;79(4):948–953. [PubMed] [Google Scholar]
- Peerschke E. I. Decreased accessibility of platelet-bound fibrinogen to antibody and enzyme probes. Blood. 1989 Aug 1;74(2):682–689. [PubMed] [Google Scholar]
- Peerschke E. I. Effect of stimulation on the stabilization of platelet-fibrinogen interactions. Thromb Haemost. 1992 Sep 7;68(3):346–351. [PubMed] [Google Scholar]
- Peerschke E. I. Events occurring after thrombin-induced fibrinogen binding to platelets. Semin Thromb Hemost. 1992 Jan;18(1):34–43. doi: 10.1055/s-2007-1002408. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Glycoprotein IIb and IIIa retention on fibrinogen-coated surfaces after lysis of adherent platelets. Blood. 1993 Dec 1;82(11):3358–3363. [PubMed] [Google Scholar]
- Peerschke E. I. Recognition of platelet-associated fibrinogen by polyclonal antibodies: correlation with platelet aggregation. Blood. 1992 Apr 15;79(8):2028–2033. [PubMed] [Google Scholar]
- Peerschke E. I. Stabilization of platelet-fibrinogen interactions: modulation by divalent cations. J Lab Clin Med. 1993 Jan;121(1):135–141. [PubMed] [Google Scholar]
- Peerschke E. I. Time-dependent association between platelet-bound fibrinogen and the Triton X-100 insoluble cytoskeleton. Blood. 1991 Feb 1;77(3):508–514. [PubMed] [Google Scholar]
- Peerschke E. I., Wainer J. A. Examination of irreversible platelet-fibrinogen interactions. Am J Physiol. 1985 May;248(5 Pt 1):C466–C472. doi: 10.1152/ajpcell.1985.248.5.C466. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Polley M. J., Leung L. L., Clark F. Y., Nachman R. L. Thrombin-induced platelet membrane glycoprotein IIb and IIIa complex formation. An electron microscope study. J Exp Med. 1981 Oct 1;154(4):1058–1068. doi: 10.1084/jem.154.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso S., Kiefel V., Mueller-Eckhardt C. Redistribution of platelet glycoproteins induced by allo- and autoantibodies. Thromb Haemost. 1987 Oct 28;58(3):866–871. [PubMed] [Google Scholar]
- Santoso S., Zimmermann U., Neppert J., Mueller-Eckhardt C. Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood. 1986 Feb;67(2):343–349. [PubMed] [Google Scholar]
- Wencel-Drake J. D. Plasma membrane GPIIb/IIIa. Evidence for a cycling receptor pool. Am J Pathol. 1990 Jan;136(1):61–70. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Escolar G. Fibrinogen receptors do not undergo spontaneous redistribution on surface-activated platelets. Arteriosclerosis. 1990 Sep-Oct;10(5):738–744. doi: 10.1161/01.atv.10.5.738. [DOI] [PubMed] [Google Scholar]
- White J. G., Escolar G. Induction of receptor clustering, patching, and capping on surface-activated platelets. Lab Invest. 1990 Sep;63(3):332–340. [PubMed] [Google Scholar]
- White J. G., Escolar G. Mobility of GPIIb-IIIa receptors within membranes of surface- and suspension-activated platelets does not depend on assembly and contraction of cytoplasmic actin. Eur J Cell Biol. 1990 Aug;52(2):341–348. [PubMed] [Google Scholar]