Abstract
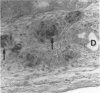
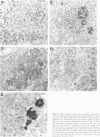
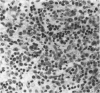
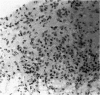
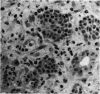
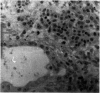
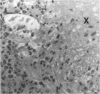
The response of non-immunosuppressed non-obese diabetic/Lt mice to an isograft (H-2g7), major histocompatibility complex-mismatched allografts (CBA, H-2K; BALB/c, H-2d), and xenograft of fetal pig pancreas was assessed by light microscopy. In non-obese diabetic mice, isografts were rapidly invaded by lymphoid cells, and the graft pathology was similar to that in the host pancreas. In prediabetic mice the graft site was invaded by small mononuclear cells (CD4 and CD8+ve T cells) and macrophages, and in diabetic mice specific beta-cell destruction was found. The allografts were invaded and destroyed within 10 to 14 days by mononuclear cells that included many blast cells. In the allograft sites the infiltrating cells soon disappeared, and within 3 weeks only a scar remained. The xenografts, in contrast, were invaded by macrophages and eosinophils with some neutrophils and mast cells and multinucleated giant cells. Xenograft destruction also occurred over 8 to 10 days, but the site remained large and swollen with a central necrotic zone and massive fibrosis forming a large granuloma, and the infiltrate persisted for many weeks. Thus, there are marked differences in the host response to a challenge with tissue that is prone to cell-specific autoimmune disease, to a graft of immunogenic allogeneic tissue, and to a transplant of discordant xenogeneic islets. Because of the differences in the host response to these grafts different immunosuppressive strategies may be needed to cope with their destruction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr Xenogeneic transplantation. A review. Transplantation. 1988 Jul;46(1):1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Baxter A. G., Koulmanda M., Mandel T. E. High and low diabetes incidence nonobese diabetic (NOD) mice: origins and characterisation. Autoimmunity. 1991;9(1):61–67. doi: 10.3109/08916939108997125. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Baxter L. A., Schuppin G. T., Smith F. E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993 Dec;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- Calne R. Y. Organ transplantation between widely disparate species. Transplant Proc. 1970 Dec;2(4):550–556. [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Charlton B., Mandel T. E. Progression from insulitis to beta-cell destruction in NOD mouse requires L3T4+ T-lymphocytes. Diabetes. 1988 Aug;37(8):1108–1112. doi: 10.2337/diab.37.8.1108. [DOI] [PubMed] [Google Scholar]
- Foster P. F., Bhattacharyya A., Sankary H. N., Coleman J., Ashmann M., Williams J. W. Eosinophil cationic protein's role in human hepatic allograft rejection. Hepatology. 1991 Jun;13(6):1117–1125. [PubMed] [Google Scholar]
- Foster P. F., Sankary H. N., Hart M., Ashmann M., Williams J. W. Blood and graft eosinophilia as predictors of rejection in human liver transplantation. Transplantation. 1989 Jan;47(1):72–74. doi: 10.1097/00007890-198901000-00016. [DOI] [PubMed] [Google Scholar]
- Groth C. G., Korsgren O., Tibell A., Tollemar J., Möller E., Bolinder J., Ostman J., Reinholt F. P., Hellerström C., Andersson A. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994 Nov 19;344(8934):1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Hom J. T., Estridge T. Antigen-induced recruitment of eosinophils: importance of CD4+ T cells, IL5, and mast cells. Clin Immunol Immunopathol. 1994 Dec;73(3):305–311. doi: 10.1006/clin.1994.1203. [DOI] [PubMed] [Google Scholar]
- Hongwei W., Nanra R. S., Stein A., Avis L., Price A., Hibberd A. D. Eosinophils in acute renal allograft rejection. Transpl Immunol. 1994;2(1):41–46. doi: 10.1016/0966-3274(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Bohman S. O., Fredens K. Activated eosinophil infiltration and deposits of eosinophil cationic protein in renal allograft rejection. Nephron. 1991;59(2):266–270. doi: 10.1159/000186563. [DOI] [PubMed] [Google Scholar]
- Iwamoto I., Nakajima H., Endo H., Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993 Feb 1;177(2):573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormendi F., Amend W. J., Jr The importance of eosinophil cells in kidney allograft rejection. Transplantation. 1988 Mar;45(3):537–539. doi: 10.1097/00007890-198803000-00007. [DOI] [PubMed] [Google Scholar]
- Kroegel C., Warner J. A., Virchow J. C., Jr, Matthys H. Pulmonary immune cells in health and disease: the eosinophil leucocyte (Part II). Eur Respir J. 1994 Apr;7(4):743–760. doi: 10.1183/09031936.94.07040743. [DOI] [PubMed] [Google Scholar]
- Langer A., Valdivia L. A., Murase N., Woo J., Celli S., Fung J. J., Starzl T. E., Demetris A. J. Humoral and cellular immunopathology of hepatic and cardiac hamster-into-rat xenograft rejection. Marked stimulation of IgM++bright/IgD+dull splenic B cells. Am J Pathol. 1993 Jul;143(1):85–98. [PMC free article] [PubMed] [Google Scholar]
- Martinez O. M., Ascher N. L., Ferrell L., Villanueva J., Lake J., Roberts J. P., Krams S. M. Evidence for a nonclassical pathway of graft rejection involving interleukin 5 and eosinophils. Transplantation. 1993 Apr;55(4):909–918. doi: 10.1097/00007890-199304000-00041. [DOI] [PubMed] [Google Scholar]
- Murase N., Starzl T. E., Demetris A. J., Valdivia L., Tanabe M., Cramer D., Makowka L. Hamster-to-rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993 Apr;55(4):701–708. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Kephart G. M., Colby T. V., Gleich G. J. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992 Feb;140(2):521–528. [PMC free article] [PubMed] [Google Scholar]
- Rohrbach M. S., Wheatley C. L., Slifman N. R., Gleich G. J. Activation of platelets by eosinophil granule proteins. J Exp Med. 1990 Oct 1;172(4):1271–1274. doi: 10.1084/jem.172.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. Interleukin-5, eosinophils, and disease. Blood. 1992 Jun 15;79(12):3101–3109. [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E., Goetz F., Michael A. F. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985 Aug;53(2):132–144. [PubMed] [Google Scholar]
- Sibley R. K., Sutherland D. E. Pancreas transplantation. An immunohistologic and histopathologic examination of 100 grafts. Am J Pathol. 1987 Jul;128(1):151–170. [PMC free article] [PubMed] [Google Scholar]
- Simeonovic C. J., Ceredig R., Wilson J. D. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell-depleted mice. Transplantation. 1990 May;49(5):849–856. doi: 10.1097/00007890-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Fung J., Tzakis A., Todo S., Demetris A. J., Marino I. R., Doyle H., Zeevi A., Warty V., Michaels M. Baboon-to-human liver transplantation. Lancet. 1993 Jan 9;341(8837):65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D. E., Goetz F. C., Sibley R. K. Recurrence of disease in pancreas transplants. Diabetes. 1989 Jan;38 (Suppl 1):85–87. doi: 10.2337/diab.38.1.s85. [DOI] [PubMed] [Google Scholar]
- Thompson S. C., Mandel T. E. Fetal pig pancreas. Preparation and assessment of tissue for transplantation, and its in vivo development and function in athymic (nude) mice. Transplantation. 1990 Mar;49(3):571–581. doi: 10.1097/00007890-199003000-00019. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J. Eosinophils in the 1990s: new perspectives on their role in health and disease. Postgrad Med J. 1994 Aug;70(826):536–552. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M. R., Hall-Craggs M., Shen S. Y., Posner J. N., Alongi S. V., Dagher F. J., Sadler J. H. The prognostic value of the eosinophil in acute renal allograft rejection. Transplantation. 1986 Jun;41(6):709–712. doi: 10.1097/00007890-198606000-00008. [DOI] [PubMed] [Google Scholar]
- Weller P. F. Cytokine regulation of eosinophil function. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S55–S59. doi: 10.1016/0090-1229(92)90041-l. [DOI] [PubMed] [Google Scholar]
- Weller P. F. The immunobiology of eosinophils. N Engl J Med. 1991 Apr 18;324(16):1110–1118. doi: 10.1056/NEJM199104183241607. [DOI] [PubMed] [Google Scholar]
- de Groen P. C., Kephart G. M., Gleich G. J., Ludwig J. The eosinophil as an effector cell of the immune response during hepatic allograft rejection. Hepatology. 1994 Sep;20(3):654–662. doi: 10.1016/0270-9139(94)90102-3. [DOI] [PubMed] [Google Scholar]