Abstract
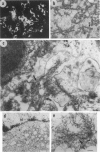
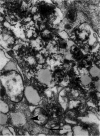
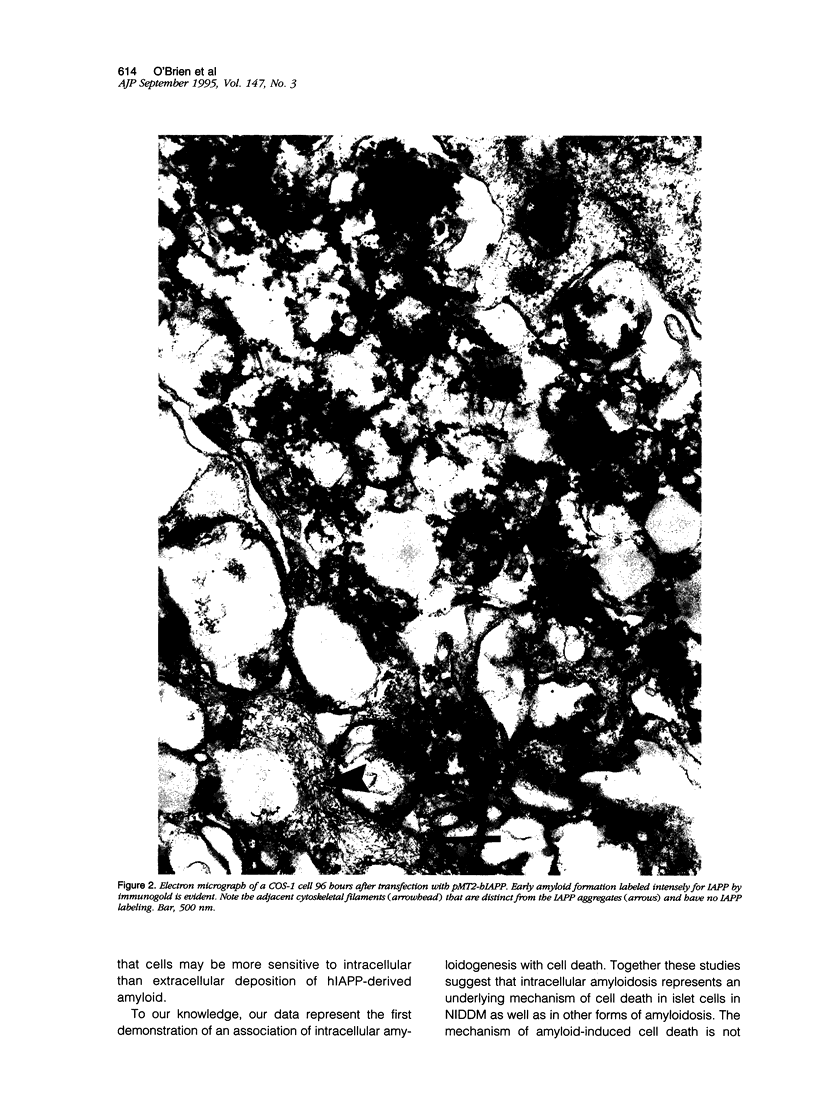
Non-insulin-dependent diabetes mellitus is characterized by concurrent loss of beta-cells and deposition of islet amyloid derived from islet amyloid polypeptide (IAPP). We have previously demonstrated that IAPP-derived amyloid forms intracellularly in humans with chronic excess insulin expression (eg, insulinoma and insulin receptor antibody-induced insulin resistance). To determine whether overexpression of IAPP results in intracellular amyloid in mammalian cells, we transfected COS cells with vectors expressing amyloidogenic human IAPP or non-amyloidogenic rat IAPP. Transfected COS-1 cells secreted comparable amounts of human IAPP and rat IAPP (2.1 to 2.8 nmol/L/48 hours). After 96 hours, 90% of cells expressing human IAPP contained amyloid fibrils and were degenerating or dead, whereas cells transfected with rat IAPP lacked amyloid and were viable. Thus, overexpression of human IAPP can result in intracellular amyloid formation that is associated with cell death, suggesting that intracellular amyloid may play a role in beta-cell loss in non-insulin-dependent diabetes mellitus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Engel W. K., Alvarez R. B. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992 Jul;141(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Svensson V., Rorsman F., Engström U., Westermark G. T., Wilander E., Johnson K., Westermark P. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989 Aug;183(2):484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Udelsman R., Feulner G. J., Norton D. D., Holbrook N. J. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Persky B., Soehnlen B. J., Grogan T. M., Salmon S. E. Amyloid production in human myeloma stem-cell culture, with morphologic evidence of amyloid secretion by associated macrophages. N Engl J Med. 1982 Dec 30;307(27):1689–1692. doi: 10.1056/NEJM198212303072706. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Munoz P. C., Gorgone G., Purcell J. J., Jr, Rodrigues M., Ghiso J., Levy E., Haltia M., Frangione B. Amyloidosis due to a mutation of the gelsolin gene in an American family with lattice corneal dystrophy type II. N Engl J Med. 1991 Dec 19;325(25):1780–1785. doi: 10.1056/NEJM199112193252505. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986 May;29(5):301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Takahashi M., Koga M., Yokota T., Yamashita Y., Uchino F. Amyloid fibril formation in the rough endoplasmic reticulum of plasma cells from a patient with localized A lambda amyloidosis. Lab Invest. 1991 Feb;64(2):265–271. [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Hayden D. W., Jordan K., Ghobrial H. K., Mahoney W. C., Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988 Jan;130(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans S. J., van Mansfeld A. D., Jansz H. S., Krans H. M., Radder J. K., Frölich M., de Boer S. F., Kreutter D. K., Andrews G. C., Maassen J. A. Amylin-induced in vivo insulin resistance in conscious rats: the liver is more sensitive to amylin than peripheral tissues. Diabetologia. 1991 Apr;34(4):218–224. doi: 10.1007/BF00405079. [DOI] [PubMed] [Google Scholar]
- Levy E., Carman M. D., Fernandez-Madrid I. J., Power M. D., Lieberburg I., van Duinen S. G., Bots G. T., Luyendijk W., Frangione B. Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990 Jun 1;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Terakado K., Usami M., Yoshikawa K. Formation of amyloid-like fibrils in COS cells overexpressing part of the Alzheimer amyloid protein precursor. Nature. 1990 Oct 11;347(6293):566–569. doi: 10.1038/347566a0. [DOI] [PubMed] [Google Scholar]
- Maury C. P., Kere J., Tolvanen R., de la Chapelle A. Finnish hereditary amyloidosis is caused by a single nucleotide substitution in the gelsolin gene. FEBS Lett. 1990 Dec 10;276(1-2):75–77. doi: 10.1016/0014-5793(90)80510-p. [DOI] [PubMed] [Google Scholar]
- May P. C., Boggs L. N., Fuson K. S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer's disease amyloid-beta neurotoxicity. J Neurochem. 1993 Dec;61(6):2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- McCracken A. A., Kruse K. B. Intracellular transport of rat serum albumin is altered by a genetically engineered deletion of the propeptide. J Biol Chem. 1989 Dec 15;264(35):20843–20846. [PubMed] [Google Scholar]
- Nichols W. C., Liepnieks J. J., Snyder E. L., Benson M. D. Senile cardiac amyloidosis associated with homozygosity for a transthyretin variant (ILE-122). J Lab Clin Med. 1991 Mar;117(3):175–180. [PubMed] [Google Scholar]
- O'Brien T. D., Butler A. E., Roche P. C., Johnson K. H., Butler P. C. Islet amyloid polypeptide in human insulinomas. Evidence for intracellular amyloidogenesis. Diabetes. 1994 Feb;43(2):329–336. doi: 10.2337/diab.43.2.329. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Butler P. C., Westermark P., Johnson K. H. Islet amyloid polypeptide: a review of its biology and potential roles in the pathogenesis of diabetes mellitus. Vet Pathol. 1993 Jul;30(4):317–332. doi: 10.1177/030098589303000401. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Rizza R. A., Carney J. A., Butler P. C. Islet amyloidosis in a patient with chronic massive insulin resistance due to antiinsulin receptor antibodies. J Clin Endocrinol Metab. 1994 Jul;79(1):290–292. doi: 10.1210/jcem.79.1.8027243. [DOI] [PubMed] [Google Scholar]
- Rother K. I., Carney J. A., Couce M., Charlesworth J., Butler P. C. Islet amyloid polypeptide in pancreatic tissue of children with persistent hyperinsulinemic hypoglycemia caused by primary islet hyperplasia and nesidioblastosis. J Clin Endocrinol Metab. 1995 Jun;80(6):1956–1959. doi: 10.1210/jcem.80.6.7539820. [DOI] [PubMed] [Google Scholar]
- Röcken C., Linke R. P., Saeger W. Immunohistology of islet amyloid polypeptide in diabetes mellitus: semi-quantitative studies in a post-mortem series. Virchows Arch A Pathol Anat Histopathol. 1992;421(4):339–344. doi: 10.1007/BF01660981. [DOI] [PubMed] [Google Scholar]
- Saitoh Y., Mori H., Matsumoto K., Ushio Y., Hayakawa T., Mori S., Arita N., Mogami H. Accumulation of amyloid in pituitary adenomas. Acta Neuropathol. 1985;68(2):87–92. doi: 10.1007/BF00688628. [DOI] [PubMed] [Google Scholar]
- Scallon B. J., Fung W. J., Tsang T. C., Li S., Kado-Fong H., Huang K. S., Kochan J. P. Primary structure and functional activity of a phosphatidylinositol-glycan-specific phospholipase D. Science. 1991 Apr 19;252(5004):446–448. doi: 10.1126/science.2017684. [DOI] [PubMed] [Google Scholar]
- Tsang W. Y., Chan J. K. Amyloid-producing squamous cell carcinoma of the uterine cervix. Arch Pathol Lab Med. 1993 Feb;117(2):199–201. [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Stagg C. A., Li D. G., Putney D. J., Holbrook N. J. Vascular heat shock protein expression in response to stress. Endocrine and autonomic regulation of this age-dependent response. J Clin Invest. 1993 Feb;91(2):465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Engström U., Johnson K. H., Westermark G. T., Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]