Abstract
Treatment of confluent monolayers of human umbilical vein endothelial cells with sublethal concentrations of hydrogen peroxide (H2O2) produces reversible cell retraction that opens gaps between adjacent cells. Despite the retraction, adjacent cells remain in contact through a network of dendrite-like processes. Retraction depends on cellular metabolism but not new protein synthesis or protein kinase C. Shape changes induced by H2O2 are accompanied by partial redistribution of actin filaments from the cell periphery in resting endothelial cells to a tangled network of centrally located filaments in H2O2-treated endothelial cells. This change in actin organization is associated with a loss of the normal distribution pattern of surface protein expression. Specifically, beta 1 and beta 3 integrins partly escape from focal adhesion plaques and migrate to the lateral and apical surface of the cell; PECAM-1 redistributes from the lateral borders to the basal surface; and ICAM-1 and ICAM-2 spread from apical caps to the basal surface and to the dendrite-like processes. The likely consequence of endothelial retraction accompanied by abnormal membrane protein distribution is a loss of normal endothelial cell functions. These changes are best considered manifestations of H2O2-induced sublethal injury that may cause endothelial dysfunction.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley J. R., Johnson D. R., Pober J. S. Endothelial activation by hydrogen peroxide. Selective increases of intercellular adhesion molecule-1 and major histocompatibility complex class I. Am J Pathol. 1993 May;142(5):1598–1609. [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Carpén O., Pallai P., Staunton D. E., Springer T. A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992 Sep;118(5):1223–1234. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Van Leuven F., Carmeliet G., Van Den Berghe H., Cassiman J. J. Cultured human fibroblasts contain a large pool of precursor beta 1-integrin but lack an intracellular pool of mature subunit. Eur J Biochem. 1991 Jul 1;199(1):25–33. doi: 10.1111/j.1432-1033.1991.tb16087.x. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W., Wong M. K., Lacey C. In vitro reendothelialization. Microfilament bundle reorganization in migrating porcine endothelial cells. Arteriosclerosis. 1984 Mar-Apr;4(2):91–96. doi: 10.1161/01.atv.4.2.91. [DOI] [PubMed] [Google Scholar]
- Grammas P., Liu G. J., Wood K., Floyd R. A. Anoxia/reoxygenation induces hydroxyl free radical formation in brain microvessels. Free Radic Biol Med. 1993 May;14(5):553–557. doi: 10.1016/0891-5849(93)90113-9. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993 Aug 1;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J Exp Med. 1992 Sep 1;176(3):819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990 Mar 9;247(4947):1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. W., Stone P., Shasby D. M. Cationic neutrophil proteins increase transendothelial albumin movement. J Appl Physiol (1985) 1987 Apr;62(4):1521–1530. doi: 10.1152/jappl.1987.62.4.1521. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Ratych R. E., Chuknyiska R. S., Bulkley G. B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987 Aug;102(2):122–131. [PubMed] [Google Scholar]
- Ritchie A. J., Johnson D. R., Ewenstein B. M., Pober J. S. Tumor necrosis factor induction of endothelial cell surface antigens is independent of protein kinase C activation or inactivation. Studies with phorbol myristate acetate and staurosporine. J Immunol. 1991 May 1;146(9):3056–3062. [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siflinger-Birnboim A., Goligorsky M. S., Del Vecchio P. J., Malik A. B. Activation of protein kinase C pathway contributes to hydrogen peroxide-induced increase in endothelial permeability. Lab Invest. 1992 Jul;67(1):24–30. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Schuger L., Gibbs D. F., Bromberg J., Johnson K. J., Ryan U. S., Ward P. A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989 Sep;135(3):435–438. [PMC free article] [PubMed] [Google Scholar]
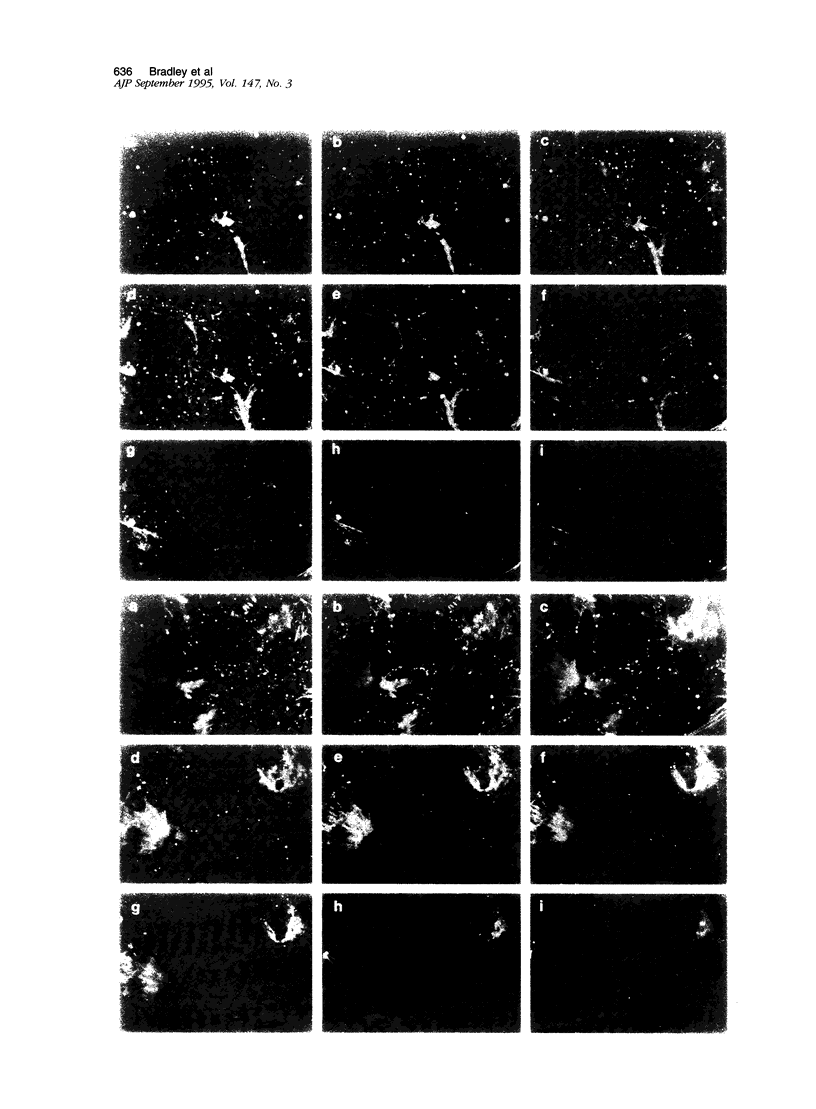
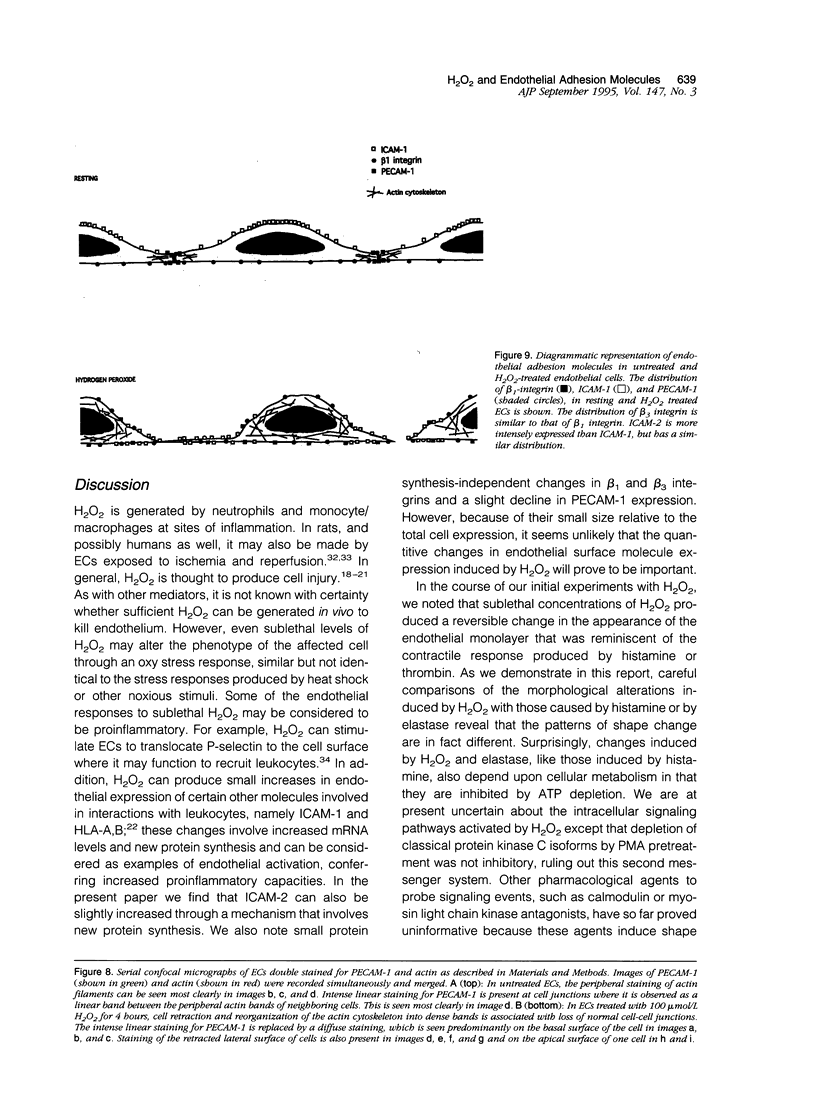
- Wong M. K., Gotlieb A. I. Endothelial cell monolayer integrity. I. Characterization of dense peripheral band of microfilaments. Arteriosclerosis. 1986 Mar-Apr;6(2):212–219. doi: 10.1161/01.atv.6.2.212. [DOI] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. Endothelial monolayer integrity. Perturbation of F-actin filaments and the dense peripheral band-vinculin network. Arteriosclerosis. 1990 Jan-Feb;10(1):76–84. doi: 10.1161/01.atv.10.1.76. [DOI] [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Inhibition of endothelial cell retraction by ATP depletion. Am J Pathol. 1988 Jul;132(1):28–37. [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. C., Gotlieb A. I. Disruption of endothelial actin microfilaments by protein kinase C inhibitors. Microvasc Res. 1992 Jan;43(1):100–111. doi: 10.1016/0026-2862(92)90009-e. [DOI] [PubMed] [Google Scholar]
- de Fougerolles A. R., Stacker S. A., Schwarting R., Springer T. A. Characterization of ICAM-2 and evidence for a third counter-receptor for LFA-1. J Exp Med. 1991 Jul 1;174(1):253–267. doi: 10.1084/jem.174.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]