Abstract
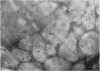
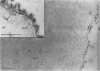
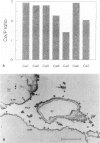
Active oxygen species including hydrogen peroxide (H2O2) play a major role in ischemia-reperfusion injury. In the present study, changes in myocardial H2O2 content as well as its subcellular distribution were examined in rat hearts subjected to ischemia-reperfusion. Isolated perfused rat hearts were made globally ischemic for 20 or 30 minutes and were reperfused for different durations. H2O2 content in these hearts was studied biochemically and changes were correlated with the recovery of function. These hearts were also analyzed for subcellular distribution of H2O2. Optimal conditions of tissue processing as well as incubation medium were established for reacting cerium chloride with H2O2 to form cerium perhydroxide, an insoluble electron-dense product. The chemical composition of these deposits was confirmed by x-ray micro-analysis. Global ischemia caused complete contractile failure in minutes and after 30 minutes of ischemia, these was a > 250% increase in the myocardial H2O2 content. Depressed contractile function recovery in the early phase of reperfusion was accompanied by approximately a 600% increase in the myocardial H2O2 content. Brief pre-fixation with low concentrations of glutaraldehyde, inhibition of alkaline phosphatase, glutathione peroxidase, and catalase, post-fixation but no post-osmication, and no counterstaining yielded the best cytochemical definition of H2O2. In normal hearts, extremely small amounts of cerium hydroperoxide precipitates were located on the endothelial cells. X-ray microanalysis confirmed the presence of cerium in the reaction product. Ischemia resulted in a stronger reaction, particularly on the sarcolemma as well as abluminal side of the endothelial cells; and upon reperfusion, cerium precipitate reaction at these sites was more intense. In the reperfused hearts, the reaction product also appeared within mitochondria between the cristae as well as on the myofibrils, but Z-lines were devoid of any precipitate. The data support a significant increase in myocardial H2O2 during both the phase of ischemia and the first few minutes of reperfusion. A stronger reaction on the sarcolemma and abluminal side of endothelial cells may also indicate enhanced H2O2 accumulation as well as vulnerability of these sites to oxidative stress injury.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angermüller S., Fahimi H. D. Ultrastructural cytochemical localization of uricase in peroxisomes of rat liver. J Histochem Cytochem. 1986 Feb;34(2):159–165. doi: 10.1177/34.2.3080517. [DOI] [PubMed] [Google Scholar]
- Babbs C. F., Cregor M. D., Turek J. J., Badylak S. F. Endothelial superoxide production in the isolated rat heart during early reperfusion after ischemia. A histochemical study. Am J Pathol. 1991 Nov;139(5):1069–1080. [PMC free article] [PubMed] [Google Scholar]
- Bolli R. Mechanism of myocardial "stunning". Circulation. 1990 Sep;82(3):723–738. doi: 10.1161/01.cir.82.3.723. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Grosso M. A., Whitman G. J., Banerjee A., Terada L. S., Repine J. E., Harken A. H. The coincidence of myocardial reperfusion injury and hydrogen peroxide production in the isolated rat heart. Surgery. 1989 Apr;105(4):496–501. [PubMed] [Google Scholar]
- Brown J. M., Terada L. S., Grosso M. A., Whitmann G. J., Velasco S. E., Patt A., Harken A. H., Repine J. E. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988 Apr;81(4):1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Thomas S. M., Garner A. Free-radical-mediated fragmentation of monoamine oxidase in the mitochondrial membrane. Roles for lipid radicals. Biochem J. 1986 Dec 1;240(2):489–494. doi: 10.1042/bj2400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal H., Kirshenbaum L. A., Randhawa A. K., Singal P. K. Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physiol. 1991 Sep;261(3 Pt 2):H632–H638. doi: 10.1152/ajpheart.1991.261.3.H632. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Gerber G., Siems W., Werner A., Stoesser R., Foeldes-Papp Z., Kowalewski J., Schneider W., Grune T. Regulation of purine nucleotide metabolism in hypoxic liver and intestine of rats: radical scavenging effects of allopurinol and oxypurinol. Adv Exp Med Biol. 1989;253B:497–504. doi: 10.1007/978-1-4684-5676-9_74. [DOI] [PubMed] [Google Scholar]
- Gossrau R., Van Noorden C. J., Frederiks W. M. Pitfalls in the light microscopical detection of NADH oxidase. Histochem J. 1990 Mar;22(3):155–161. doi: 10.1007/BF01003535. [DOI] [PubMed] [Google Scholar]
- Gupta M., Singal P. K. Time course of structure, function, and metabolic changes due to an exogenous source of oxygen metabolites in rat heart. Can J Physiol Pharmacol. 1989 Dec;67(12):1549–1559. doi: 10.1139/y89-250. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Hess M. L., Manson N. H., Okabe E. Involvement of free radicals in the pathophysiology of ischemic heart disease. Can J Physiol Pharmacol. 1982 Nov;60(11):1382–1389. doi: 10.1139/y82-206. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Woollard A. C., Wolff S. P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990 Jul 30;268(1):69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- Kaul N., Siveski-Iliskovic N., Hill M., Slezak J., Singal P. K. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993 Oct;30(2):55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum L. A., Singal P. K. Changes in antioxidant enzymes in isolated cardiac myocytes subjected to hypoxia-reoxygenation. Lab Invest. 1992 Dec;67(6):796–803. [PubMed] [Google Scholar]
- Kloner R. A., Przyklenk K., Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989 Nov;80(5):1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Arroyo C. M., Dickens B. F., Weglicki W. B. Spin-trapping evidence that graded myocardial ischemia alters post-ischemic superoxide production. Free Radic Biol Med. 1987;3(2):153–159. doi: 10.1016/s0891-5849(87)80011-4. [DOI] [PubMed] [Google Scholar]
- Kramer J. H., Mak I. T., Weglicki W. B. Differential sensitivity of canine cardiac sarcolemmal and microsomal enzymes to inhibition by free radical-induced lipid peroxidation. Circ Res. 1984 Jul;55(1):120–124. doi: 10.1161/01.res.55.1.120. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Whittenburg D., Repine J. E. Role of xanthine oxidase in ischemia/reperfusion injury. Am J Physiol. 1990 Mar;258(3 Pt 2):F711–F716. doi: 10.1152/ajprenal.1990.258.3.F711. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Microperoxisomes and peroxisomes in relation to lipid metabolism. Ann N Y Acad Sci. 1982;386:138–152. doi: 10.1111/j.1749-6632.1982.tb21412.x. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Cohen M. V., Mueller H. S. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J Mol Cell Cardiol. 1983 Oct;15(10):713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Tanaka M., Murry C. E., Richard V. J., Jennings R. B. Evaluation of free radical injury in myocardium. Toxicol Pathol. 1990;18(4 Pt 1):470–480. [PubMed] [Google Scholar]
- Robinson J. M., Karnovsky M. J. Ultrastructural localization of several phosphatases with cerium. J Histochem Cytochem. 1983 Oct;31(10):1197–1208. doi: 10.1177/31.10.6309949. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Brosamer K., Forder J. R., Simon R. H., Ward P. A., Grum C. M. Cerium chloride as a histochemical marker of hydrogen peroxide in reperfused ischemic hearts. J Mol Cell Cardiol. 1990 Jan;22(1):83–97. doi: 10.1016/0022-2828(90)90974-7. [DOI] [PubMed] [Google Scholar]
- Singal P. K., Deally C. M., Weinberg L. E. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987 Aug;19(8):817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- Slezak J., Tribulova N., Ravingerova T., Singal P. K. Myocardial heterogeneity and regional variations in response to injury. Lab Invest. 1992 Sep;67(3):322–330. [PubMed] [Google Scholar]
- Takemura G., Onodera T., Millard R. W., Ashraf M. Demonstration of hydroxyl radical and its role in hydrogen peroxide-induced myocardial injury: hydroxyl radical dependent and independent mechanisms. Free Radic Biol Med. 1993 Jul;15(1):13–25. doi: 10.1016/0891-5849(93)90121-a. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Hess M. L. The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis. 1986 May-Jun;28(6):449–462. doi: 10.1016/0033-0620(86)90027-7. [DOI] [PubMed] [Google Scholar]
- Vandeplassche G., Hermans C., Thoné F., Borgers M. Mitochondrial hydrogen peroxide generation by NADH-oxidase activity following regional myocardial ischemia in the dog. J Mol Cell Cardiol. 1989 Apr;21(4):383–392. doi: 10.1016/0022-2828(89)90649-4. [DOI] [PubMed] [Google Scholar]
- Vaughan D. M., Koke J. R., Bittar N. Ultrastructure, peroxisomes and lipid peroxidation in reperfused myocardium. Cytobios. 1988;55(221):71–80. [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]