Abstract
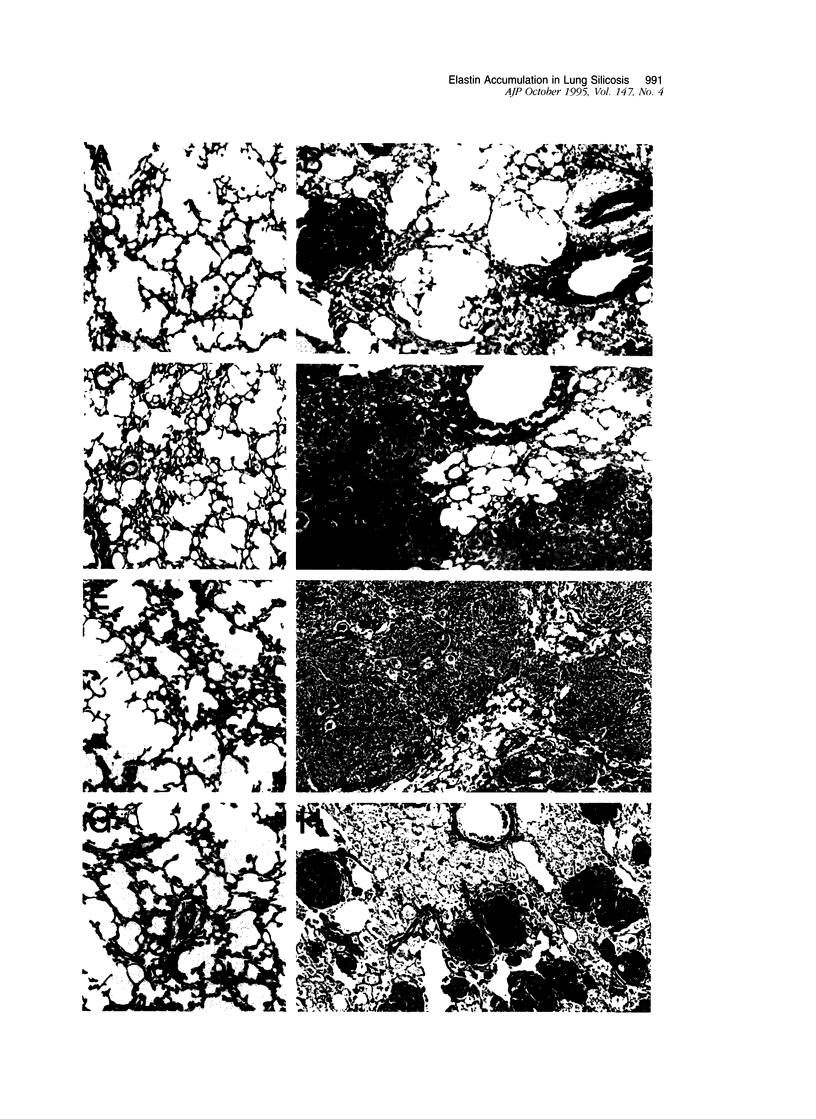
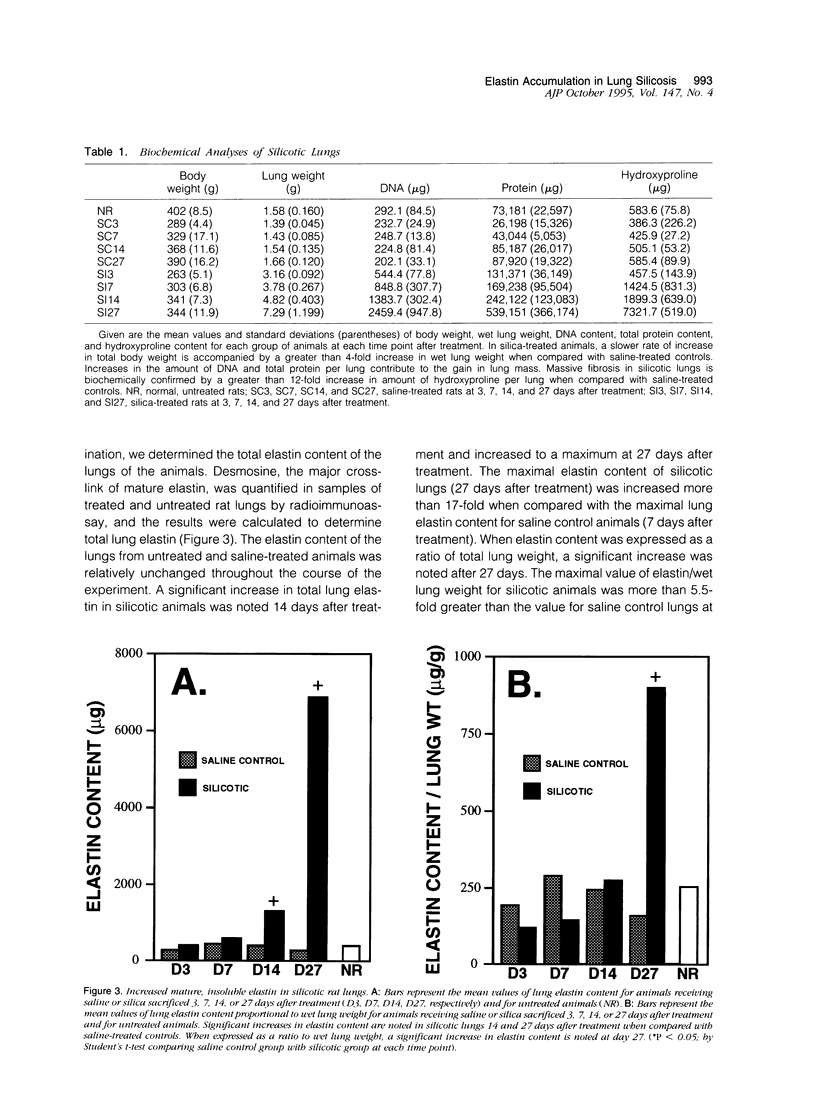
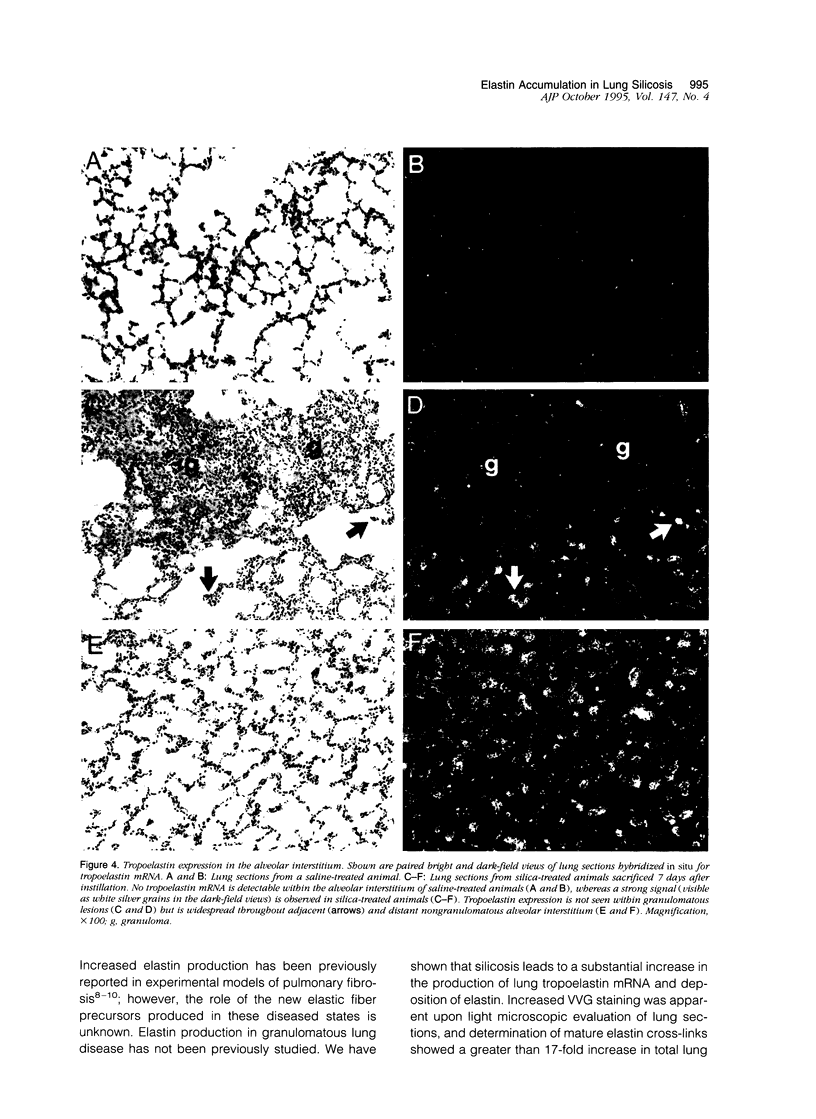
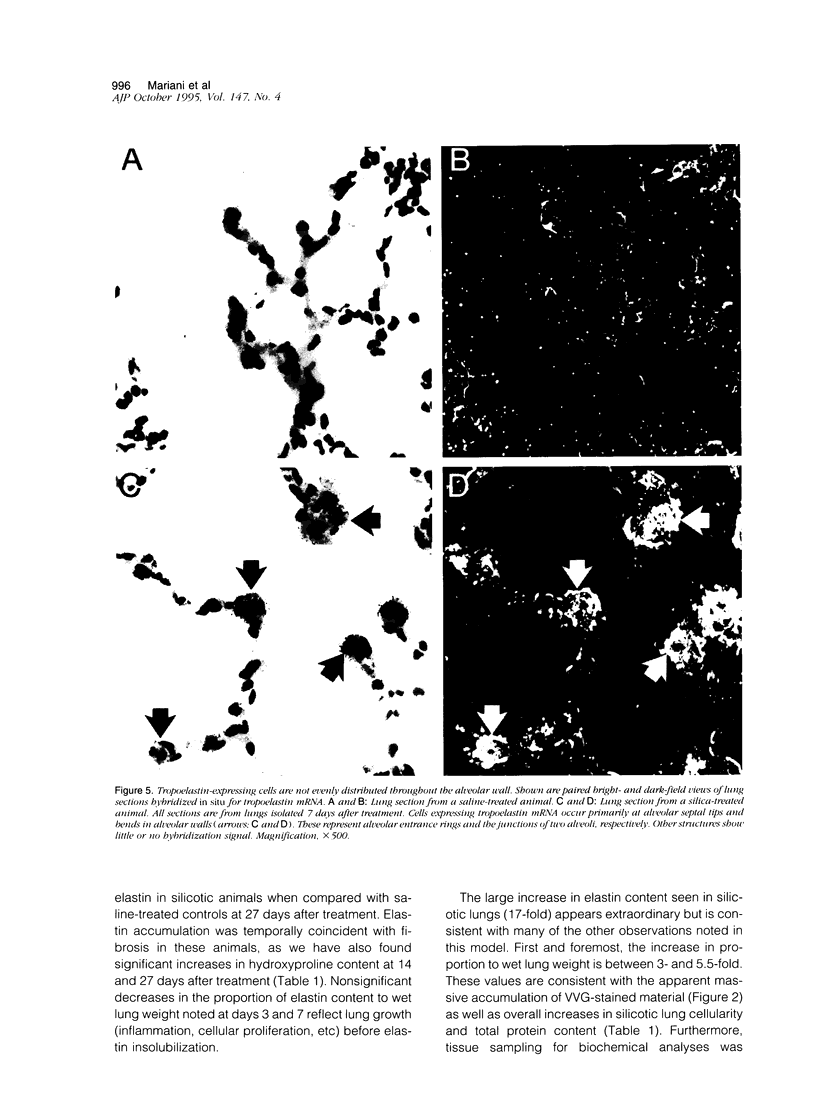
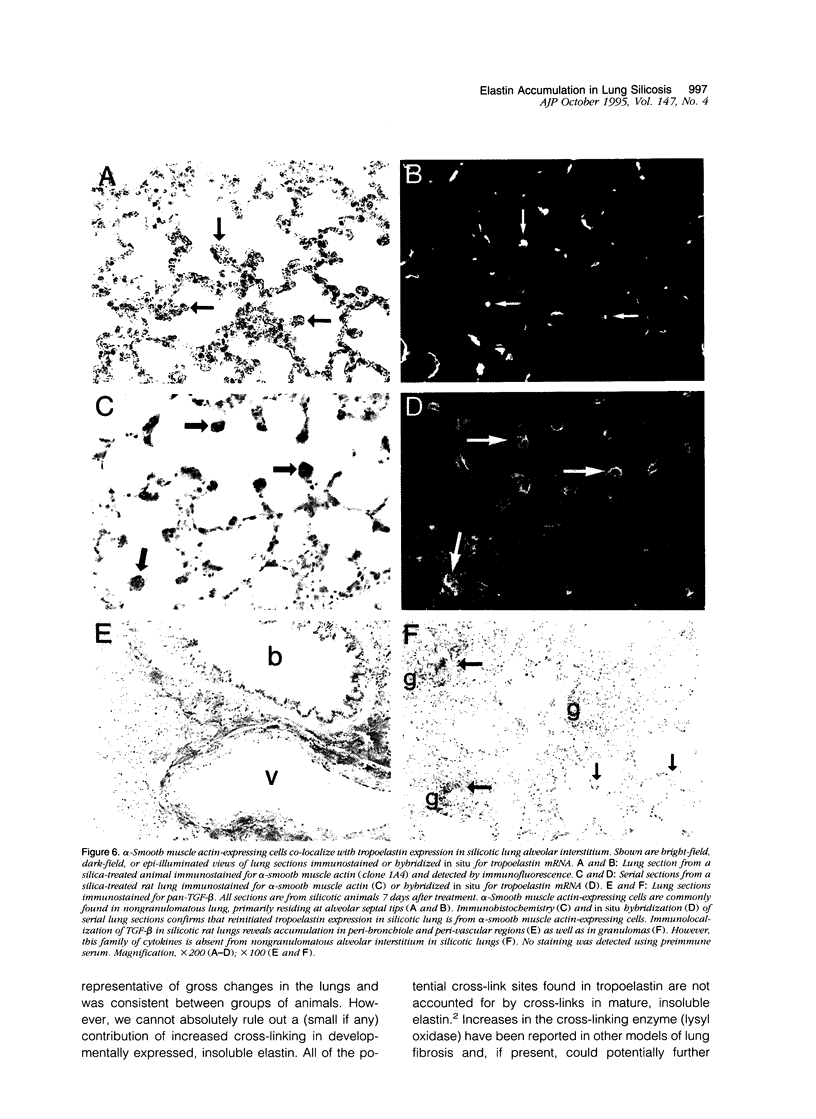
In the normal, healthy lung, elastin production is restricted to periods of development and growth. However, elastin expression in the adult lung has been observed in some forms of pulmonary injury, including pulmonary fibrosis. Here, we report that elastin production is significantly increased within precise interstitial compartments of the lung in an experimental model of granulomatous lung disease. An increase in the number and volume of elastic fibers within the alveolar walls was apparent on histological examination of Verhoeff-van Gieson-stained sections of silicotic rat lungs. Quantitation of mature elastin cross-links indicated that silicosis was accompanied by a 17-fold increase in lung elastin content when compared with values from saline-treated controls. In situ hybridization for tropoelastin mRNA revealed that elastin production was absent from granulomatous lesions yet was prominent at nonfibrotic alveolar septal tips, where a high density of elastic fibers is seen in the normal lung. Immunohistochemistry indicated tropoelastin was being expressed by alpha-smooth muscle actin-containing cells. Transforming growth factor-beta was immunolocalized to granulomatous regions of the silicotic lung but was absent from regions showing increased tropoelastin expression. These data indicate that the reinitiation of tropoelastin gene expression is associated with granulomatous lung disease, and this expression leads to the aberrant accumulation of mature elastin in the lung.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton I., Tomlinson A., Colville-Nash P. R., Willoughby D. A. Temporal and spatial immunolocalization of cytokines in murine chronic granulomatous tissue. Implications for their role in tissue development and repair processes. Lab Invest. 1993 Oct;69(4):405–414. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle P. T., Thurlbeck W. M. Postpneumonectomy compensatory lung growth. Am Rev Respir Dis. 1988 Nov;138(5):1314–1326. doi: 10.1164/ajrccm/138.5.1314. [DOI] [PubMed] [Google Scholar]
- Crouch E., Persson A., Chang D., Parghi D. Surfactant protein D. Increased accumulation in silica-induced pulmonary lipoproteinosis. Am J Pathol. 1991 Oct;139(4):765–776. [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Ribaux C., Chaponnier C., Gabbiani G. Cytoskeletal features of alveolar myofibroblasts and pericytes in normal human and rat lung. J Histochem Cytochem. 1992 Dec;40(12):1955–1963. doi: 10.1177/40.12.1333502. [DOI] [PubMed] [Google Scholar]
- King G. S., Mohan V. S., Starcher B. C. Radioimmunoassay for desmosine. Connect Tissue Res. 1980;7(4):263–267. doi: 10.3109/03008208009152362. [DOI] [PubMed] [Google Scholar]
- Last J. A., Gelzleichter T. R., Pinkerton K. E., Walker R. M., Witschi H. A new model of progressive pulmonary fibrosis in rats. Am Rev Respir Dis. 1993 Aug;148(2):487–494. doi: 10.1164/ajrccm/148.2.487. [DOI] [PubMed] [Google Scholar]
- Manthey C. L., Allen J. B., Ellingsworth L. R., Wahl S. M. In situ expression of transforming growth factor beta in streptococcal cell wall-induced granulomatous inflammation and hepatic fibrosis. Growth Factors. 1990;4(1):17–26. doi: 10.3109/08977199009011006. [DOI] [PubMed] [Google Scholar]
- McGowan S. E. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992 Aug;6(11):2895–2904. [PubMed] [Google Scholar]
- McGowan S. E. Influences of endogenous and exogenous TGF-beta on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am J Physiol. 1992 Aug;263(2 Pt 1):L257–L263. doi: 10.1152/ajplung.1992.263.2.L257. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Lange G. Antibodies to insoluble and solubilized elastin. Methods Enzymol. 1982;82(Pt A):744–759. doi: 10.1016/0076-6879(82)82099-5. [DOI] [PubMed] [Google Scholar]
- Mecham R. P., Whitehouse L. A., Wrenn D. S., Parks W. C., Griffin G. L., Senior R. M., Crouch E. C., Stenmark K. R., Voelkel N. F. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987 Jul 24;237(4813):423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- Mercer R. R., Crapo J. D. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol (1985) 1990 Aug;69(2):756–765. doi: 10.1152/jappl.1990.69.2.756. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest. 1978 Feb;38(2):188–200. [PubMed] [Google Scholar]
- Noguchi A., Samaha H. Developmental changes in tropoelastin gene expression in the rat lung studied by in situ hybridization. Am J Respir Cell Mol Biol. 1991 Dec;5(6):571–578. doi: 10.1165/ajrcmb/5.6.571. [DOI] [PubMed] [Google Scholar]
- Oldmixon E. H., Hoppin F. G., Jr Distribution of elastin and collagen in canine lung alveolar parenchyma. J Appl Physiol (1985) 1989 Nov;67(5):1941–1949. doi: 10.1152/jappl.1989.67.5.1941. [DOI] [PubMed] [Google Scholar]
- Parks W. C., Roby J. D. Consequences of prolonged inhalation of ozone on F344/N rats: collaborative studies. Part IV: Effects on expression of extracellular matrix genes. Res Rep Health Eff Inst. 1994 Oct;(65 Pt 4):3–29. [PubMed] [Google Scholar]
- Perez R. L., Jeon Y. J., Staton G. W., Jr, Roman J. Role of extracellular matrices, matrix receptors, and cytokines in granulomatous lung inflammation. Chest. 1993 Feb;103(2 Suppl):86S–87S. doi: 10.1378/chest.103.2_supplement.86s-a. [DOI] [PubMed] [Google Scholar]
- Pierce R. A., Deak S. B., Stolle C. A., Boyd C. D. Heterogeneity of rat tropoelastin mRNA revealed by cDNA cloning. Biochemistry. 1990 Oct 16;29(41):9677–9683. doi: 10.1021/bi00493a024. [DOI] [PubMed] [Google Scholar]
- Pierce R. A., Mariencheck W. I., Sandefur S., Crouch E. C., Parks W. C. Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am J Physiol. 1995 Mar;268(3 Pt 1):L491–L500. doi: 10.1152/ajplung.1995.268.3.L491. [DOI] [PubMed] [Google Scholar]
- Poiani G. J., Tozzi C. A., Yohn S. E., Pierce R. A., Belsky S. A., Berg R. A., Yu S. Y., Deak S. B., Riley D. J. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res. 1990 Apr;66(4):968–978. doi: 10.1161/01.res.66.4.968. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Rannels S. R. Compensatory growth of the lung following partial pneumonectomy. Exp Lung Res. 1988;14(2):157–182. doi: 10.3109/01902148809115122. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Kovacs S. O., Pentland A. P., Olerud J. E., Welgus H. G., Parks W. C. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993 Dec;92(6):2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. D., Endicott S. K., Province M. A., Pierce J. A., Campbell E. J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991 May;87(5):1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider G. L. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 2. Am Rev Respir Dis. 1992 Dec;146(6):1615–1622. doi: 10.1164/ajrccm/146.6.1615. [DOI] [PubMed] [Google Scholar]
- Starcher B. C. Elastin and the lung. Thorax. 1986 Aug;41(8):577–585. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcher B., Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res. 1995;31(2):133–140. doi: 10.3109/03008209509028401. [DOI] [PubMed] [Google Scholar]
- Sutcliffe M. C., Davidson J. M. Effect of static stretching on elastin production by porcine aortic smooth muscle cells. Matrix. 1990 Jul;10(3):148–153. doi: 10.1016/s0934-8832(11)80163-0. [DOI] [PubMed] [Google Scholar]
- Todorovich-Hunter L., Johnson D. J., Ranger P., Keeley F. W., Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest. 1988 Feb;58(2):184–195. [PubMed] [Google Scholar]
- Tozzi C. A., Poiani G. J., Harangozo A. M., Boyd C. D., Riley D. J. Pressure-induced connective tissue synthesis in pulmonary artery segments is dependent on intact endothelium. J Clin Invest. 1989 Sep;84(3):1005–1012. doi: 10.1172/JCI114221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyalov S. L., Gabbiani G., Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol. 1993 Dec;143(6):1754–1765. [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Williams A. O., Flanders K. C., Saffiotti U. Immunohistochemical localization of transforming growth factor-beta 1 in rats with experimental silicosis, alveolar type II hyperplasia, and lung cancer. Am J Pathol. 1993 Jun;142(6):1831–1840. [PMC free article] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Adler K. B., Low R. B. Immunohistochemical identification of cell types in normal and in bleomycin-induced fibrotic rat lung. Cellular origins of interstitial cells. Am Rev Respir Dis. 1984 Nov;130(5):910–916. doi: 10.1164/arrd.1984.130.5.910. [DOI] [PubMed] [Google Scholar]
- Zhang K., Rekhter M. D., Gordon D., Phan S. H. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994 Jul;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]