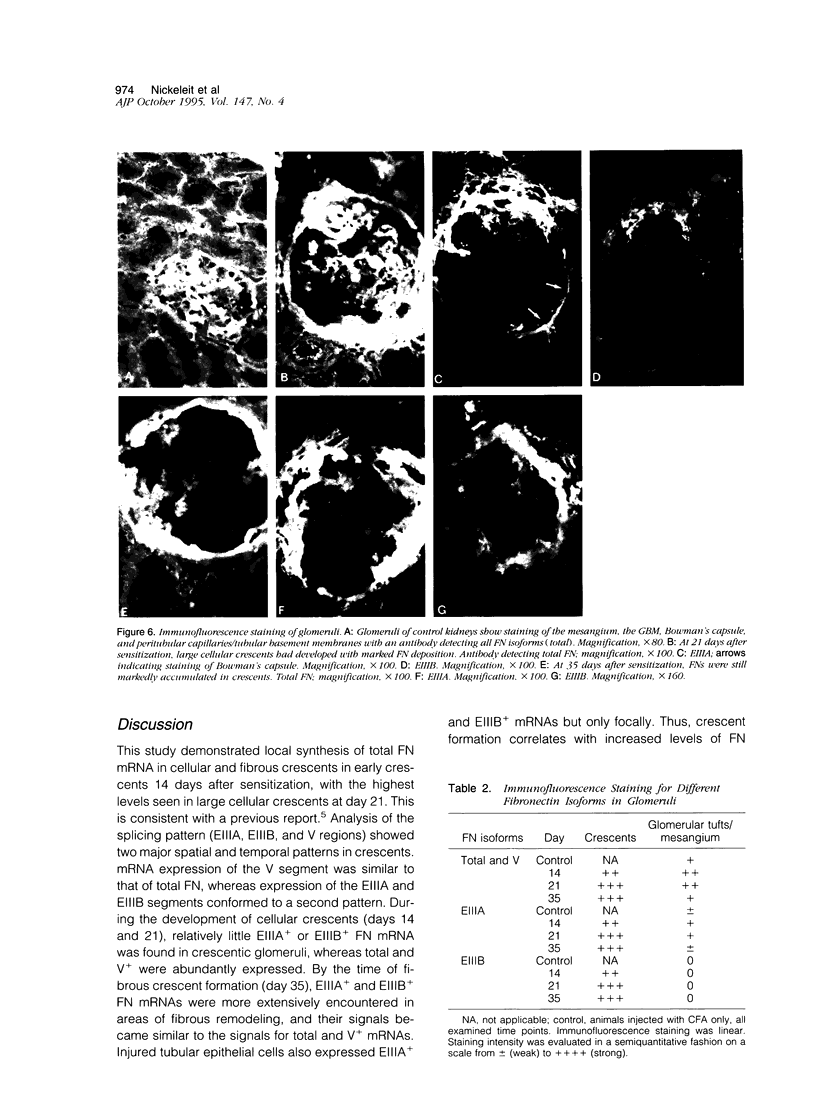
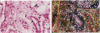Abstract
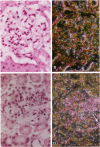
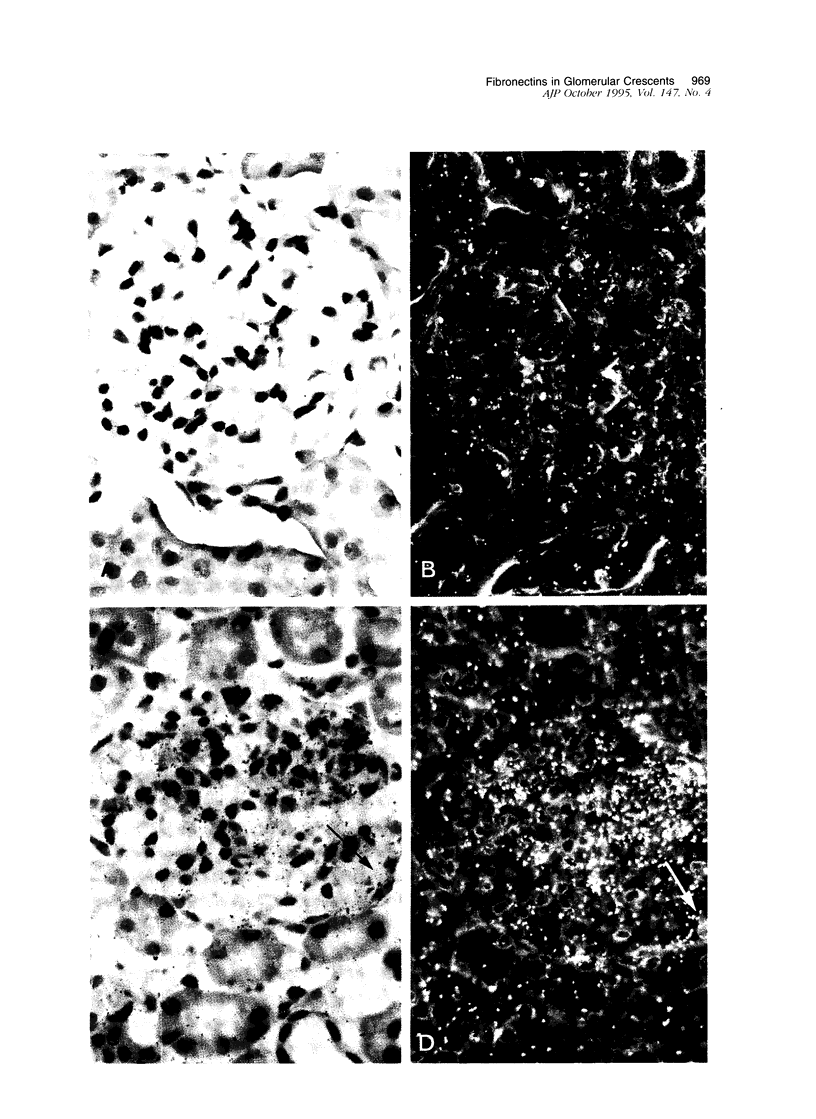
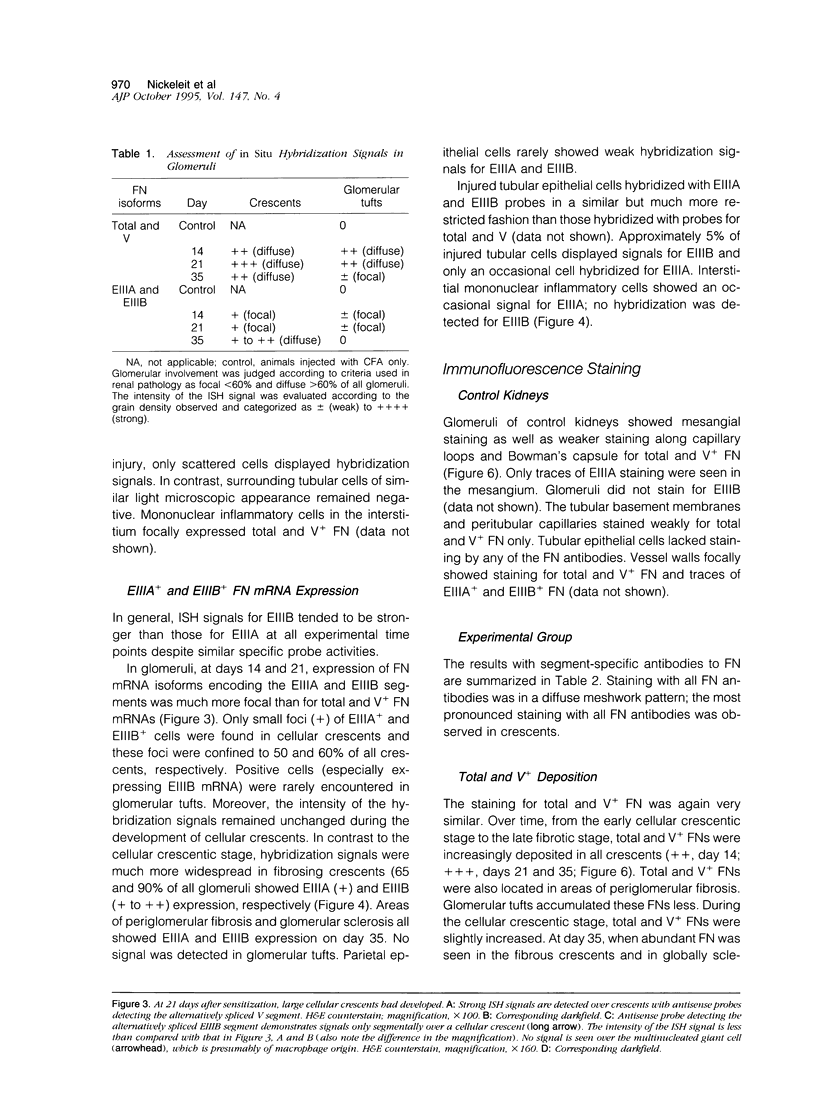
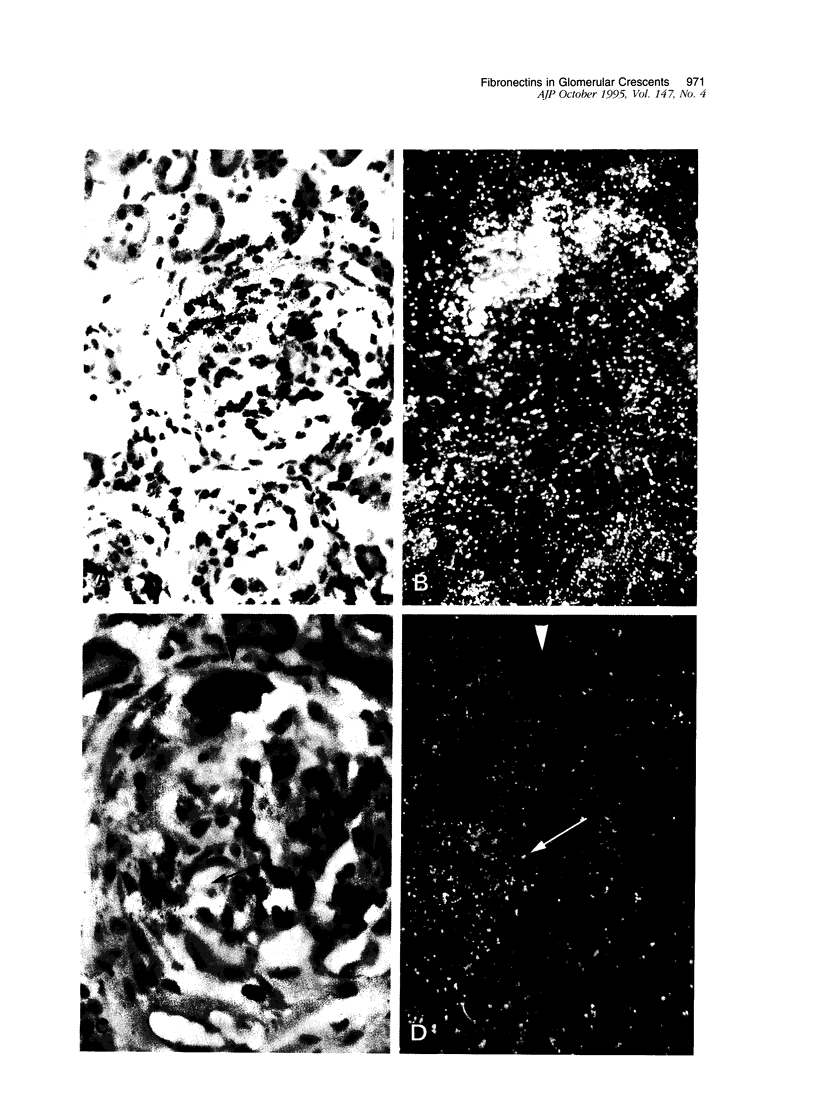
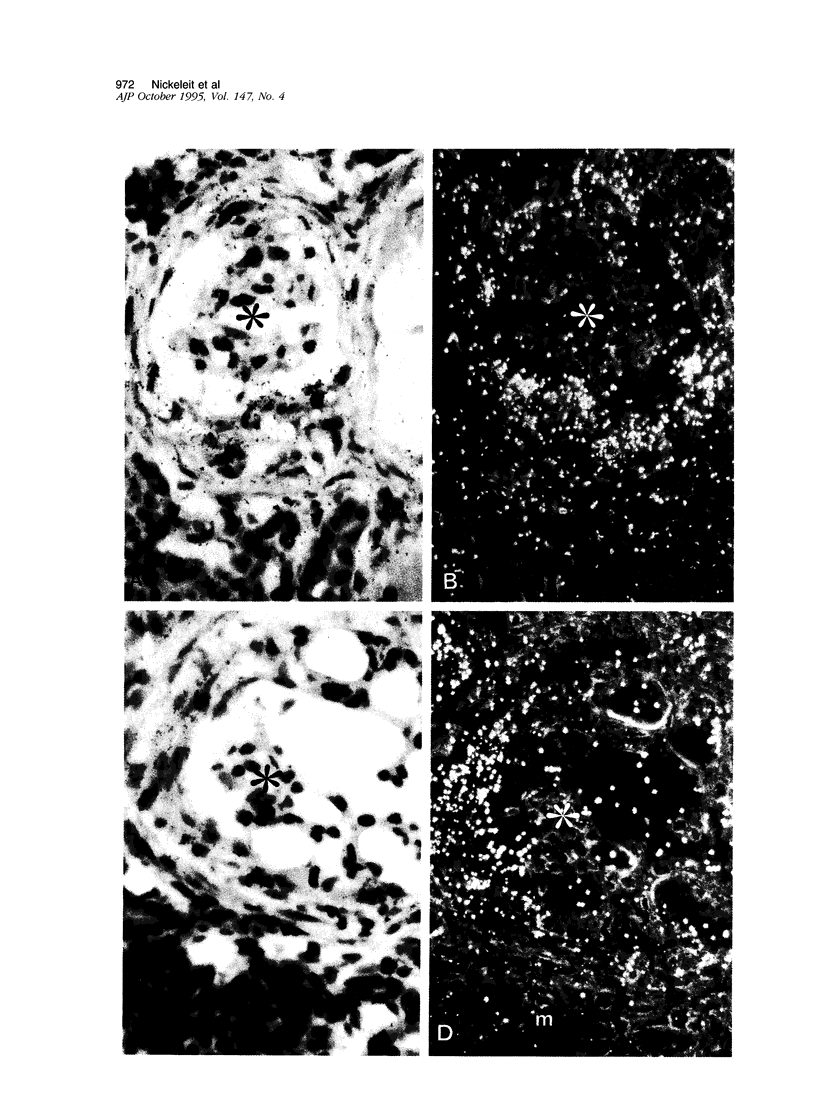
Crescents are a severe and stereotyped glomerular response to injury that occur in several forms of glomerulonephritis that progress to renal failure. The key pathogenetic step that leads to glomerular scarring in unknown, but fibronectin (FN), the clotting system, macrophages, and proliferating parietal epithelial cells are known to participate. This study was designed to determine whether FN is synthesized locally, and in what molecular isoform, and whether cytokines known to promote FN synthesis are present in the crescent. Rats immunized with bovine glomerular basement membrane develop cellular crescents by 14 days and fibrous crescents and glomerulosclerosis by 35 days. In situ hybridization was performed with oligonucleotides specific for sequences common to all FN isoforms (total FN) or sequences specific for the alternatively spliced segments (EIIIA, EIIIB, and V). Throughout the time period (14, 21, and 35 days) all crescents and glomerular tufts contained cells with strong ISH signals for total and V+ mRNA, with the strongest signals present in large cellular crescents at day 21. In contrast, EIIIA+ and EIIIB+ mRNAs showed maximal abundance within sclerosing crescents at 35 days. Protein deposition of EIIIA+, EIIIB+, and V+ FN isoforms was confirmed by immunofluorescence with segment-specific FN antibodies. Transforming growth factor-beta and interleukin-1 beta, both known to promote FN synthesis, were found in cellular crescents (days 14 and 21) and were still present, but greatly diminished, in the sclerotic phase (day 35). In summary, EIIIA-, EIIIB-, and V+ FN mRNA plasma isoforms predominate in cellular crescents, whereas in the fibrosing stage, mainly the oncofetal EIIIA+, EIIIB+, and V+ isoforms are synthesized and accumulate.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres G. A., Accinni L., Beiser S. M., Christian C. L., Cinotti G. A., Erlanger B. F., Hsu K. C., Seegal B. C. Localization of fluorescein-labeled antinucleoside antibodies in glomeruli of patients with active systemic lupus erythematosus nephritis. J Clin Invest. 1970 Nov;49(11):2106–2118. doi: 10.1172/JCI106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad L., Schwartz M. M., Virtanen I., Gould V. E. Immunolocalization of tenascin and cellular fibronectins in diverse glomerulopathies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(5):307–316. doi: 10.1007/BF02899277. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Balza E., Borsi L., Allemanni G., Zardi L. Transforming growth factor beta regulates the levels of different fibronectin isoforms in normal human cultured fibroblasts. FEBS Lett. 1988 Feb 8;228(1):42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Hastings R. R., De la Garza M. A. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994 Sep;145(3):585–597. [PMC free article] [PubMed] [Google Scholar]
- Border W. A., Noble N. A., Yamamoto T., Harper J. R., Yamaguchi Y. u., Pierschbacher M. D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992 Nov 26;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Castellani P., Rosellini C., Vecchio D., Allemanni G., Chang S. E., Taylor-Papadimitriou J., Pande H., Zardi L. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987 Mar;104(3):595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Dubin D., Lavigne L., Logan B., Dvorak H. F., Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993 Mar;142(3):793–801. [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Nielsen L. D., Howell S. E., Folkvord J. M. Human keratinocytes that have not terminally differentiated synthesize laminin and fibronectin but deposit only fibronectin in the pericellular matrix. J Cell Biochem. 1985;28(2):127–141. doi: 10.1002/jcb.240280206. [DOI] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Rabinovitch M. Increased interleukin-1 beta and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol. 1993 Jun;142(6):1772–1786. [PMC free article] [PubMed] [Google Scholar]
- Clausell N., Rabinovitch M. Upregulation of fibronectin synthesis by interleukin-1 beta in coronary artery smooth muscle cells is associated with the development of the post-cardiac transplant arteriopathy in piglets. J Clin Invest. 1993 Oct;92(4):1850–1858. doi: 10.1172/JCI116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra T., Wiggins R., Noh J. W., Merritt S., Phan S. H. Transforming growth factor-beta production in anti-glomerular basement membrane disease in the rabbit. Am J Pathol. 1991 Jan;138(1):223–234. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J., Clark R. A. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol. 1994 Oct;6(5):717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Goyal M., Wiggins R. Fibronectin mRNA and protein accumulation, distribution, and breakdown in rabbit anti-glomerular basement membrane disease. J Am Soc Nephrol. 1991 Jun;1(12):1334–1342. doi: 10.1681/ASN.V1121334. [DOI] [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- James M. P., Herdson P. B., Gavin J. B. The development of glomerular crescents in sheep. Pathology. 1981 Jul;13(3):441–448. doi: 10.3109/00313028109059062. [DOI] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Lester J., Fink S., Aronin N., DiFiglia M. Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res. 1993 Sep 3;621(1):106–110. doi: 10.1016/0006-8993(93)90303-5. [DOI] [PubMed] [Google Scholar]
- Lianos E. A., Orphanos V., Cattell V., Cook T., Anagnou N. Glomerular expression and cell origin of transforming growth factor-beta 1 in anti-glomerular basement membrane disease. Am J Med Sci. 1994 Jan;307(1):1–5. doi: 10.1097/00000441-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Magnuson V. L., Young M., Schattenberg D. G., Mancini M. A., Chen D. L., Steffensen B., Klebe R. J. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991 Aug 5;266(22):14654–14662. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M., Liao F., Hough G. P., Ross L. A., Van Lenten B. J., Rajavashisth T. B., Lusis A. J., Laks H., Drinkwater D. C., Fogelman A. M. Interaction of monocytes with cocultures of human aortic wall cells involves interleukins 1 and 6 with marked increases in connexin43 message. J Clin Invest. 1991 May;87(5):1763–1772. doi: 10.1172/JCI115195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Guo Y. J., Miyasaka M., Tamatani T., Collins A. B., Sy M. S., McCluskey R. T., Andres G. Antibodies to intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 prevent crescent formation in rat autoimmune glomerulonephritis. J Exp Med. 1993 Mar 1;177(3):667–677. doi: 10.1084/jem.177.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Tamkun J. W., Hynes R. O. Repeating modular structure of the fibronectin gene: relationship to protein structure and subunit variation. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6571–6575. doi: 10.1073/pnas.82.19.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Peters J. H., Trevithick J. E., Johnson P., Hynes R. O. Expression of the alternatively spliced EIIIB segment of fibronectin. Cell Adhes Commun. 1995 Feb;3(1):67–89. doi: 10.3109/15419069509081278. [DOI] [PubMed] [Google Scholar]
- Pettersson E. E., Colvin R. B. Cold-insoluble globulin (fibronectin, LETS protein) in normal and diseased human glomeruli: papain-sensitive attachment to normal glomeruli and deposition in crescents. Clin Immunol Immunopathol. 1978 Dec;11(4):425–436. doi: 10.1016/0090-1229(78)90170-8. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Paul J. I., Hynes R. O. On the origin of species of fibronectin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1424–1428. doi: 10.1073/pnas.82.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- VASSALLI P., MCCLUSKEY R. T. THE PATHOGENIC ROLE OF THE COAGULATION PROCESS IN RABBIT MASUGI NEPHRITIS. Am J Pathol. 1964 Oct;45:653–677. [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Murakami K., Okada M., Hino S., Miyamoto H., Maki S. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993 Feb;68(2):154–163. [PubMed] [Google Scholar]