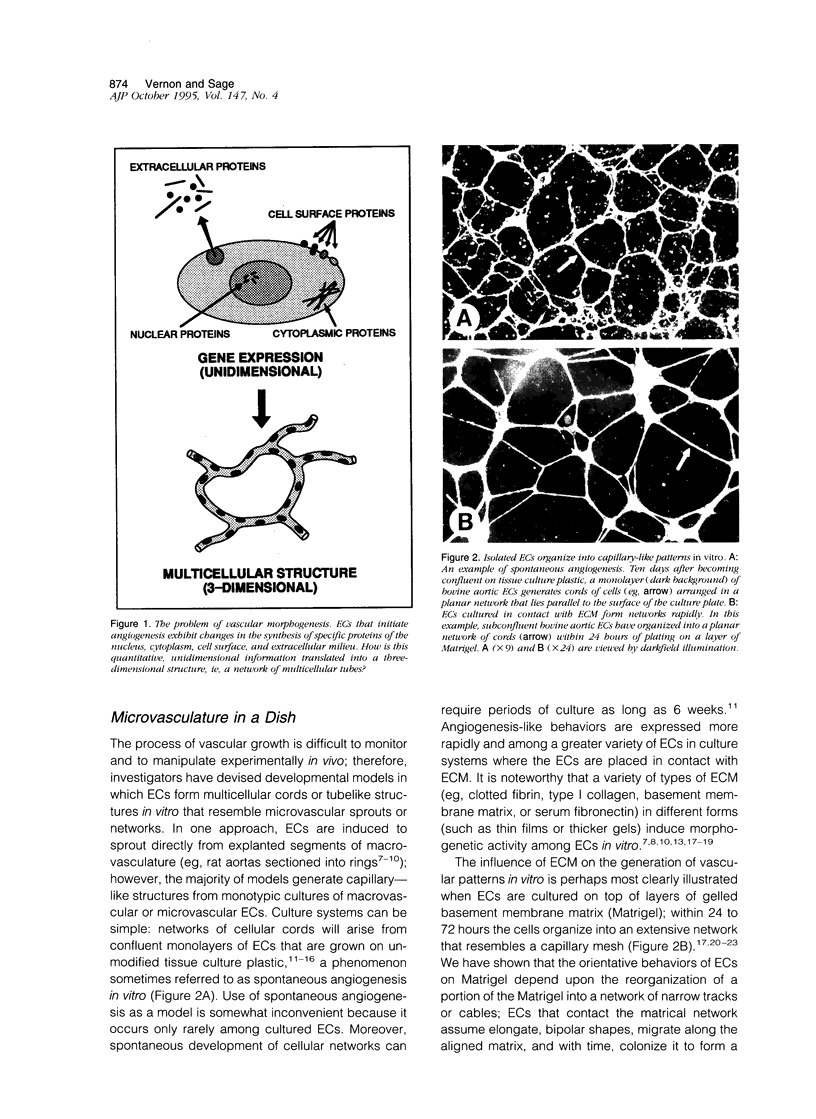
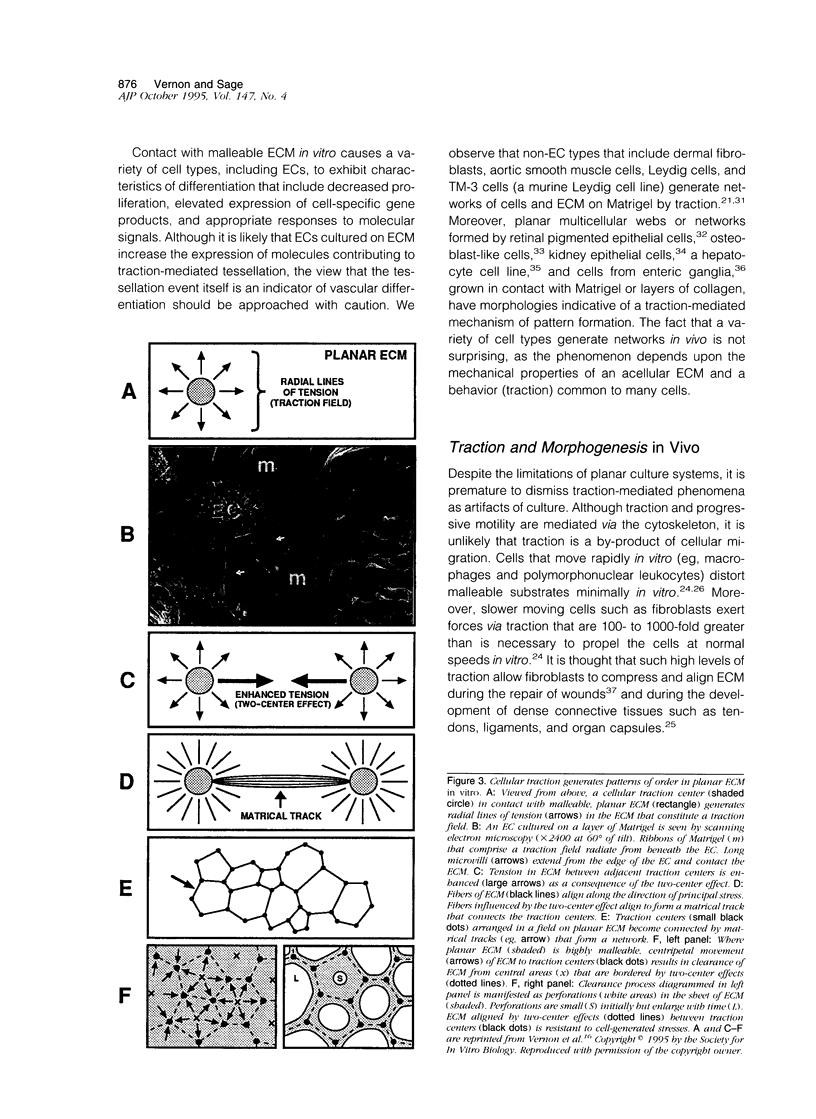
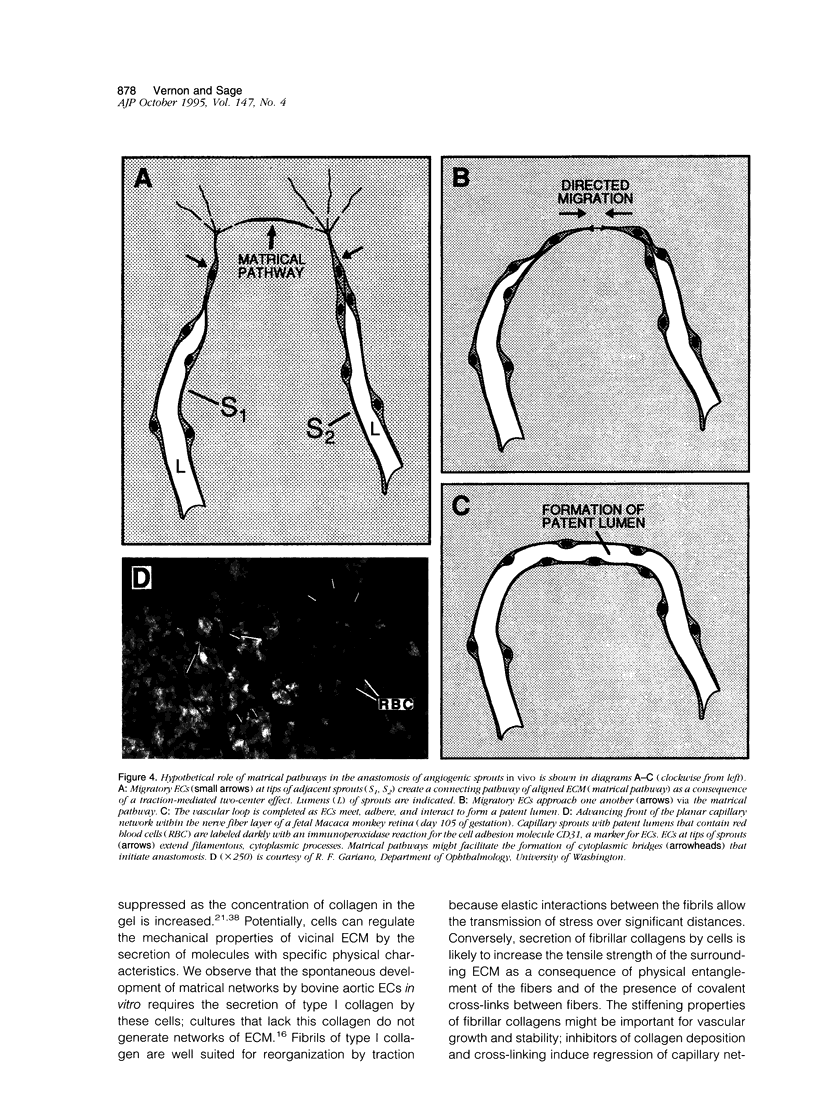
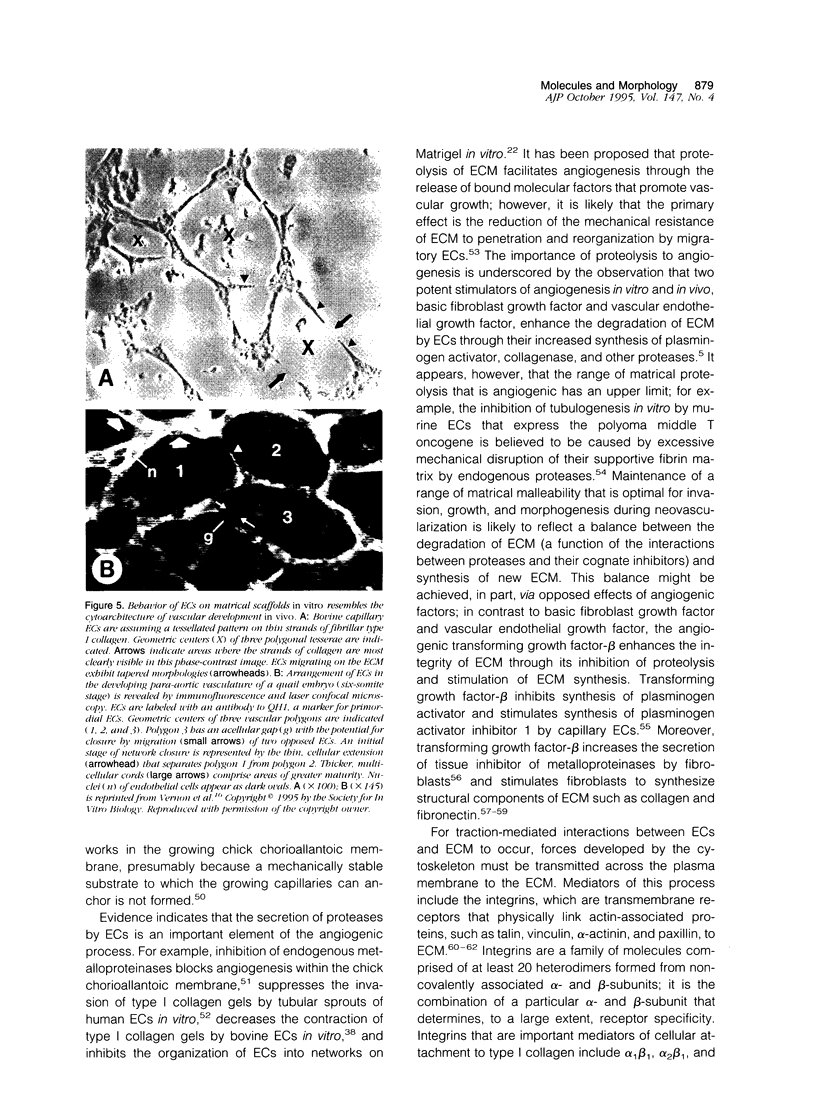
Abstract
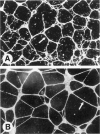
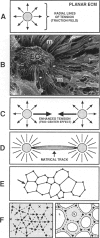
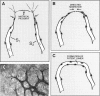
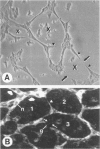
In response to an angiogenic stimulus, ECs initiate programs of gene expression that result in the quantitative alteration of gene products within nuclear, cytoplasmic, cell surface, and extracellular compartments. During the formation of new microvasculature, patterns of molecular expression among individual ECs must direct the creation of complex, multicellular morphologies in two and three dimensions. Studies in vitro indicate that cell-generated forces of tension can organize ECM into structures that direct the behavior of single cells (via influences on cellular elongation, alignment, and migration) and that provide positional information for the creation of multicellular patterns. Significantly, the formation of organized matrical structures is controlled by gene products (of ECs or other cell types that populate the ECM) that influence the balance between the forces of cellular tension and the viscoelastic resistance of the ECM. Regulation of relevant genes could be accomplished by soluble molecular signals (eg, growth factors) and/or solid-state signals arising from specific arrangements of cytoskeletal structure that, in turn, are a function of the equilibrium between cellular tension and matrical resistance. Within cells, information for the construction of complex organelles is encoded in the shapes of the constituent molecules. Similarly, the creation of complex vascular architecture must be mediated by molecular shapes, a fact that is readily apparent in simple receptor-ligand interactions such as the binding of growth factors to ECs or the attachment of ECs to one another. However, between molecules and morphology also exists a set of multilayered, interactive, multimolecular systems that establish vascular form at unicellular and multicellular levels. Characterization of these systems is an elusive target that resides at the frontier of vascular biology; the identification of models in vitro that accurately reproduce macroscale events of vascular morphogenesis should advance considerably our understanding of vascular development and lead to an elucidation of its regulation in vivo.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlén K., Rubin K. Platelet-derived growth factor-BB stimulates synthesis of the integrin alpha 2-subunit in human diploid fibroblasts. Exp Cell Res. 1994 Dec;215(2):347–353. doi: 10.1006/excr.1994.1351. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Ausprunk D. H., Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977 Jul;14(1):53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Battegay E. J., Rupp J., Iruela-Arispe L., Sage E. H., Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994 May;125(4):917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J., Margolis M., Schreiner C., Edgell C. J., Azizkhan J., Lazarowski E., Juliano R. L. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J Cell Physiol. 1992 Dec;153(3):437–449. doi: 10.1002/jcp.1041530302. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C., Yeh R. K. Talin: role at sites of cell-substratum adhesion. Cell Motil Cytoskeleton. 1990;16(1):7–13. doi: 10.1002/cm.970160103. [DOI] [PubMed] [Google Scholar]
- Bergsteinsdottir K., Hashimoto Y., Brennan A., Mirsky R., Jessen K. R. The effect of three dimensional collagen type I preparation on the structural organization of guinea pig enteric ganglia in culture. Exp Cell Res. 1993 Nov;209(1):64–75. doi: 10.1006/excr.1993.1286. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Burri P. H. Intussusceptive microvascular growth, a new mechanism of capillary network formation. EXS. 1992;61:32–39. doi: 10.1007/978-3-0348-7001-6_8. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. E. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Davis G. E., Camarillo C. W. Regulation of endothelial cell morphogenesis by integrins, mechanical forces, and matrix guidance pathways. Exp Cell Res. 1995 Jan;216(1):113–123. doi: 10.1006/excr.1995.1015. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Grammas P., Giacomelli F., Wiener J. Angiogenesis in rat aorta ring explant cultures. Lab Invest. 1989 Apr;60(4):523–531. [PubMed] [Google Scholar]
- Drake C. J., Davis L. A., Little C. D. Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn. 1992 Jan;193(1):83–91. doi: 10.1002/aja.1001930111. [DOI] [PubMed] [Google Scholar]
- Enenstein J., Kramer R. H. Confocal microscopic analysis of integrin expression on the microvasculature and its sprouts in the neonatal foreskin. J Invest Dermatol. 1994 Sep;103(3):381–386. doi: 10.1111/1523-1747.ep12395390. [DOI] [PubMed] [Google Scholar]
- Feder J., Marasa J. C., Olander J. V. The formation of capillary-like tubes by calf aortic endothelial cells grown in vitro. J Cell Physiol. 1983 Jul;116(1):1–6. doi: 10.1002/jcp.1041160102. [DOI] [PubMed] [Google Scholar]
- Fisher C., Gilbertson-Beadling S., Powers E. A., Petzold G., Poorman R., Mitchell M. A. Interstitial collagenase is required for angiogenesis in vitro. Dev Biol. 1994 Apr;162(2):499–510. doi: 10.1006/dbio.1994.1104. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Fournier N., Doillon C. J. In vitro angiogenesis in fibrin matrices containing fibronectin or hyaluronic acid. Cell Biol Int Rep. 1992 Dec;16(12):1251–1263. doi: 10.1016/s0309-1651(06)80042-1. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Matthias L. J., Meyer G., Kaur P., Russ G., Faull R., Berndt M. C., Vadas M. A. Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol. 1993 May;121(4):931–943. doi: 10.1083/jcb.121.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Davis G. E., Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992 Mar;10(2):111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Warner P. Generation of spatially periodic patterns by a mechanical instability: a mechanical alternative to the Turing model. J Embryol Exp Morphol. 1984 Apr;80:1–20. [PubMed] [Google Scholar]
- Harris A. K., Stopak D., Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981 Mar 19;290(5803):249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- Harris A. K., Wild P., Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980 Apr 11;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ingber D. E., Dike L., Hansen L., Karp S., Liley H., Maniotis A., McNamee H., Mooney D., Plopper G., Sims J. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D., Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988 Jul;59(1):44–51. [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Diglio C. A., Sage E. H. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- Jackson C. J., Jenkins K. L. Type I collagen fibrils promote rapid vascular tube formation upon contact with the apical side of cultured endothelium. Exp Cell Res. 1991 Jan;192(1):319–323. doi: 10.1016/0014-4827(91)90194-y. [DOI] [PubMed] [Google Scholar]
- Kennedy A., Frank R. N., Sotolongo L. B., Das A., Zhang N. L. Proliferative response and macromolecular synthesis by ocular cells cultured on extracellular matrix materials. Curr Eye Res. 1990 Apr;9(4):307–322. doi: 10.3109/02713689008999619. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Dressel D., Steinmayer T., Mauch C., Eckes B., Krieg T., Bankert R. B., Weber L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991 Dec;115(5):1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Giancotti F. G., Presta M., Albelda S. M., Buck C. A., Rifkin D. B. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993 Oct;4(10):973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konerding M. A., van Ackern C., Steinberg F., Streffer C. Combined morphological approaches in the study of network formation in tumor angiogenesis. EXS. 1992;61:40–58. doi: 10.1007/978-3-0348-7001-6_9. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald R. R., Fitzharris T. P., Bolender D. L., Bernanke D. H. Sturctural analysis of cell:matrix association during the morphogenesis of atrioventricular cushion tissue. Dev Biol. 1979 Apr;69(2):634–654. doi: 10.1016/0012-1606(79)90317-8. [DOI] [PubMed] [Google Scholar]
- Marx M., Perlmutter R. A., Madri J. A. Modulation of platelet-derived growth factor receptor expression in microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1994 Jan;93(1):131–139. doi: 10.1172/JCI116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Orci L. Phorbol esters induce angiogenesis in vitro from large-vessel endothelial cells. J Cell Physiol. 1987 Feb;130(2):284–291. doi: 10.1002/jcp.1041300215. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Montesano R., Pepper M. S., Möhle-Steinlein U., Risau W., Wagner E. F., Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990 Aug 10;62(3):435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Sadahira Y., Kawasaki S., Hayashi T., Notohara K., Awai M. Capillary growth from reversed rat aortic segments cultured in collagen gel. Acta Pathol Jpn. 1988 Dec;38(12):1503–1512. doi: 10.1111/j.1440-1827.1988.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Rifkin D. B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Moses M. A., Sudhalter J., Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990 Jun 15;248(4961):1408–1410. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- Nehls V., Schuchardt E., Drenckhahn D. The effect of fibroblasts, vascular smooth muscle cells, and pericytes on sprout formation of endothelial cells in a fibrin gel angiogenesis system. Microvasc Res. 1994 Nov;48(3):349–363. doi: 10.1006/mvre.1994.1061. [DOI] [PubMed] [Google Scholar]
- Nicosia R. F., Tchao R., Leighton J. Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Res. 1983 May;43(5):2159–2166. [PubMed] [Google Scholar]
- Olivo M., Bhardwaj R., Schulze-Osthoff K., Sorg C., Jacob H. J., Flamme I. A comparative study on the effects of tumor necrosis factor-alpha (TNF-alpha), human angiogenic factor (h-AF) and basic fibroblast growth factor (bFGF) on the chorioallantoic membrane of the chick embryo. Anat Rec. 1992 Sep;234(1):105–115. doi: 10.1002/ar.1092340112. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Paku S., Paweletz N. First steps of tumor-related angiogenesis. Lab Invest. 1991 Sep;65(3):334–346. [PubMed] [Google Scholar]
- Patan S., Alvarez M. J., Schittny J. C., Burri P. H. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histol Cytol. 1992;55 (Suppl):65–75. doi: 10.1679/aohc.55.suppl_65. [DOI] [PubMed] [Google Scholar]
- Paweletz N., Knierim M. Tumor-related angiogenesis. Crit Rev Oncol Hematol. 1989;9(3):197–242. doi: 10.1016/s1040-8428(89)80002-2. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Gharaee-Kermani M., Wolber F., Ryan U. S. Stimulation of rat endothelial cell transforming growth factor-beta production by bleomycin. J Clin Invest. 1991 Jan;87(1):148–154. doi: 10.1172/JCI114964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. J., Vernon R. B., Abrass I. B., Sage E. H. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994 Jan;158(1):169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Sage E. H., Vernon R. B. Regulation of angiogenesis by extracellular matrix: the growth and the glue. J Hypertens Suppl. 1994 Dec;12(10):S145–S152. [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Rifkin D. B. The opposing effects of basic fibroblast growth factor and transforming growth factor beta on the regulation of plasminogen activator activity in capillary endothelial cells. J Cell Biol. 1987 Aug;105(2):957–963. doi: 10.1083/jcb.105.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiro J. A., Chan B. M., Roswit W. T., Kassner P. D., Pentland A. P., Hemler M. E., Eisen A. Z., Kupper T. S. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991 Oct 18;67(2):403–410. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- Schnaper H. W., Grant D. S., Stetler-Stevenson W. G., Fridman R., D'Orazi G., Murphy A. N., Bird R. E., Hoythya M., Fuerst T. R., French D. L. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993 Aug;156(2):235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- Stopak D., Harris A. K. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol. 1982 Apr;90(2):383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- Taub M., Wang Y., Szczesny T. M., Kleinman H. K. Epidermal growth factor or transforming growth factor alpha is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc Natl Acad Sci U S A. 1990 May;87(10):4002–4006. doi: 10.1073/pnas.87.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. E., Glenney J. R., Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990 Sep;111(3):1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. B., Angello J. C., Iruela-Arispe M. L., Lane T. F., Sage E. H. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992 May;66(5):536–547. [PubMed] [Google Scholar]
- Vernon R. B., Lane T. F., Angello J. C., Sage H. Adhesion, shape, proliferation, and gene expression of mouse Leydig cells are influenced by extracellular matrix in vitro. Biol Reprod. 1991 Jan;44(1):157–170. doi: 10.1095/biolreprod44.1.157. [DOI] [PubMed] [Google Scholar]
- Vernon R. B., Lara S. L., Drake C. J., Iruela-Arispe M. L., Angello J. C., Little C. D., Wight T. N., Sage E. H. Organized type I collagen influences endothelial patterns during "spontaneous angiogenesis in vitro": planar cultures as models of vascular development. In Vitro Cell Dev Biol Anim. 1995 Feb;31(2):120–131. doi: 10.1007/BF02633972. [DOI] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Kleinman H. K., Reddi A. H. Differentiation of canalicular cell processes in bone cells by basement membrane matrix components: regulation by discrete domains of laminin. Cell. 1990 Oct 19;63(2):437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- Zagzag D. Angiogenic growth factors in neural embryogenesis and neoplasia. Am J Pathol. 1995 Feb;146(2):293–309. [PMC free article] [PubMed] [Google Scholar]
- Zutter M. M., Santoro S. A. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990 Jul;137(1):113–120. [PMC free article] [PubMed] [Google Scholar]