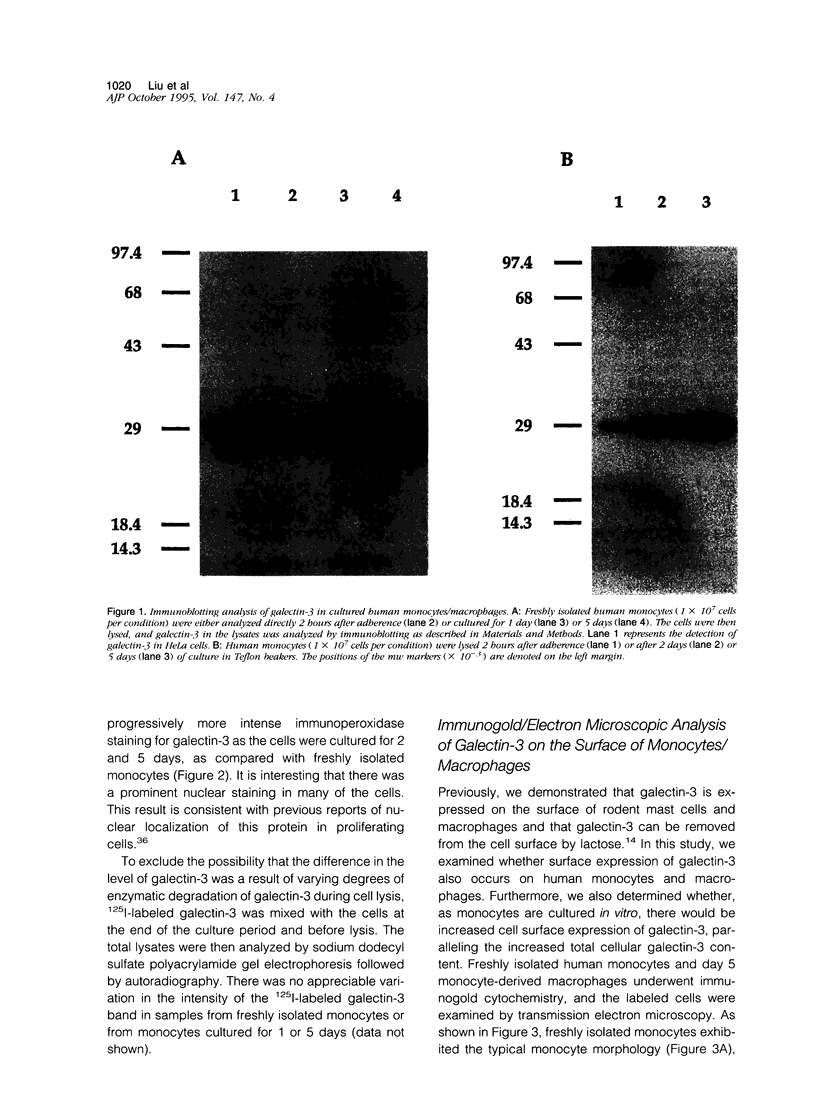
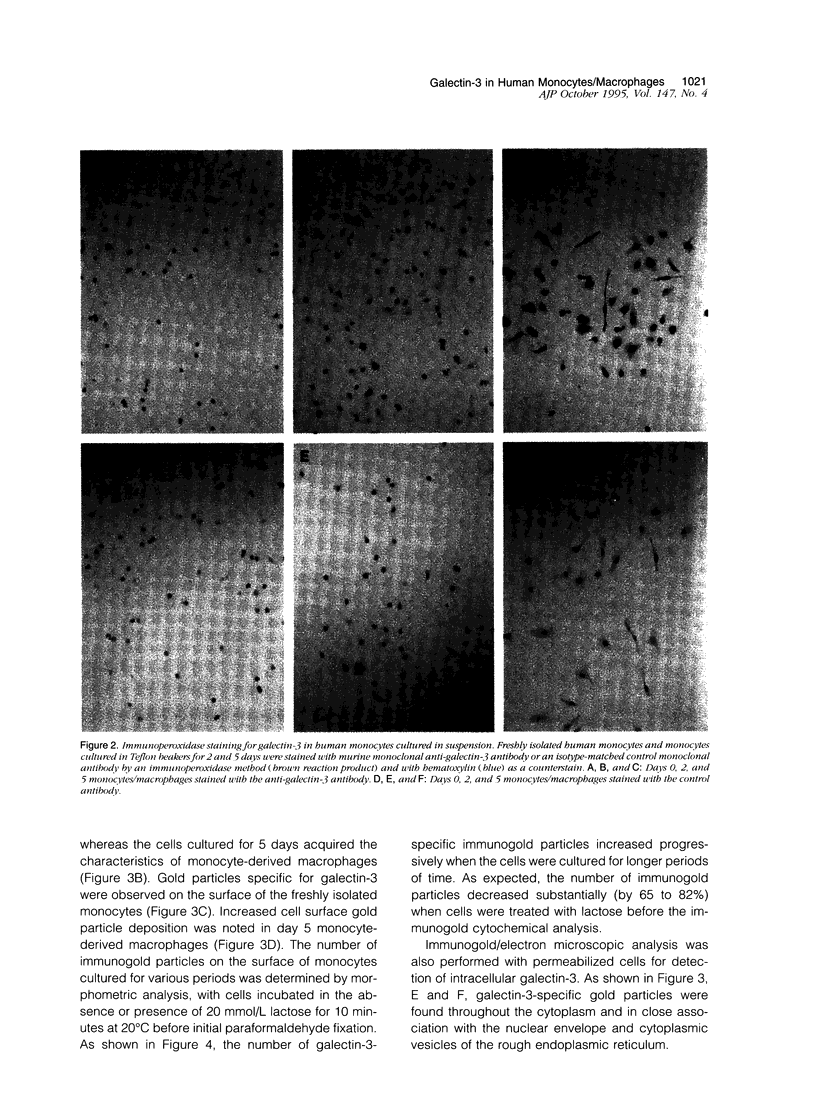
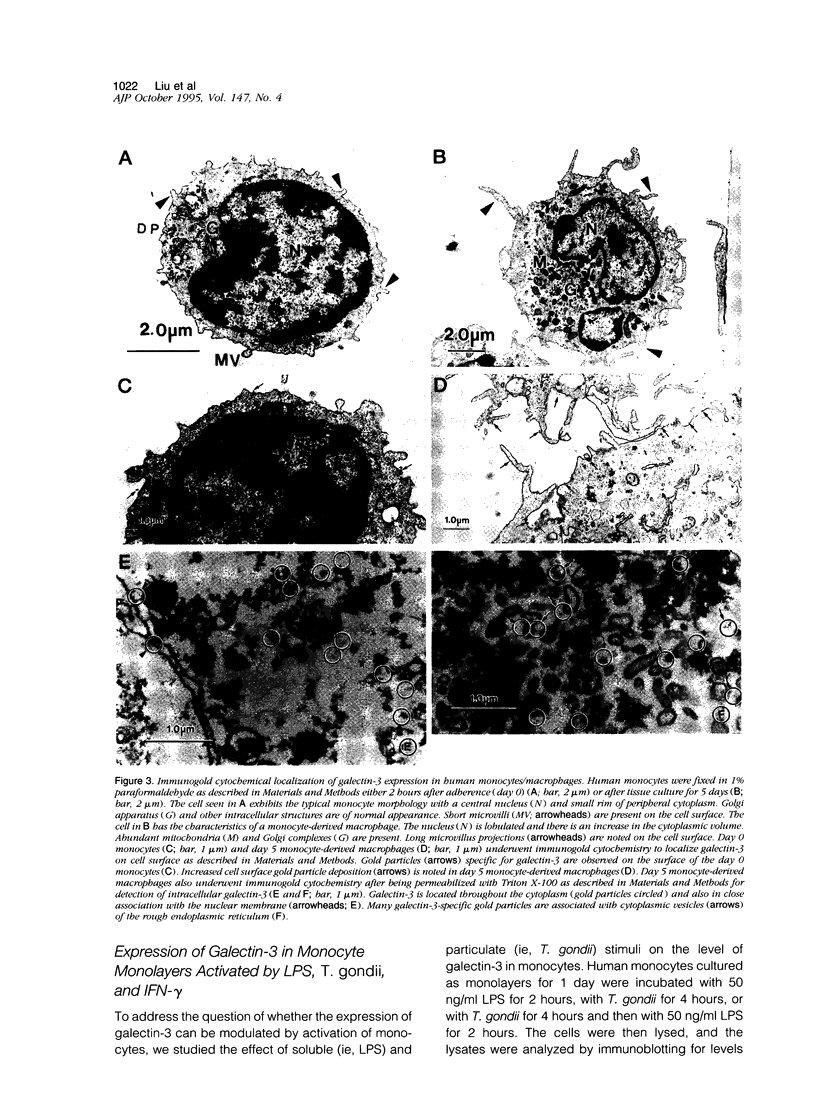
Abstract
A family of beta-galactoside-binding animal lectins has recently been designated as galectins. One member of this family, galectin-3, has been known as epsilon BP for its IgE-binding activity and as Mac-2, a macrophage surface antigen, CBP35, CBP30, L-29, and L-34. Although much information has accumulated on the expression of this lectin in murine macrophages and human monocytic cell lines, little is known about the expression and function of this protein in normal human monocytes/macrophages. We now report that galectin-3 is expressed in normal human peripheral blood monocytes and its level increases dramatically as human monocytes differentiate into macrophages upon culturing in vitro. Immunoblot analysis showed that there was a 5-fold increase in the level of galectin-3 after 1 day of culture and greater than a 12-fold increase after 5 days. Immunocytochemical analysis confirmed this progressive increase of galectin-3 expression in cultured monocytes. Immunogold cytochemistry/electron microscopy analysis revealed that galectin-3 was expressed on the surface of human monocytes and that the level of cell surface galectin-3 increased progressively as these cells differentiated into macrophages. The level of galectin-3 in human monocytes/macrophages was modulated by stimuli such as lipopolysaccharide and interferon-gamma, and galectin-3 was secreted when monocytes were stimulated by calcium ionophore A23187 Soluble galectin-3 caused superoxide release from human monocytes; this activity was dependent on the lectin property of galectin-3, as it was inhibitable by lactose. Thus, galectin-3 may modulate the function of this cell type in an autocrine or paracrine fashion through binding to cell surface glycoconjugates.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Albrandt K., Orida N. K., Liu F. T. An IgE-binding protein with a distinctive repetitive sequence and homology with an IgG receptor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6859–6863. doi: 10.1073/pnas.84.19.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994 Feb 25;76(4):597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807–20810. [PubMed] [Google Scholar]
- Cherayil B. J., Chaitovitz S., Wong C., Pillai S. Molecular cloning of a human macrophage lectin specific for galactose. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7324–7328. doi: 10.1073/pnas.87.18.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil B. J., Weiner S. J., Pillai S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J Exp Med. 1989 Dec 1;170(6):1959–1972. doi: 10.1084/jem.170.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Barondes S. H. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990 May;110(5):1681–1691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Flotte T. J., Springer T. A., Thorbecke G. J. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983 Apr;111(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Frigeri L. G., Liu F. T. Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J Immunol. 1992 Feb 1;148(3):861–867. [PubMed] [Google Scholar]
- Frigeri L. G., Robertson M. W., Liu F. T. Expression of biologically active recombinant rat IgE-binding protein in Escherichia coli. J Biol Chem. 1990 Dec 5;265(34):20763–20769. [PubMed] [Google Scholar]
- Frigeri L. G., Zuberi R. I., Liu F. T. Epsilon BP, a beta-galactoside-binding animal lectin, recognizes IgE receptor (Fc epsilon RI) and activates mast cells. Biochemistry. 1993 Aug 3;32(30):7644–7649. doi: 10.1021/bi00081a007. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y., Takahashi T., Kuroishi T., Hibi K., Suyama M., Niimi T., Shimokata K., Yamakawa K., Nakamura Y., Ueda R. Prognostic significance of p53 mutations and 3p deletions in primary resected non-small cell lung cancer. Cancer Res. 1993 Jan 1;53(1):1–4. [PubMed] [Google Scholar]
- Hsu D. K., Zuberi R. I., Liu F. T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992 Jul 15;267(20):14167–14174. [PubMed] [Google Scholar]
- Hughes R. C. Mac-2: a versatile galactose-binding protein of mammalian tissues. Glycobiology. 1994 Feb;4(1):5–12. doi: 10.1093/glycob/4.1.5. [DOI] [PubMed] [Google Scholar]
- Jia S., Wang J. L. Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J Biol Chem. 1988 May 5;263(13):6009–6011. [PubMed] [Google Scholar]
- Koths K., Taylor E., Halenbeck R., Casipit C., Wang A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J Biol Chem. 1993 Jul 5;268(19):14245–14249. [PubMed] [Google Scholar]
- Leenen P. J., Jansen A. M., van Ewijk W. Murine macrophage cell lines can be ordered in a linear differentiation sequence. Differentiation. 1986;32(2):157–164. doi: 10.1111/j.1432-0436.1986.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Leffler H., Masiarz F. R., Barondes S. H. Soluble lactose-binding vertebrate lectins: a growing family. Biochemistry. 1989 Nov 14;28(23):9222–9229. doi: 10.1021/bi00449a039. [DOI] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981 Jun 10;256(11):5735–5740. [PubMed] [Google Scholar]
- Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E., Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993 Jun 5;268(16):11750–11757. [PubMed] [Google Scholar]
- Liu F. T., Albrandt K., Mendel E., Kulczycki A., Jr, Orida N. K. Identification of an IgE-binding protein by molecular cloning. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4100–4104. doi: 10.1073/pnas.82.12.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T. S-type mammalian lectins in allergic inflammation. Immunol Today. 1993 Oct;14(10):486–490. doi: 10.1016/0167-5699(93)90263-K. [DOI] [PubMed] [Google Scholar]
- Mayo L. A., Curnutte J. T. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mehul B., Bawumia S., Martin S. R., Hughes R. C. Structure of baby hamster kidney carbohydrate-binding protein CBP30, an S-type animal lectin. J Biol Chem. 1994 Jul 8;269(27):18250–18258. [PubMed] [Google Scholar]
- Metcalfe D. D., Thompson H. L., Klebanoff S. J., Henderson W. R., Jr Oxidative degradation of rat mast-cell heparin proteoglycan. Biochem J. 1990 Nov 15;272(1):51–57. doi: 10.1042/bj2720051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsatsos I. K., Wade M., Schindler M., Wang J. L. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6452–6456. doi: 10.1073/pnas.84.18.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill M. A., Henderson W. R., Klebanoff S. J. Oxidative degradation of leukotriene C4 by human monocytes and monocyte-derived macrophages. J Exp Med. 1985 Nov 1;162(5):1634–1644. doi: 10.1084/jem.162.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P. H., Leijh P. C., van Furth R. Quantitative immunocytochemical characterization of mononuclear phagocytes. I. Monoblasts, promonocytes, monocytes, and peritoneal and alveolar macrophages. Cell Immunol. 1987 Apr 1;105(2):374–385. doi: 10.1016/0008-8749(87)90085-2. [DOI] [PubMed] [Google Scholar]
- Oda S., Sato M., Toyoshima S., Osawa T. Binding of activated macrophages to tumor cells through a macrophage lectin and its role in macrophage tumoricidal activity. J Biochem. 1989 Jun;105(6):1040–1043. doi: 10.1093/oxfordjournals.jbchem.a122763. [DOI] [PubMed] [Google Scholar]
- Oda S., Sato M., Toyoshima S., Osawa T. Purification and characterization of a lectin-like molecule specific for galactose/N-acetyl-galactosamine from tumoricidal macrophages. J Biochem. 1988 Oct;104(4):600–605. doi: 10.1093/oxfordjournals.jbchem.a122518. [DOI] [PubMed] [Google Scholar]
- Raz A., Pazerini G., Carmi P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 1989 Jul 1;49(13):3489–3493. [PubMed] [Google Scholar]
- Robertson M. W., Albrandt K., Keller D., Liu F. T. Human IgE-binding protein: a soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry. 1990 Sep 4;29(35):8093–8100. doi: 10.1021/bi00487a015. [DOI] [PubMed] [Google Scholar]
- Sato S., Burdett I., Hughes R. C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993 Jul;207(1):8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Control of Mac-2 surface expression on murine macrophage cell lines. Eur J Immunol. 1994 Jan;24(1):216–221. doi: 10.1002/eji.1830240134. [DOI] [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J Biol Chem. 1994 Feb 11;269(6):4424–4430. [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins as cell recognition molecules. Science. 1989 Oct 13;246(4927):227–234. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- Stahl P. D. The mannose receptor and other macrophage lectins. Curr Opin Immunol. 1992 Feb;4(1):49–52. doi: 10.1016/0952-7915(92)90123-v. [DOI] [PubMed] [Google Scholar]
- Truong M. J., Gruart V., Kusnierz J. P., Papin J. P., Loiseau S., Capron A., Capron M. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J Exp Med. 1993 Jan 1;177(1):243–248. doi: 10.1084/jem.177.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M. J., Gruart V., Liu F. T., Prin L., Capron A., Capron M. IgE-binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur J Immunol. 1993 Dec;23(12):3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- Woo H. J., Shaw L. M., Messier J. M., Mercurio A. M. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem. 1990 May 5;265(13):7097–7099. [PubMed] [Google Scholar]
- Yamaoka A., Kuwabara I., Frigeri L. G., Liu F. T. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J Immunol. 1995 Apr 1;154(7):3479–3487. [PubMed] [Google Scholar]
- Yong E. C., Chi E. Y., Fritsche T. R., Henderson W. R., Jr Human platelet-mediated cytotoxicity against Toxoplasma gondii: role of thromboxane. J Exp Med. 1991 Jan 1;173(1):65–78. doi: 10.1084/jem.173.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi R. I., Frigeri L. G., Liu F. T. Activation of rat basophilic leukemia cells by epsilon BP, an IgE-binding endogenous lectin. Cell Immunol. 1994 Jun;156(1):1–12. doi: 10.1006/cimm.1994.1148. [DOI] [PubMed] [Google Scholar]







