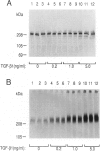
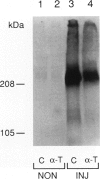
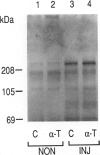
Abstract
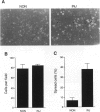
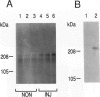
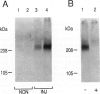
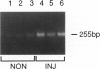
The arterial response to injury is characterized by a short period of increased proliferation and migration of vascular smooth muscle cells, followed by an extended period of extracellular matrix accumulation in the intima. Transforming growth factor-beta (TGF-beta) has been implicated as a causative factor in the formation of extracellular matrix in this process, which leads to progressive thickening of the intima, known as intimal hyperplasia. In vitro analysis of vascular smooth muscle cells harvested from normal rat aortas and from aortas injured 14 days earlier showed that both types of cells attached equally well to culture dishes but that the initial spreading of the cells was increased in cells derived from injured vessels. Cells from the injured arteries produced more fibronectin and proteoglycans into the culture medium than the cells from normal arteries and contained more TGF-beta 1 mRNA. TGF-beta 1 increased proteoglycan synthesis by normal smooth muscle cells, and the presence of a neutralizing anti-TGF-beta 1 antibody reduced proteoglycan synthesis by the cells from injured arteries in culture. Fibronectin synthesis was not altered by these treatments. These results indicate that the accumulation of extracellular matrix components in neointimal lesions is at least partially caused by autocrine TGF-beta activity in vascular smooth muscle cells.
Full text
PDF

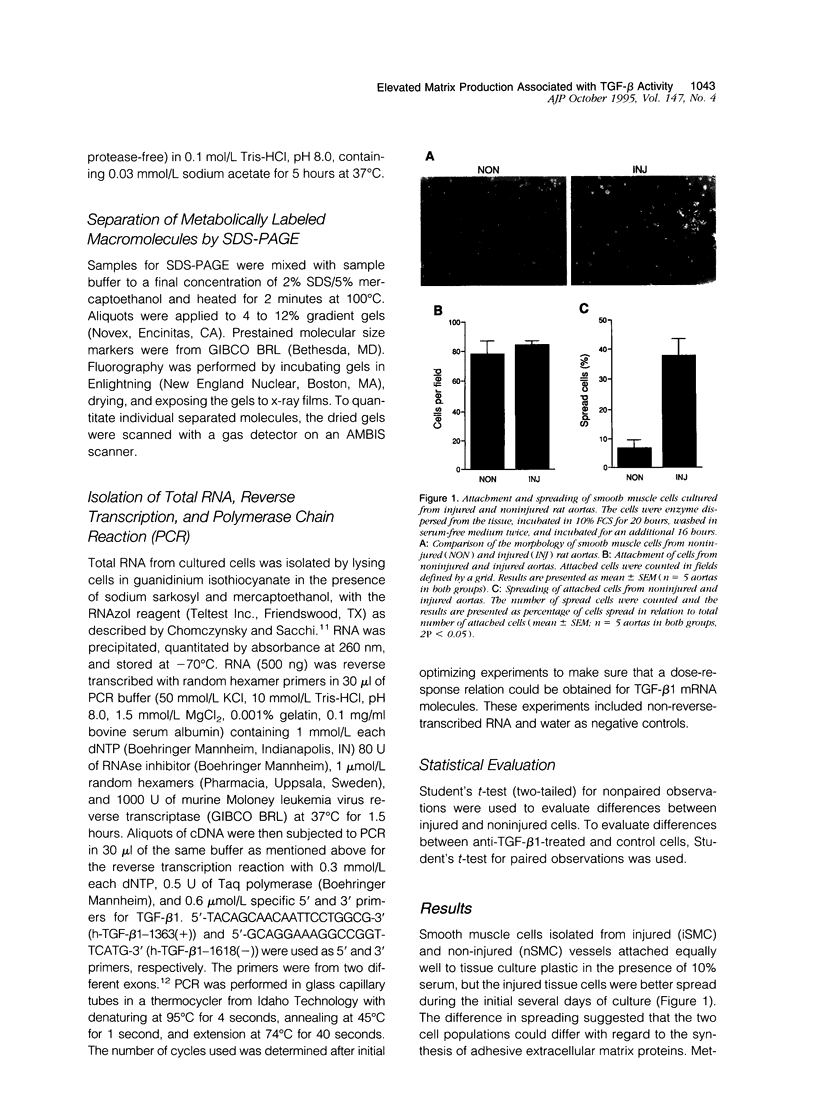
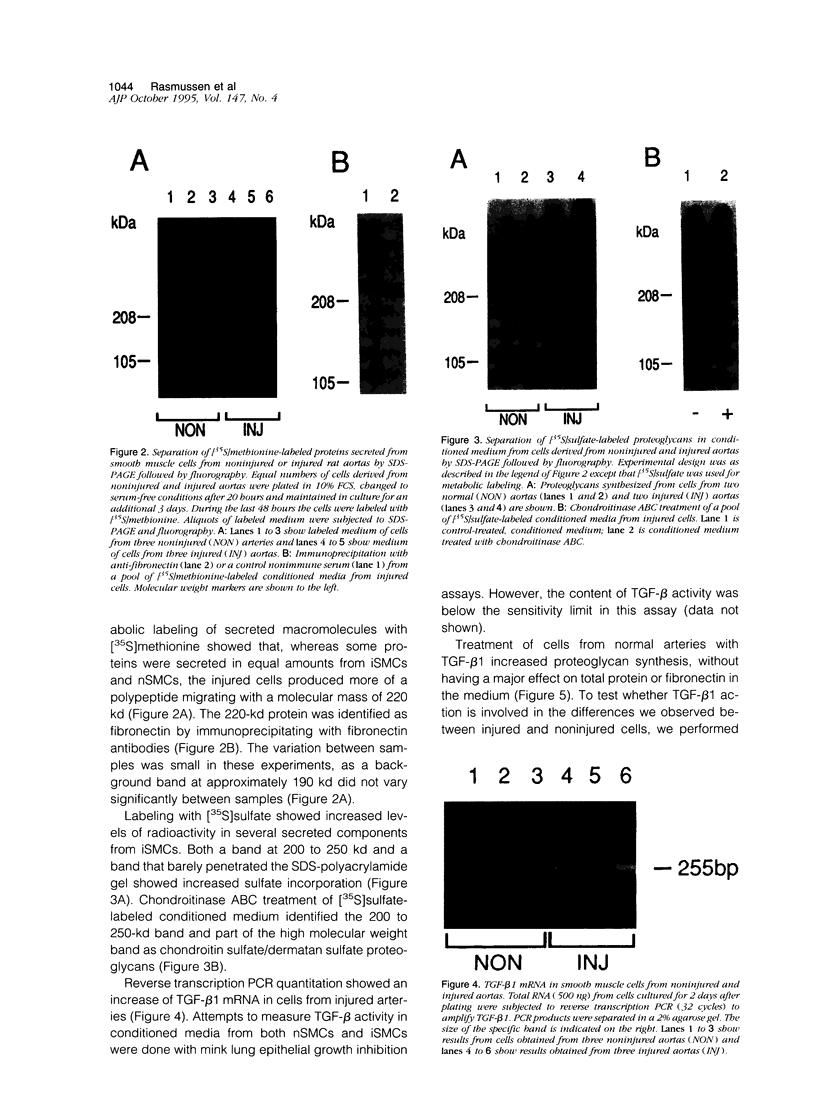
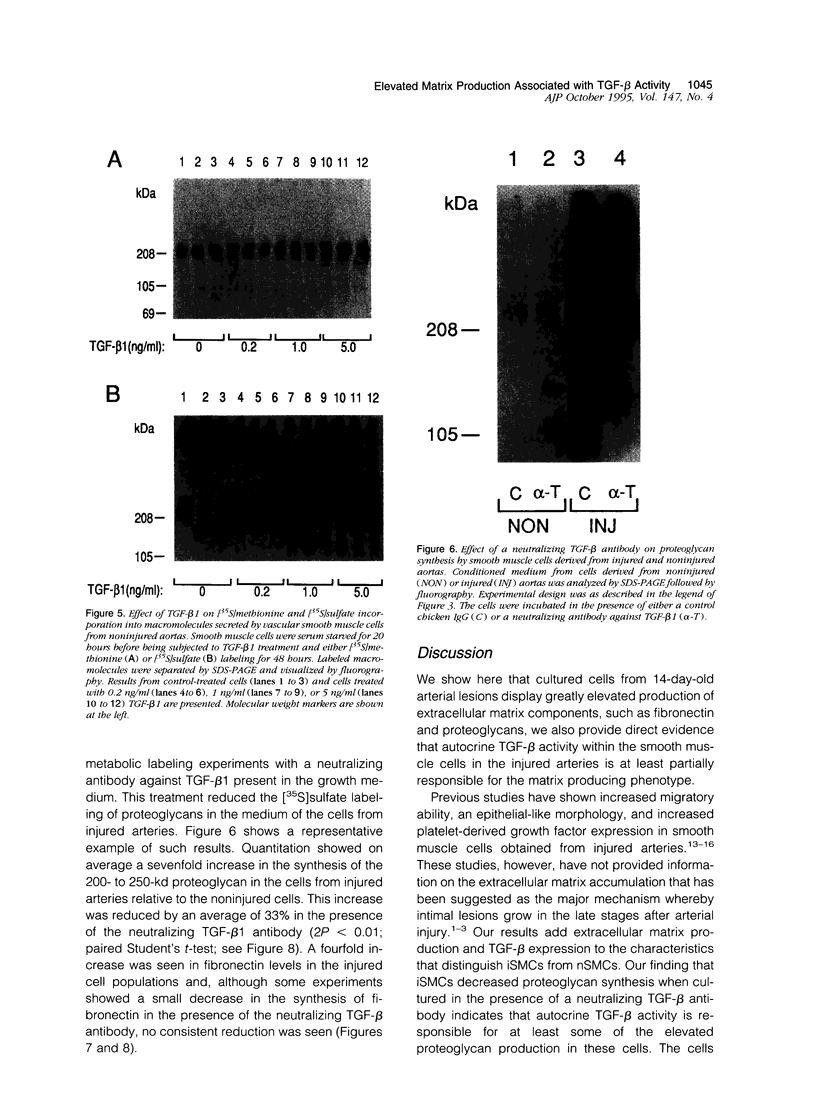
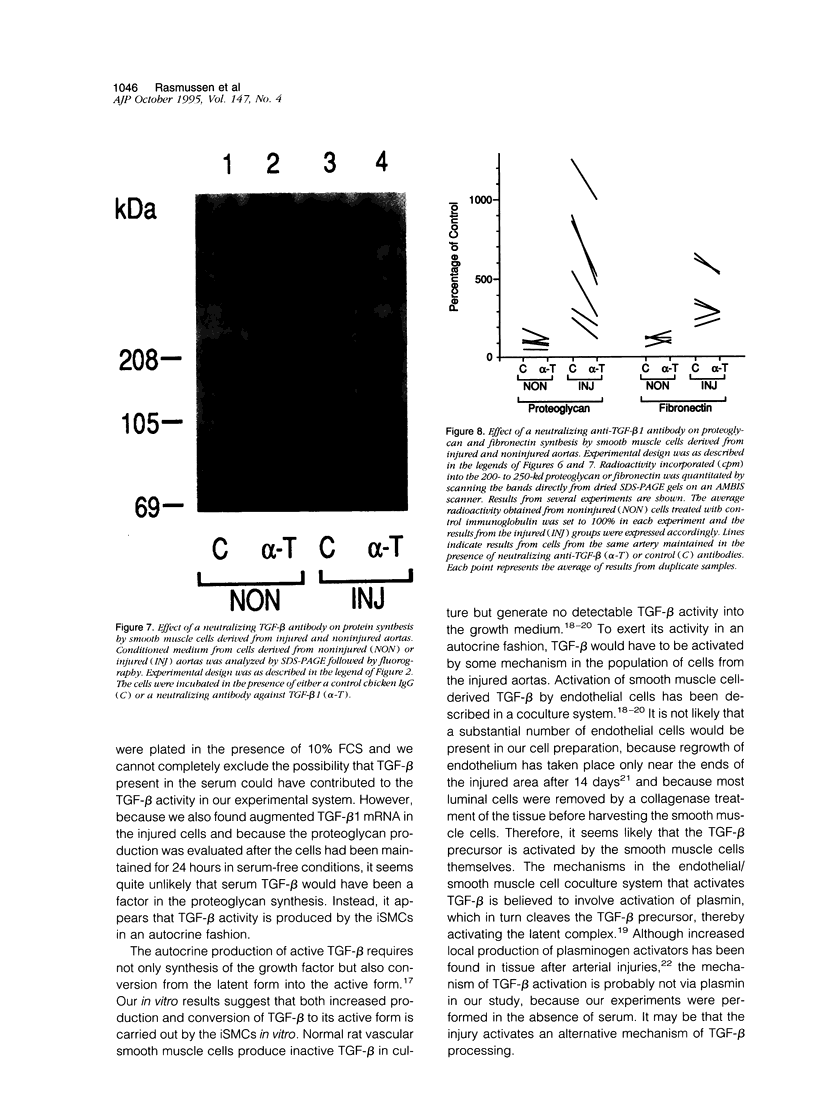


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauriedel G., Windstetter U., DeMaio S. J., Jr, Kandolf R., Höfling B. Migratory activity of human smooth muscle cells cultivated from coronary and peripheral primary and restenotic lesions removed by percutaneous atherectomy. Circulation. 1992 Feb;85(2):554–564. doi: 10.1161/01.cir.85.2.554. [DOI] [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Derynck R., Rhee L., Chen E. Y., Van Tilburg A. Intron-exon structure of the human transforming growth factor-beta precursor gene. Nucleic Acids Res. 1987 Apr 10;15(7):3188–3189. doi: 10.1093/nar/15.7.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Vasilevskaya T. D., Grunwald J., Saginati M., Koteliansky V. E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989 Jul;109(1):357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D. J., Kemp P. R., Liu A. C., Lawn R. M., Metcalfe J. C. Activation of transforming growth factor-beta is inhibited in transgenic apolipoprotein(a) mice. Nature. 1994 Aug 11;370(6489):460–462. doi: 10.1038/370460a0. [DOI] [PubMed] [Google Scholar]
- Grainger D. J., Kirschenlohr H. L., Metcalfe J. C., Weissberg P. L., Wade D. P., Lawn R. M. Proliferation of human smooth muscle cells promoted by lipoprotein(a). Science. 1993 Jun 11;260(5114):1655–1658. doi: 10.1126/science.8503012. [DOI] [PubMed] [Google Scholar]
- Grünwald J., Haudenschild C. C. Intimal injury in vivo activates vascular smooth muscle cell migration and explant outgrowth in vitro. Arteriosclerosis. 1984 May-Jun;4(3):183–188. doi: 10.1161/01.atv.4.3.183. [DOI] [PubMed] [Google Scholar]
- Liau G., Chan L. M. Regulation of extracellular matrix RNA levels in cultured smooth muscle cells. Relationship to cellular quiescence. J Biol Chem. 1989 Jun 15;264(17):10315–10320. [PubMed] [Google Scholar]
- Lindner V., Reidy M. A., Fingerle J. Regrowth of arterial endothelium. Denudation with minimal trauma leads to complete endothelial cell regrowth. Lab Invest. 1989 Nov;61(5):556–563. [PubMed] [Google Scholar]
- Magnuson V. L., Young M., Schattenberg D. G., Mancini M. A., Chen D. L., Steffensen B., Klebe R. J. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991 Aug 5;266(22):14654–14662. [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Shum L., Pompili V. J., Yang Z. Y., San H., Shu H. B., Liptay S., Gold L., Gordon D., Derynck R. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikol S., Isner J. M., Pickering J. G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992 Oct;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi A., Ehrlich H. P., Ropraz P., Spagnoli L. G., Gabbiani G. Rat aortic smooth muscle cells isolated from different layers and at different times after endothelial denudation show distinct biological features in vitro. Arterioscler Thromb. 1994 Jun;14(6):982–989. doi: 10.1161/01.atv.14.6.982. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. H., Garbarsch C., Chemnitz J., Christensen B. C., Lorenzen I. Injury and repair of smaller muscular and elastic arteries. Immunohistochemical demonstration of fibronectin and fibrinogen/fibrin and their degradation products in rabbit femoral and common carotid arteries following a dilatation injury. Virchows Arch A Pathol Anat Histopathol. 1989;415(6):579–585. doi: 10.1007/BF00718654. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y. G., Rasmussen L. M., Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest. 1994 Mar;93(3):1172–1178. doi: 10.1172/JCI117070. [DOI] [PMC free article] [PubMed] [Google Scholar]