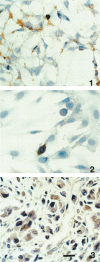
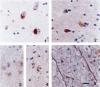
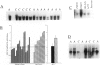Abstract
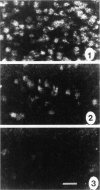
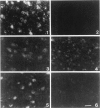
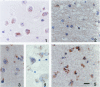
GAP-43 is a growth-associated phosphoprotein expressed at high levels in neurons during development, axonal regeneration, and neuritic sprouting. GAP-43 gene expression in mature neurons is probably functionally important for the structural remodeling of synapses as required for learning and establishing new memory. The widespread aberrant neuritic growth accompanied by impaired synaptic plasticity in Alzheimer's disease (AD) suggests that abnormal GAP-43 gene expression may contribute to the cascade of neurodegeneration. In the present study, end-stage AD brains exhibited reduced neuronal expression but increased glial cell levels of GAP-43 mRNA and protein. Glial cell localization of GAP-43 gene expression was confirmed by in situ hybridization of cerebral tissue, Northern blot analysis of microdissected cerebral white matter, and independent analysis of astrocytoma cell lines and primary malignant astrocytomas. In addition, in AD, GAP-43 immunoreactivity was translocated from the cytosol to membranes of swollen neuritic (dendritic) and glial cell processes throughout cerebral cortex and white matter. Downregulated and aberrant neuronal GAP-43 gene expression appears to reflect an important molecular lesion that precedes and progresses with the widespread synaptic disconnection and dementia in AD. At the same time, the presence of similar neuronal abnormalities in Pick's disease, diffuse Lewy body disease, Parkinson's disease, and Down syndrome suggests common mechanisms in the respective cascades of neurodegeneration. Finally, the finding of aberrantly increased glial cell GAP-43 gene expression in AD exposes a previously unrecognized neurodegenerative change that may account for the axonal loss and white matter atrophy detected early in the course of disease.
Full text
PDF


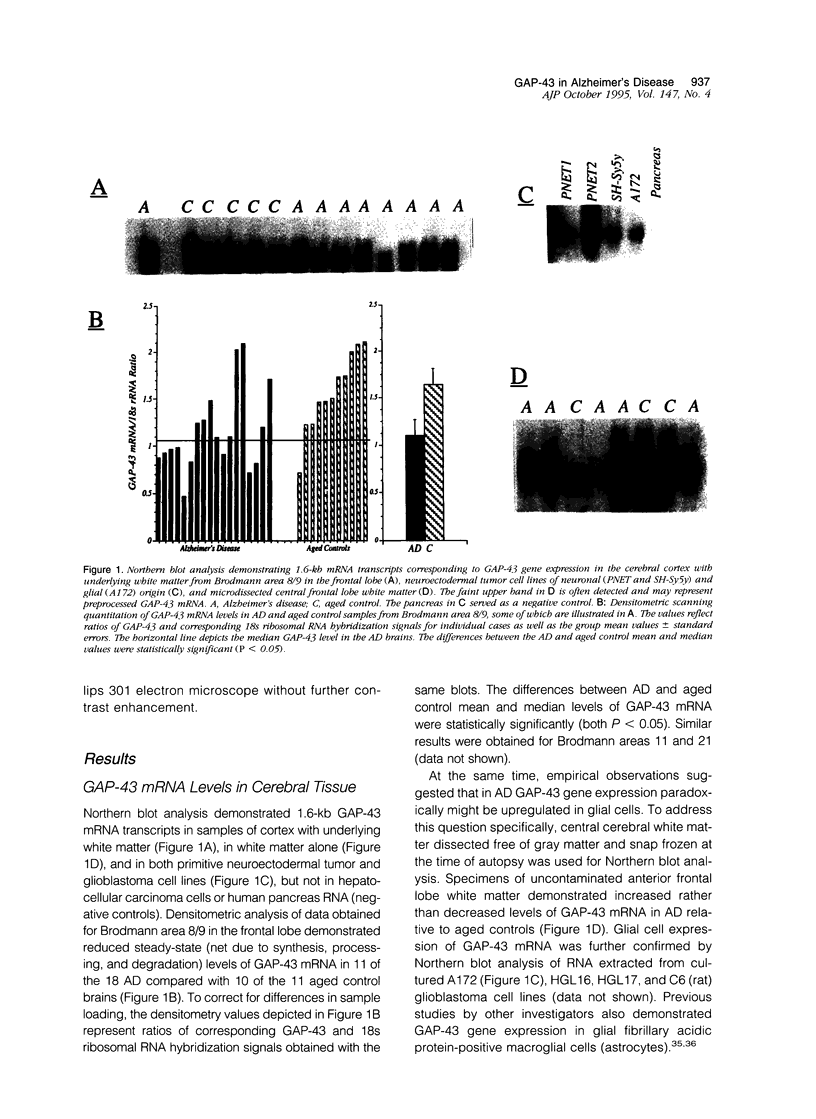

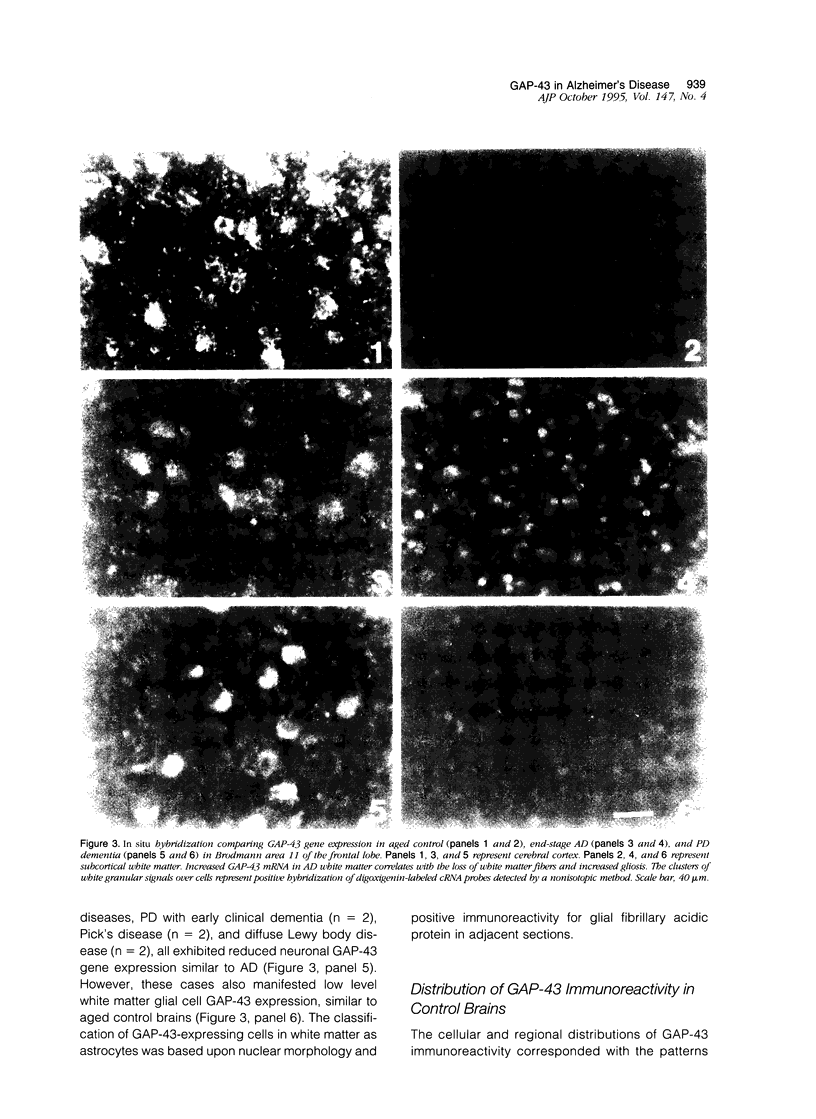
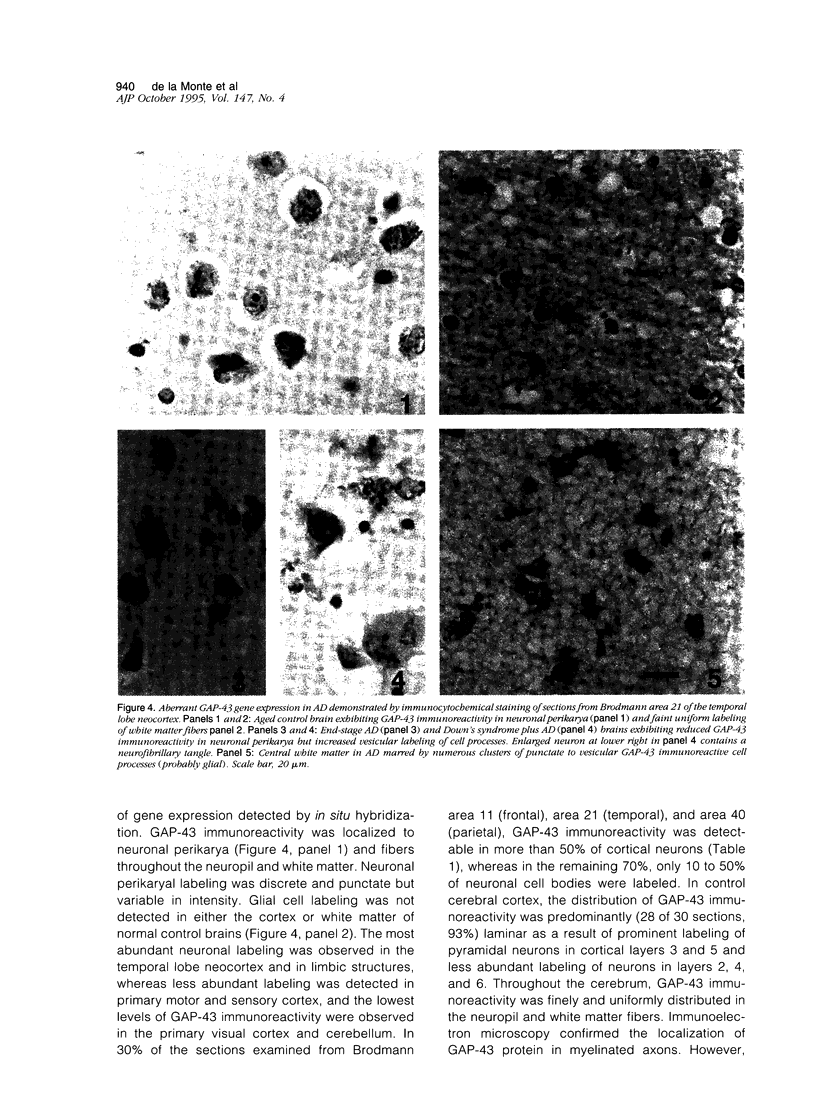
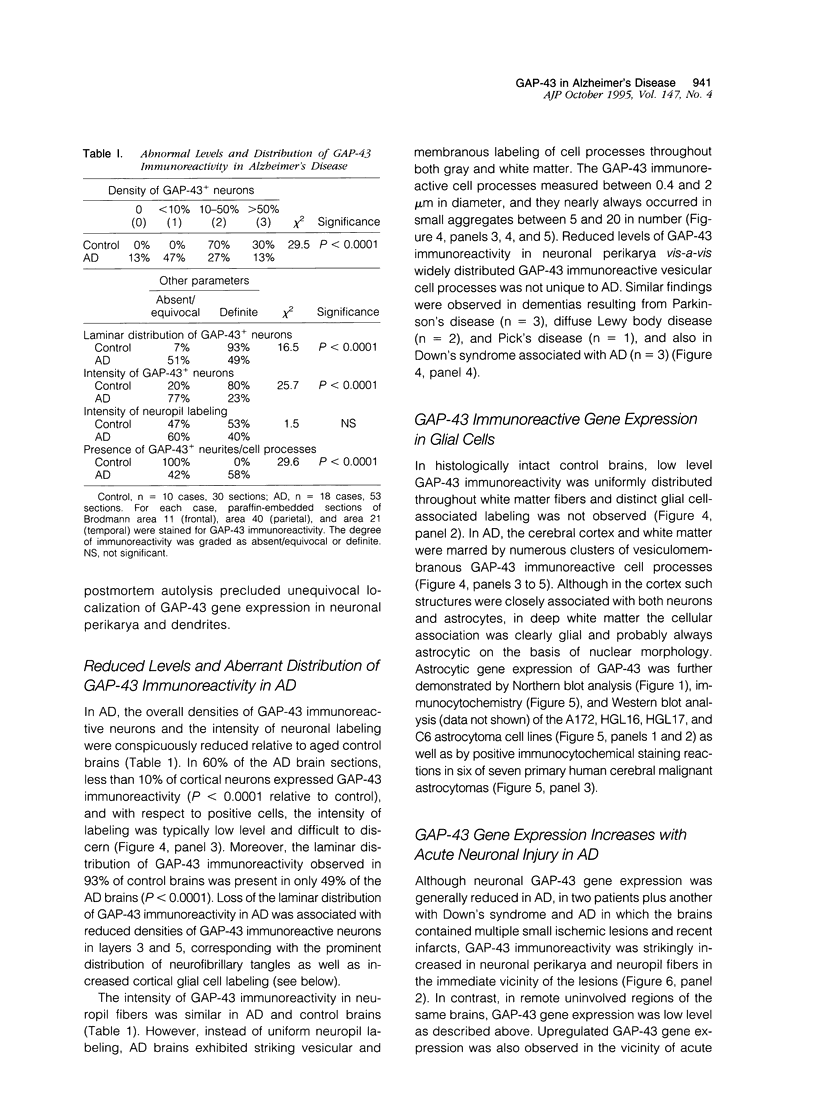
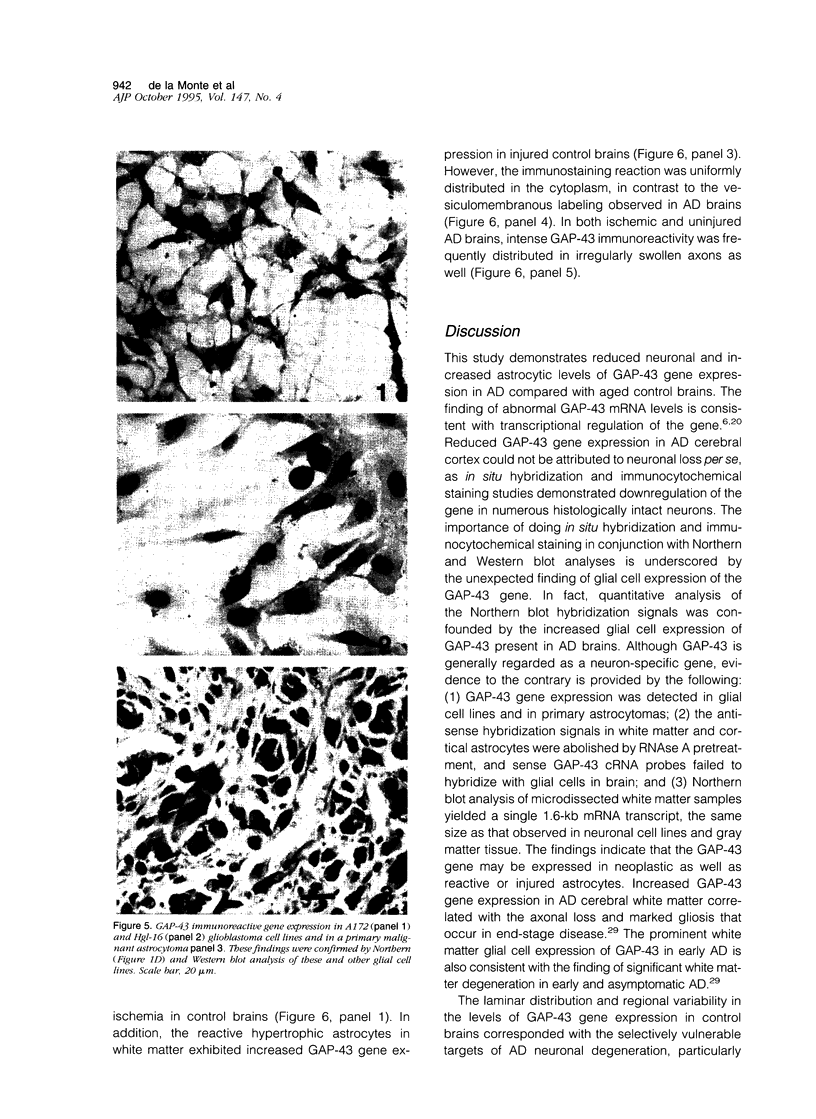
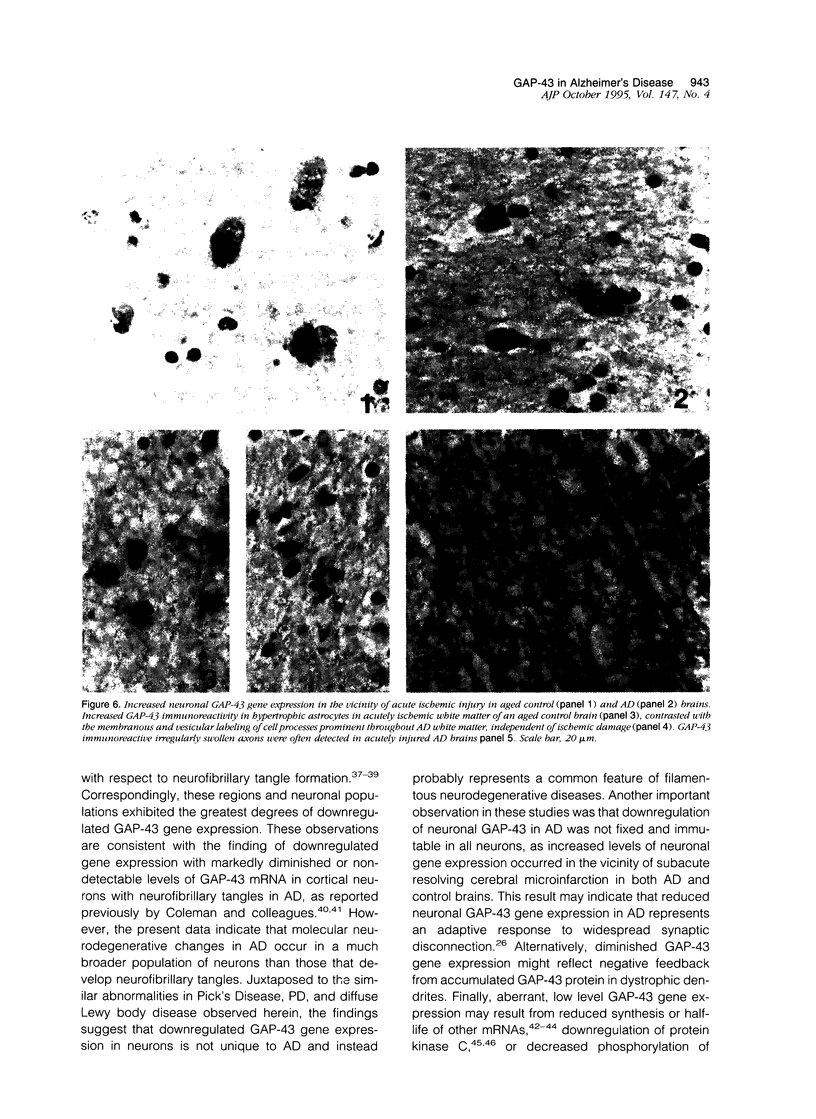



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K. A., Cimler B. M., Meier K. E., Storm D. R. Regulation of calmodulin binding to P-57. A neurospecific calmodulin binding protein. J Biol Chem. 1987 May 5;262(13):6108–6113. [PubMed] [Google Scholar]
- Barcikowska M., Wisniewski H. M., Bancher C., Grundke-Iqbal I. About the presence of paired helical filaments in dystrophic neurites participating in the plaque formation. Acta Neuropathol. 1989;78(3):225–231. doi: 10.1007/BF00687751. [DOI] [PubMed] [Google Scholar]
- Basi G. S., Jacobson R. D., Virág I., Schilling J., Skene J. H. Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell. 1987 Jun 19;49(6):785–791. doi: 10.1016/0092-8674(87)90616-7. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N., Finklestein S. P., Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988 Jan;8(1):339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L. I., Lewis E. R. Increased transport of 44,000- to 49,000-dalton acidic proteins during regeneration of the goldfish optic nerve: a two-dimensional gel analysis. J Neurosci. 1983 Nov;3(11):2153–2163. doi: 10.1523/JNEUROSCI.03-11-02153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz L. I., Shashoua V. E., Yoon M. G. Specific changes in rapidly transported proteins during regeneration of the goldfish optic nerve. J Neurosci. 1981 Mar;1(3):300–307. doi: 10.1523/JNEUROSCI.01-03-00300.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E., Kalus P. Alzheimer's disease: areal and laminar pathology in the occipital isocortex. Acta Neuropathol. 1989;77(5):494–506. doi: 10.1007/BF00687251. [DOI] [PubMed] [Google Scholar]
- Brun A., Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986 Mar;19(3):253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Callahan L. M., Selski D. J., Martzen M. R., Cheetham J. E., Coleman P. D. Preliminary evidence: decreased GAP-43 message in tangle-bearing neurons relative to adjacent tangle-free neurons in Alzheimer's disease parahippocampal gyrus. Neurobiol Aging. 1994 May-Jun;15(3):381–386. doi: 10.1016/0197-4580(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Cimler B. M., Giebelhaus D. H., Wakim B. T., Storm D. R., Moon R. T. Characterization of murine cDNAs encoding P-57, a neural-specific calmodulin-binding protein. J Biol Chem. 1987 Sep 5;262(25):12158–12163. [PubMed] [Google Scholar]
- Cole G., Dobkins K. R., Hansen L. A., Terry R. D., Saitoh T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988 Jun 14;452(1-2):165–174. doi: 10.1016/0006-8993(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Coleman P. D., Kazee A. M., Lapham L., Eskin T., Rogers K. Reduced GAP-43 message levels are associated with increased neurofibrillary tangle density in the frontal association cortex (area 9) in Alzheimer's disease. Neurobiol Aging. 1992 Nov-Dec;13(6):631–639. doi: 10.1016/0197-4580(92)90085-c. [DOI] [PubMed] [Google Scholar]
- De la Monte S. M., Federoff H. J., Ng S. C., Grabczyk E., Fishman M. C. GAP-43 gene expression during development: persistence in a distinctive set of neurons in the mature central nervous system. Brain Res Dev Brain Res. 1989 Apr 1;46(2):161–168. doi: 10.1016/0165-3806(89)90279-4. [DOI] [PubMed] [Google Scholar]
- Englund E., Brun A., Alling C. White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain. 1988 Dec;111(Pt 6):1425–1439. doi: 10.1093/brain/111.6.1425. [DOI] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Florez J. C., Nelson R. B., Routtenberg A. Contrasting patterns of protein phosphorylation in human normal and Alzheimer brain: focus on protein kinase C and protein F1/GAP-43. Exp Neurol. 1991 Jun;112(3):264–272. doi: 10.1016/0014-4886(91)90126-w. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Johnson A. B., Wisniewski H. M., Terry R. D., Iqbal K. Evidence that Alzheimer neurofibrillary tangles originate from neurotubules. Lancet. 1979 Mar 17;1(8116):578–580. doi: 10.1016/s0140-6736(79)91006-7. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Barton A. J., Najlerahim A., McDonald B., Pearson R. C. Regional and neuronal reductions of polyadenylated messenger RNA in Alzheimer's disease. Psychol Med. 1991 Nov;21(4):855–866. doi: 10.1017/s0033291700029858. [DOI] [PubMed] [Google Scholar]
- Ihara Y. Massive somatodendritic sprouting of cortical neurons in Alzheimer's disease. Brain Res. 1988 Aug 30;459(1):138–144. doi: 10.1016/0006-8993(88)90293-4. [DOI] [PubMed] [Google Scholar]
- Jacobson R. D., Virág I., Skene J. H. A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in rat CNS. J Neurosci. 1986 Jun;6(6):1843–1855. doi: 10.1523/JNEUROSCI.06-06-01843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K., Skene J. H. Elevated synthesis of an axonally transported protein correlates with axon outgrowth in normal and injured pyramidal tracts. J Neurosci. 1986 Sep;6(9):2563–2570. doi: 10.1523/JNEUROSCI.06-09-02563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns L. R., Ng S. C., Freeman J. A., Fishman M. C. Cloning of complementary DNA for GAP-43, a neuronal growth-related protein. Science. 1987 May 1;236(4801):597–600. doi: 10.1126/science.2437653. [DOI] [PubMed] [Google Scholar]
- Katz F., Ellis L., Pfenninger K. H. Nerve growth cones isolated from fetal rat brain. III. Calcium-dependent protein phosphorylation. J Neurosci. 1985 Jun;5(6):1402–1411. doi: 10.1523/JNEUROSCI.05-06-01402.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Two monoclonal antibodies recognize Alzheimer's neurofibrillary tangles, neurofilament, and microtubule-associated proteins. J Neurochem. 1987 Feb;48(2):455–462. doi: 10.1111/j.1471-4159.1987.tb04114.x. [DOI] [PubMed] [Google Scholar]
- Langstrom N. S., Anderson J. P., Lindroos H. G., Winblad B., Wallace W. C. Alzheimer's disease-associated reduction of polysomal mRNA translation. Brain Res Mol Brain Res. 1989 Jun;5(4):259–269. doi: 10.1016/0169-328x(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Love S., Saitoh T., Quijada S., Cole G. M., Terry R. D. Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol. 1988 Jul;47(4):393–405. doi: 10.1097/00005072-198807000-00001. [DOI] [PubMed] [Google Scholar]
- Masliah E., Cole G., Shimohama S., Hansen L., DeTeresa R., Terry R. D., Saitoh T. Differential involvement of protein kinase C isozymes in Alzheimer's disease. J Neurosci. 1990 Jul;10(7):2113–2124. doi: 10.1523/JNEUROSCI.10-07-02113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Iimoto D. S., Saitoh T., Hansen L. A., Terry R. D. Increased immunoreactivity of brain spectrin in Alzheimer disease: a marker for synapse loss? Brain Res. 1990 Oct 29;531(1-2):36–44. doi: 10.1016/0006-8993(90)90755-z. [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., Albright T., DeTeresa R., Terry R., Baudier J., Saitoh T. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991 May;6(5):729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., Alford M., DeTeresa R., Hansen L. A. Cortical and subcortical patterns of synaptophysinlike immunoreactivity in Alzheimer's disease. Am J Pathol. 1991 Jan;138(1):235–246. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., DeTeresa R. M., Hansen L. A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989 Aug 28;103(2):234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- McLachlan D. R., Lukiw W. J., Wong L., Bergeron C., Bech-Hansen N. T. Selective messenger RNA reduction in Alzheimer's disease. Brain Res. 1988 Jun;427(3):255–261. doi: 10.1016/0169-328x(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Meiri K. F., Pfenninger K. H., Willard M. B. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986 May;83(10):3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve R. L., Finch E. A., Bird E. D., Benowitz L. I. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc Natl Acad Sci U S A. 1988 May;85(10):3638–3642. doi: 10.1073/pnas.85.10.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. C., de la Monte S. M., Conboy G. L., Karns L. R., Fishman M. C. Cloning of human GAP-43: growth association and ischemic resurgence. Neuron. 1988 Apr;1(2):133–139. doi: 10.1016/0896-6273(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero N. I., Finklestein S. P., Benowitz L. I. Synthesis of a growth-associated protein by embryonic rat cerebrocortical neurons in vitro. J Neurosci. 1986 Dec;6(12):3721–3730. doi: 10.1523/JNEUROSCI.06-12-03721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Morrison J. H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer's disease. J Neurosci. 1985 Oct;5(10):2801–2808. doi: 10.1523/JNEUROSCI.05-10-02801.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Chan S. Y., Henzel W., Haskell C., Kuang W. J., Chen E., Wilcox J. N., Ullrich A., Goeddel D. V., Routtenberg A. Primary structure and mRNA localization of protein F1, a growth-related protein kinase C substrate associated with synaptic plasticity. EMBO J. 1987 Dec 1;6(12):3641–3646. doi: 10.1002/j.1460-2075.1987.tb02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel A. B., Tomiyasu U. Dendritic sprouting in Alzheimer's presenile dementia. Exp Neurol. 1978 May 15;60(1):1–8. doi: 10.1016/0014-4886(78)90164-4. [DOI] [PubMed] [Google Scholar]
- Skene J. H. Growth-associated proteins and the curious dichotomies of nerve regeneration. Cell. 1984 Jul;37(3):697–700. doi: 10.1016/0092-8674(84)90404-5. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Jacobson R. D., Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986 Aug 15;233(4765):783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Virág I. Posttranslational membrane attachment and dynamic fatty acylation of a neuronal growth cone protein, GAP-43. J Cell Biol. 1989 Feb;108(2):613–624. doi: 10.1083/jcb.108.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981 Apr;89(1):96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J Cell Biol. 1981 Apr;89(1):86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., Chan S. Y., McGuire C. B., Costello B. R., Norden J. J., Freeman J. A., Routtenberg A. Evidence for the coidentification of GAP-43, a growth-associated protein, and F1, a plasticity-associated protein. J Neurosci. 1987 Dec;7(12):4066–4075. doi: 10.1523/JNEUROSCI.07-12-04066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaton M., Mandybur T. I., Perry G., Onorato M., Autilio-Gambetti L., Gambetti P. The widespread alteration of neurites in Alzheimer's disease may be unrelated to amyloid deposition. Ann Neurol. 1989 Dec;26(6):771–778. doi: 10.1002/ana.410260614. [DOI] [PubMed] [Google Scholar]
- Vitković L., Steisslinger H. W., Aloyo V. J., Mersel M. The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8296–8300. doi: 10.1073/pnas.85.21.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R., Lassmann H., Fischer P., Jellinger K., Winkler H. A high ratio of chromogranin A to synaptin/synaptophysin is a common feature of brains in Alzheimer and Pick disease. FEBS Lett. 1990 Apr 24;263(2):337–339. doi: 10.1016/0014-5793(90)81408-g. [DOI] [PubMed] [Google Scholar]
- Zuber M. X., Goodman D. W., Karns L. R., Fishman M. C. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989 Jun 9;244(4909):1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]
- da Cunha A., Vitković L. Regulation of immunoreactive GAP-43 expression in rat cortical macroglia is cell type specific. J Cell Biol. 1990 Jul;111(1):209–215. doi: 10.1083/jcb.111.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graan P. N., van Hooff C. O., Tilly B. C., Oestreicher A. B., Schotman P., Gispen W. H. Phosphoprotein B-50 in nerve growth cones from fetal rat brain. Neurosci Lett. 1985 Nov 11;61(3):235–241. doi: 10.1016/0304-3940(85)90470-7. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer's disease. Ann Neurol. 1989 May;25(5):450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]