Abstract
Human erythropoietin, a widely used and important therapeutic glycoprotein, has a relatively short plasma half-life due to clearance by glomerular filtration as well as by other mechanisms. We hypothesized that an erythropoietin species with a larger molecular size would exhibit an increased plasma half-life and, potentially, an enhanced biological activity. We now report the production of biologically active erythropoietin dimers and trimers by chemical crosslinking of the conventional monomeric form. We imparted free sulfhydryl residues to a pool of erythropoietin monomer by chemical modification. A second pool was reacted with another modifying reagent to yield monomer with maleimido groups. Upon mixing these two pools, covalently linked dimers and trimers were formed that were biologically active in vitro. The plasma half-life of erythropoietin dimers in rabbits was >24 h compared with 4 h for the monomers. Importantly, erythropoietin dimers were biologically active in vivo as shown by their ability to increase the hematocrits of mice when injected subcutaneously. In addition, the dimers exhibited >26-fold higher activity in vivo than did the monomers and were very effective after only one dose. Dimeric and other oligomeric forms of Epo may have an important role in therapy.
Human erythropoietin (Epo) was first purified from the urine of anemic human donors (1). This important accomplishment was made possible by a salient feature of the protein, namely, that its molecular size of 31–37 kDa allows it to pass through the renal glomerulus and to be excreted in the urine.
The advent of recombinant human erythropoietin (2–4), an important therapeutic for correcting certain types of anemia, also made possible more detailed studies of erythropoietin physiology and pharmacokinetics. When administered intravenously to rodents, radiolabeled Epo seemed to localize to several different tissues (5). Pharmacokinetic studies in human volunteers with normal renal function have yielded a plasma half-life of 4–13 h (6, 7). This half-life is much shorter than that of other, larger proteins used therapeutically, for example, serum albumin and Ig.
We hypothesized that an Epo species with a larger molecular size would have a reduced rate of clearance, thereby lengthening its plasma survival and increasing its in vivo biological activity. Here, we report the synthesis of Epo dimers and trimers using heterobifunctional crosslinking reagents. These Epo species are biologically active. Moreover, Epo dimers exhibit a markedly enhanced plasma survival and are much more efficacious in vivo than is the conventional monomeric Epo.
MATERIALS AND METHODS
Preparation of Epo Containing Free Sulfhydryl Groups (SH-Epo) or Maleimido Groups (maleimido-Epo).
Recombinant human Epo (1.0 mg/ml) was incubated in the presence of 10-fold molar excess of succinimidyl 6-[3-(2-pyridyldithio)propionamido] hexanoate (LC-SPDP) (Pierce) or in the presence of 10-fold molar excess of succinimidyl 4-(N-maleimido methyl)cyclohexane-1-carboxylate (SMCC) (Pierce) in PBS (20 mM sodium phosphate/100 mM sodium chloride, pH 7.5) for 30 min at 23°C to yield LC-SPDP-Epo and maleimido-Epo, respectively. DTT (1 mM, final concentration) was added only to LC-SPDP-Epo, and the incubation was continued for 10 min at 23°C to yield SH-Epo. Both reactions were stopped by the addition of cold PBS (final volume 0.5 ml) followed by dialysis against 3 × 1.0 liter of PBS, 16–18 h, at 4°C. Preliminary experiments were carried out to determine the concentrations of reactants that would yield chemically modified Epo with full biological activity.
Production of Epo Oligomers and Separation by HPLC.
To effect oligomerization, equal volumes of SH-Epo and maleimido-Epo were incubated together for 1.0 h at 23°C and then subjected to size exclusion HPLC (SE-HPLC). SE-HPLC was carried out on a Progel TSK 3000SWxl column equilibrated with 100 mM sodium phosphate, 0.15 M sodium chloride, pH 7.0. The elution profile was monitored at 278 nm. Fractions (0.2 ml) were collected, and BSA was added to a final concentration of 1 mg/ml. An aliquot of the mixture was also subjected to SDS/PAGE for analysis.
Bioassay.
The bioactivity of each sample (in international units, IU) was determined in vitro essentially as described by Krystal (8). The standard curve was calibrated against the World Health Organization Second International Reference Preparation. Each sample was diluted with bioassay medium containing 78% α-MEM, 20% heat-inactivated fetal bovine serum, 1% β-mercaptoethanol, and 1% penicillin/streptomycin/fungizone (GIBCO).
Western Blotting.
Samples were subjected to SDS/PAGE and transferred electrophoretically to 0.45-μm nitrocellulose membranes in 25 mM Tris⋅HCl, 192 mM glycine, 10% methanol. Membranes were then rinsed twice with distilled water and incubated overnight at 4°C in 20 mM Tris⋅HCl, 0.5 M NaCl, 0.5% Tween 20 (TBST), 10% nonfat dry milk, pH 7.5. The membranes were rinsed twice with TBST, washed once with TBST for 15 min, and washed twice for 5 min each. The membranes were then incubated with anti-erythropoietin monoclonal antibody AE-7A5 (9) (Genzyme), 0.7 μg per ml of TBST, 5% nonfat dry milk for 1 h at 23°C. Rinsing and washing were carried out as above followed by incubation with a horseradish peroxidase-conjugated goat anti-mouse IgG (Cappel) diluted 1:1000 in TBST, 5% nonfat dry milk for 1 h at 23°C. Rinsing and washing were again carried out as above plus two additional washes for 5 min each. Protein bands recognized by the primary antibody were detected using an ECL luminescence kit (Amersham).
In Vivo Half-Life Determination.
New Zealand White rabbits were injected intravenously with specified amounts of monomeric or dimeric Epo. Blood samples were obtained at specified times, and serum was prepared. Serum erythropoietin levels were determined by in vitro bioassay.
In Vivo Biological Activity.
Groups of three to four C57BL/6J mice (8–10-week-old females) were used. Before administration of Epo, each animal was anesthetized, and its hematocrit was determined using blood obtained by filling two heparinized microhematocrit tubes from the retro-orbital venous plexus. An identifying mark was placed on each animal to permit the determination of its pre- and posttreatment hematocrit individually. The animals were weighed, and each received an identical amount (in international units) of Epo monomer or dimer by subcutaneous injection of the protein in sterile Dulbecco’s phosphate-buffered saline (0.5 ml). The biologic activities of the treatment samples were verified in triplicate by in vitro bioassay. The frequency of treatment was either thrice weekly (days 1, 3, and 5) or once only (day 1). On day 8, the posttreatment hematocrit of each animal was determined.
RESULTS
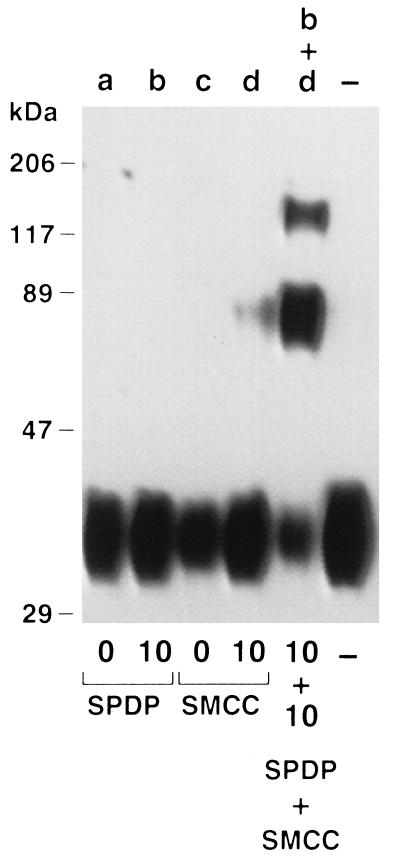
We hypothesized that an Epo species with a larger molecular size would exhibit an increased plasma half-life due, in part, to reduced filtration through the renal glomerulus. To produce this species, we chose to crosslink Epo monomers using a pair of heterobifunctional reagents that would take advantage of known aspects of the hormone’s physicochemical properties. Human Epo contains four cysteines that form two disulfide bridges (10). There is no convincing evidence that either of these disulfides is accessible to solvent or other reactive species unless the molecule is otherwise denatured (11). Therefore, we chose to impart free sulfhydryls to erythropoietin using LC-SPDP. The product of this reaction, SH-Epo, was then reacted with maleimido-Epo, which had been produced by modifying a separate pool of Epo with the crosslinking reagent SMCC. The selective reaction of the new thiol group(s) of SH-Epo with the maleimido-Epo resulted in erythropoietin oligomers linked by stable covalent bonds. SDS/PAGE analysis and Western blotting of the reaction mixture with anti-erythropoietin monoclonal antibody revealed new erythropoietin species of ∼70 kDa and ∼117 kDa, consistent with the formation of Epo–Epo dimers and Epo–Epo–Epo trimers (Fig. 1).
Figure 1.
SDS/PAGE and Western blot analysis of Epo monomers, chemically modified monomers, and reaction mixture consisting of SH-Epo and maleimido Epo. Monomers (lanes a and c) were treated with 10-fold molar excess of LC-SPDP (lane b) to yield SH-Epo or with 10-fold molar excess of SMCC (lane d) to yield maleimido-Epo. Modified Epo pools (lanes b and d) were mixed and allowed to react (lane b+d). Lane — is untreated monomer. Note new Epo species of ∼70 kDa (dimers) and ∼117 kDa (trimers) in lane b+d.
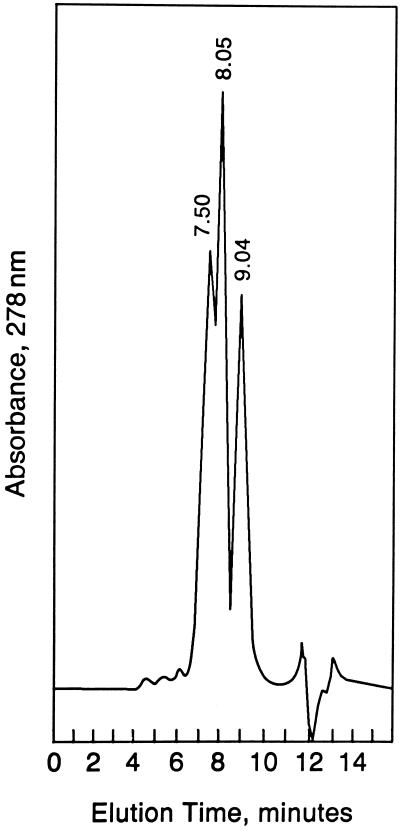
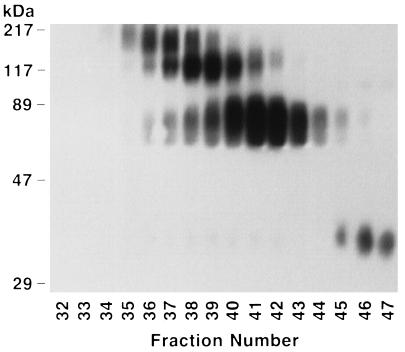
We purified Epo dimers and trimers from Epo monomers by SE-HPLC. Fifty micrograms of SH-Epo and 50 μg of maleimido-Epo were reacted, equilibrated with 0.1 M sodium phosphate, 0.15 M NaCl, pH 7.0, and subjected to SE-HPLC. The mixture was resolved into three major protein peaks with elution times of 7.50, 8.03, and 9.04 min, respectively (Fig. 2). Preliminary experiments demonstrated that monomeric Epo eluted at 8.90–9.20 min. Thus, we expected that the 9.04-min peak would prove to be monomeric Epo, whereas the 7.50- and 8.03-min peaks would prove to be trimeric and dimeric Epo, respectively. The results obtained from SDS/PAGE and Western blotting of the 0.2-ml fractions collected during SE-HPLC demonstrated that this was correct (Fig. 3). Epo trimers of ∼117 kDa were found in fractions of 37–41, coinciding with elution times of 6.8–7.4 min (allowing for appropriate retention volume between the detector and fraction collector). Next, dimers appeared, reaching a maximum in fractions 41–42 to coincide with the prominent 8.03-min peak. Finally, monomeric Epo appeared in fractions 45–47, coinciding with the 9.04-min peak, as expected.
Figure 2.
Size exclusion HPLC chromatogram of mixture after reaction of SH-Epo and maleimido-Epo. The 9.04-min peak corresponds to Epo monomer. Note two peaks eluting at 7.50 and 8.03 min, respectively.
Figure 3.
SDS/PAGE and Western blot analyses of fractions collected during SE-HPLC of reaction mixture. Note the higher molecular weight Epo oligomers in earlier fractions. Epo trimers (∼117 kDa) and dimers (∼70 kDa) are well separated from Epo monomers (∼35 kDa). In this preparation, Epo tetramers were also detected in the earliest fractions.
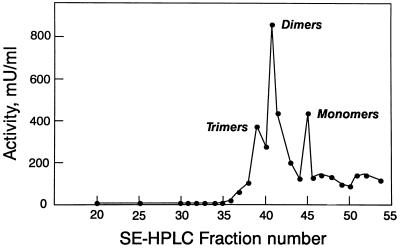
Importantly, these three species were biologically active. Portions of each fraction were subjected to bioassay. When plotted versus fraction number (Fig. 4), the profile obtained coincided with the protein elution profile, with activity maxima in fractions 39, 41, and 45. We used the integrated areas from the three chromatography peaks in Fig. 2 to calculate the amount of protein represented by each peak. These results were used to estimate the specific activities of the three Epo species. They were as follows: trimer = 100 IU/μg; dimer = 210 IU/μg; monomer = 160 IU/μg.
Figure 4.
Biological activity profile of fractions collected during SE-HPLC of reaction mixture. Note the peaks of activity corresponding to Epo trimers, dimers, and monomers.
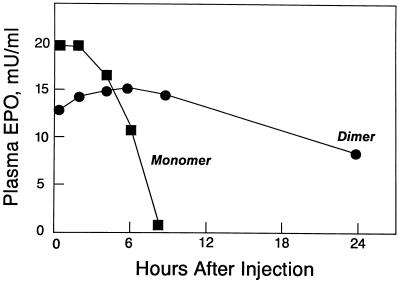
Erythropoietin dimers exhibit a markedly prolonged in vivo half-life. We injected two groups of New Zealand White rabbits intravenously either with monomeric Epo or with the dimeric form purified by SE-HPLC. At specified times, blood samples were collected, and the Epo biological activity in the serum was determined by in vitro bioassay (Fig. 5). The serum Epo levels of animals injected with monomer decreased rapidly with an approximate half-life of 4 h. In marked contrast, the serum Epo levels of animals injected with dimer were decreased only moderately after 24 h.
Figure 5.
Epo levels in plasma of rabbits injected intravenously with dimers (•) or monomers (▪) at 0 h. Each point represents the mean biological activity determined in triplicate for groups of three to four animals. Standard deviation was equal to or less than ±20% for all values. Note rapid clearance of Epo monomers compared with dimers.
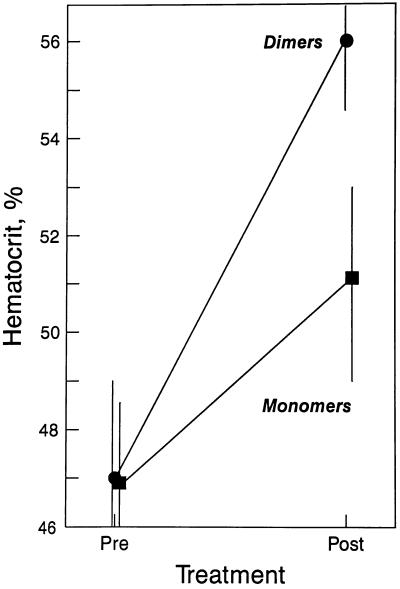
We compared the in vivo efficacy of Epo dimers and monomers by measuring the change in hematocrit (Hct) of mice injected subcutaneously with either form. Using at first a relatively high dosage regimen of 300 IU/kg, days 1, 3, and 5 (Fig. 6), the mean Hct of monomer-treated animals rose from 46.9% to 51.0% for a mean absolute increase of 4.1%. In contrast, the mean Hct of dimer-treated animals rose from 46.9% to 56.1% for a mean absolute increase of 9.2%, 2.2-fold that observed for monomers.
Figure 6.
In vivo efficacy of Epo dimers (•) and monomers (▪) in mice injected subcutaneously with 300 IU/kg, days 1, 3, and 5. Hematocrits were obtained pretreatment (Pre) and on day 8 (Post). Points represent the mean Hct of four animals in each group. The vertical bar represents the range of the highest and lowest value.
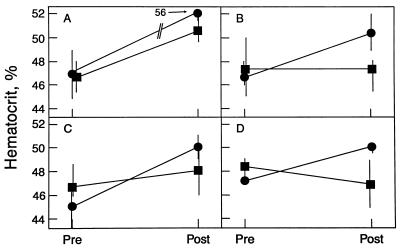
Next, we compared the efficacy of dimers and monomers using additional dosage regimens (Fig. 7, A–D). At 300 IU/kg, day 1 only, the mean Hct of dimer-treated animals rose from 46.6% to 50.3%, for a mean absolute increase of 3.7% (Fig. 7B). In contrast, no significant change was observed in the mean Hct of monomer-treated animals [47.4% pretreatment; 47.2% posttreatment (Fig. 7B)]. This difference between dimer- and monomer-treated animals is consistent with the prolonged plasma half-life of the dimeric form (Fig. 5). This result also shows that a single injection of Epo dimers can result in a significant Hct increase. Further in vivo experiments confirmed the superiority of Epo dimers over monomers. At a lower dosage of 30 IU/kg, days 1, 3, and 5 (Fig. 7C), the mean Hct of dimer-treated animals rose from 44.9% to 50.0% for a mean absolute increase of 5.1%, whereas the mean Hct of monomer-treated animals rose slightly from 47.1% to 48.2% for a 1.1% mean absolute increase. At the lowest dose tested, 30 IU/kg, day 1 only (Fig. 7D), the mean Hct of dimer-treated animals rose from 47.2% to 49.9%, for a mean absolute increase of 2.7%. As expected, the mean Hct of monomer-treated animals did not increase (48.6% pretreatment, 47.0% posttreatment). The results indicate that Epo dimers are more than 10-fold as efficacious as Epo monomers on a per unit basis and that dimers can be administered less frequently with good therapeutic results.
Figure 7.
In vivo efficacy of Epo dimers (•) and monomers (▪) using different dosage regimens. (A) 300 IU/kg, days 1, 3, and 5 (same data as seen in Fig. 6 replotted for comparison). (B) 300 IU/kg, day 1 only. (C) 30 IU/kg, days 1, 3, and 5. (D) 30 IU/kg, day 1 only. Note that for all dosage regimens, Epo dimers are superior to monomers. See legend to Fig. 6.
DISCUSSION
The present study demonstrates that crosslinking Epo molecules using suitable heterobifunctional linkers results in the production of biologically active oligomers (dimers and trimers). The data show that Epo dimers have a prolonged plasma half-life and have markedly enhanced efficacy over conventional monomeric Epo.
It is important to note the different potencies of the various Epo forms in vitro. The specific activities of monomer, dimer, and trimer were 160 IU/μg, 210 IU/μg, and 100 IU/μg, respectively, yielding a potency ratio of 1.0:1.3:0.6 when calculated on the basis of molecular mass. However, when calculated on a molar basis, the relative in vitro potencies of monomer:dimer:trimer are 1.0:2.6:1.8 (i.e., Epo oligomers have an increased specific activity). This fact should also be considered in evaluating the in vivo results. We administered equal dosages of monomer or dimer measured as units of activity (Figs. 6 and 7). Because a single injection of 30 IU/kg of dimer raised the Hct, whereas a single injection of 300 IU/kg of monomer did not, we calculated that the dimer is >10-fold as efficacious as monomer. Additionally, on a molar basis the dimer dose was only 38% that of the monomer due to the higher specific activity of the dimer. Thus, the activity of the dimers in vivo was >10/0.38, or >26-fold that of the monomers.
In designing these studies, we considered several potential approaches to increasing the size of the Epo molecule. They included derivatization with polyethylene glycol (12) and chemical conjugation to a carrier protein such as serum albumin. We chose, instead, to crosslink erythropoietin to itself. We did so with the desire of maintaining its receptor binding domain accessible and, perhaps, facilitating receptor dimerization, a phenomenon believed to be necessary for generating Epo’s intracellular signal (13, 14). Indeed, as one Epo molecule may result in the dimerization of two receptor units, the use of an Epo–Epo dimer could induce the formation of higher orders of receptor oligomers, thereby amplifying the intracellular signal. In this regard, we have shown previously that the induction of such receptor clusters results in positive cooperativity and an enhanced biological response (15, 16). The enhanced in vitro potency of the oligomers may reflect this process.
The challenge in this study was to derivatize Epo and to oligomerize it without compromising its biological function. We initially ruled out the use of homobifunctional crosslinkers such as disuccinimidyl suberate. Such compounds have been used to achieve intrachain crosslinking with some formation of dimers as unwanted byproducts (17). We viewed such homobifunctional reagents as likely to reduce the flexibility of the Epo molecule, leading to a pronounced loss of biological activity. Rather, we wanted a means of controlling the interaction of Epo monomers. The lack of free sulfhydryl groups in human Epo made this possible. Thus, we chose the heterobifunctional crosslinking pair of LC-SPDP and SMCC to achieve controlled oligomerization.
Initial studies on the therapeutic use of recombinant Epo in humans employed intravenous administration three times weekly (18). This mode of administration results in extremely high plasma levels that fall off rapidly. Subcutaneous administration achieves a more sustained plasma Epo concentration due to the relatively slow release from the subcutaneous site (19). Indeed, less frequent subcutaneous administration can be efficacious for maintenance in some clinical settings. However, once the subcutaneously administered Epo molecule reaches the circulation, it is subject to the same plasma clearance as is the intravenously administered form. Therefore, neither in rodents nor in humans has a single dose of subcutaneously administered monomeric Epo been reported to result in a significant increase in hematocrit. In marked contrast, in the present study a single dose of subcutaneously administered Epo dimers was found to be very efficacious in causing an increase in hematocrit. This observation, which we have confirmed repeatedly, suggests that dimeric and other oligomeric forms of Epo may have an important value in therapy. The use of such molecular forms could lead to reduced frequency of administration and, thus, have an important effect on cost. We suggest that this approach may be generally applicable to other therapeutic proteins, including, but not limited to, hematopoietic growth factors and coagulation factors. Exploration of these possibilities should lead to important advances in our understanding of the biology and pharmacokinetics of these proteins as well as to an enhancement of their use in clinical situations.
Acknowledgments
We thank Elanex Pharmaceuticals, Inc., Bothell, WA, and Merckle Pharmaceuticals GmbH, Germany, for samples of recombinant human erythropoietin and Rosemary Panza for expert administrative support. This work was supported in part by U.S. Navy Grant N0014-93-1-0776 and National Institutes of Health Grant DK38841 (A.J.S.).
ABBREVIATIONS
- Epo
erythropoietin
- Hct
hematocrit
- SMCC
succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
- LC-SPDP
succinimidyl 6-[(3′-(2-pyridyldithio)propionamido]hexanoate
References
- 1.Miyake T, Kung C K, Goldwasser E. J Biol Chem. 1977;252:5558–5564. [PubMed] [Google Scholar]
- 2.Jacobs K, Shoemaker C, Rudersdorf R, Neill S D, Kaufman R J, Mufson A, Seehra J, Jones S S, Hewick R, Fritsch E F, et al. Nature. 1985;313:806–810. doi: 10.1038/313806a0. [DOI] [PubMed] [Google Scholar]
- 3.Lin F K, Suggs S, Lin C H, Browne J K, Smalling R, Egrie J C, Chen K K, Fox G M, Martin F, Stabinsky Z, et al. Proc Natl Acad Sci USA. 1985;82:7580–7584. doi: 10.1073/pnas.82.22.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell J S, Berkner K L, Lebo R V, Adamson J W. Proc Natl Acad Sci USA. 1986;83:6465–6469. doi: 10.1073/pnas.83.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spivak J L, Hogans B B. Blood. 1989;73:90–99. [PubMed] [Google Scholar]
- 6.Flaharty K K. Pharmacotherapy. 1990;10:9S–14S. [PubMed] [Google Scholar]
- 7.Flaharty K K, Caro J, Erslev A, Whalen J J, Morris E M, Bjornsson T D, Vlasses P H. Clin Pharmacol Ther. 1990;47:557–564. doi: 10.1038/clpt.1990.76. [DOI] [PubMed] [Google Scholar]
- 8.Krystal G. Exp Haematol. 1983;11:649–660. [PubMed] [Google Scholar]
- 9.Sytkowski A J, Fisher J W. J Biol Chem. 1985;260:14727–14731. [PubMed] [Google Scholar]
- 10.Lai P H, Everett R, Wang F F, Arakawa T, Goldwasser E. J Biol Chem. 1986;261:3116–3121. [PubMed] [Google Scholar]
- 11.Sytkowski A J. Biochem Biophys Res Commun. 1980;96:143–149. doi: 10.1016/0006-291x(80)91192-4. [DOI] [PubMed] [Google Scholar]
- 12.Katre N V. J Immunol. 1990;144:209–213. [PubMed] [Google Scholar]
- 13.Watowich S S, Yoshimura A, Longmore G D, Hilton D J, Yoshimura Y, Lodish H F. Proc Natl Acad Sci USA. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livnah O, Stura E A, Johnson D L, Middleton S A, Mulcahy L S, Wrighton N C, Dower W J, Jolliffe L K, Wilson I A. Science. 1996;273:464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- 15.Chern Y, Yonekura S, Sytkowski A J. Blood. 1990;76:2204–2209. [PubMed] [Google Scholar]
- 16.Yonekura S, Chern Y, Donahue K A, Feldman L, Vanasse G J, Sytkowski A J. Proc Natl Acad Sci USA. 1991;88:2535–2539. doi: 10.1073/pnas.88.6.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haniu M, Narhi L O, Arakawa T, Elliott S, Rohde M F. Protein Sci. 1993;2:1441–1451. doi: 10.1002/pro.5560020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eschbach J W, Egrie J C, Downing M R, Browne J K, Adamson J W. New Engl J Med. 1987;316:73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 19.McMahon F G, Vargas R, Ryan M, Jain A K, Abels R I, Perry B, Smith I L. Blood. 1990;76:1718–1722. [PubMed] [Google Scholar]