Abstract
The formation of new associations between items is critical for establishing episodic memories. It has been suggested that the hippocampus is essential for creating such associations but is not involved, or is much less involved, in memory for single items. In Experiment 1, we tested controls and amnesic patients with bilateral lesions thought to be limited primarily to the hippocampal region in both single-item and associative recognition memory tasks. In the single-item task, a conventional recognition memory task was administered in which participants studied either houses or faces and were tested for their ability to recognize the individual items. In the associative task, participants studied paired pictures of houses and faces with instructions that encouraged associating the two stimuli, and were tested for their ability to recognize the specific pairings that were presented at study. Like the controls, the amnesic patients performed more poorly on the associative task. Relative to the controls, the amnesic patients were impaired to a similar extent on the single-item and associative tasks. In Experiment 2, the performance of the amnesic patients was improved by increasing the number of presentations of the study lists (eight presentations instead of one). On both the single-item and associative tests, the performance of the amnesic patients after eight presentations was now identical to the performance of the controls who had been given only one presentation of the study list. Thus, the associative condition was not disproportionally difficult for the amnesic patients. These results are consistent with the idea that the hippocampus is similarly involved in single-item and associative memory.
Declarative memory (memory for facts and events) relies upon structures in the medial temporal lobes, including the hippocampal region (the hippocampus proper, the dentate gyrus, the subiculum) and the adjacent structures that lie along the parahippocampal gyrus (the entorhinal, perirhinal, and parahippocampal cortices). Currently, the precise contribution that these structures make to declarative memory is not well understood. There have been several efforts to distinguish the role of the hippocampal region from the role of the adjacent structures. Many of these share a common thread, proposing that the hippocampus is particularly involved in declarative memory tasks that require the formation and use of associations between the separate components of presented material (e.g., Sutherland and Rudy 1989; Eichenbaum et al. 1994; Vargha-Khadem et al. 1997; Henke et al. 1999; Eldridge et al. 2000; Brown and Aggleton 2001; Yonelinas 2002). In strong versions of this view, the hippocampus is proposed to be essential for overtly associative tasks such as recall or paired-associate learning, and it also supports the recollective component of recognition memory. In contrast, the capacity for single-item declarative memory tasks (including familiarity-based recognition) is supported by adjacent structures in the parahippocampal gyrus (e.g., the perirhinal cortex).
An alternate view is that the hippocampus and parahippocampal gyrus are both broadly important for declarative memory (e.g., Reed and Squire 1999; Stark and Squire 2001; Broadbent et al. 2002; Stark and Squire, in press). According to this view, all tasks of declarative memory, including familiarity-based recognition, depend on forming associations, and both the hippocampus and the adjacent cortex are important for such tasks. Accordingly, a simple distinction between single-item and associative memory does not capture the division of labor between the hippocampus and adjacent structures in the medial temporal lobe.
RESULTS
Experiment 1
In Experiment 1, we examined the status of single-item and associative recognition memory in four amnesic patients with damage thought to be limited primarily to the hippocampal region. In the single-item condition, participants studied 10 pictures of either faces or houses. After a brief delay, a yes/no recognition memory test was given. Associative memory was also tested with a yes/no recognition memory task. In the associative condition, participants studied 10 pairs of houses and faces with instructions that encouraged forming an association between the items of each pair. At test, participants were shown house-face pairs and asked to determine whether each pair was an intact repetition of a studied pair or whether it was the recombination of a house and a face that had been studied as part of different pairs. If the hippocampal region provides a specific ability to explicitly associate components in declarative memory, damage to the hippocampal region should impair performance more severely on the associative house-face task than on the single-item task.
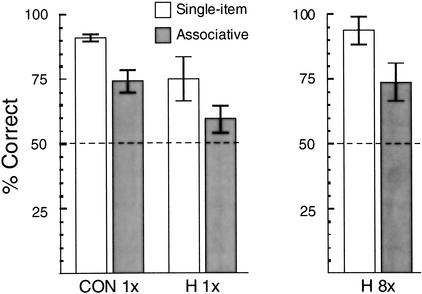
Figure 1 (left panel) shows the results. The controls scored 91% correct (correct “yes” responses plus correct “no” responses) in the single-item condition (hit rate = 90%; correct rejection rate = 92%) and 74% correct in the associative condition (hit rate = 77%; correct rejection rate = 72%). Amnesic patients with damage to the hippocampal region scored 75% correct in the single-item condition (hit rate = 76%; correct rejection rate = 74%) and 59% correct in the associative condition (hit rate = 64%; correct rejection rate = 55%). A repeated-measures analysis of variance (ANOVA) revealed an effect of group (F(1,12) = 7.6, P<.02), indicating that the amnesic patients were impaired overall relative to the controls, and an effect of test condition (F(1,12) = 21.0, P<.005), indicating that performance was poorer in the associative condition than in the single-item condition. However, there was no interaction between group and test condition (F(1,12) = 0.03), indicating that the relative difficulty of the associative condition was similar for the two groups.
Figure 1.
Percent correct scores for control volunteers (CON; n = 10) and for amnesic patients with damage to the hippocampal region (H; n = 4) on a recognition memory test for pictures of houses or faces presented in isolation (single-item; white bars) and on an associative recognition test involving house-face pairs (associative; gray bars). In Experiment 1 (left), control volunteers and amnesic patients studied each item (single items or face-house pairs) only once (1×). In Experiment 2 (right), the amnesic patients studied each item eight times (8×). Error bars indicate the standard error of the mean, and the dashed lines indicate chance performance.
Nevertheless, whereas performance of the controls was reliably above chance in both the single-item (t(9) = 31.0, P<.001) and associative (t(9) = 5.6, P<.001) conditions, the performance of the amnesic patients was not. In neither the single-item condition (t(3) = 2.9, P = .06) nor the associative condition (t(3) = 1.8, P = .18) was the performance of the patients reliably above chance.
Experiment 2
In Experiment 1, amnesic patients with damage to the hippocampal region were impaired in both the single-item and the associative conditions, and the relative difficulty of the associative condition was similar for amnesic patients and controls. Yet, because the performance of the amnesic patients was not reliably above chance, it is possible that the patients might have been disproportionally impaired in the associative condition and that a particularly severe impairment in this condition was obscured by a floor effect. In Experiment 2, we attempted to elevate the performance of the amnesic patients by increasing the amount of their exposure to the study items (for a discussion of various methods of equating amnesic and control performance, see Giovanello and Verfaellie 2001).
Figure 1 (right panel) shows the results, averaged across the two test sessions. The increase in study exposure (from 1 to 8 presentations) significantly improved the performance of the amnesic patients (67.2% to 83.2% overall; t(3) = 4.2, P<.05). The patients scored 92% correct in the single-item condition (hit rate = 92%; correct rejection rate = 93%) and 74% correct in the associative condition (hit rate = 83%; correct rejection rate = 65%). Their scores for both the single-item condition (t(3) = 8.3, P<.005) and the associative condition (t(3) = 3.7, P<.05) were reliably above chance levels. Further, eight repetitions of the study items effectively equated the score of the amnesic patients with the scores of the controls in Experiment 1, who had seen the study items only once (single-item condition: 92% correct for the patients, 91% correct for the controls; associative condition: 74% correct for the patients, 74% correct for the controls). Accordingly, an ANOVA comparing the performance of these two groups found no effect of group (F(1,12) = 0.01) and no interaction of group and test condition (F(1,12) = 0.06). The effect of test condition (F(1,12) = 28.1, P<.001) confirmed that the associative condition was more difficult than the single-item condition.
DISCUSSION
In two experiments, we examined the status of single-item memory (memory for individual houses or faces) and associative recognition memory (memory for house-face pairs) in amnesic patients with damage thought to be limited primarily to the hippocampal region. In this way, we tested the hypothesis that the hippocampal region is particularly involved in the formation and use of associations, but is not critical (or is less critical) for single-item recognition memory. If the hippocampal region were more involved in associative than in single-item memory, one would have predicted an impairment limited to, or more severe in, the overtly associative house-face task. In Experiment 1, amnesic patients and controls performed more poorly on the associative task than on the single-item task. In addition, the amnesic patients were impaired overall relative to the controls. However, there was no evidence of a differential impairment for the associative task. In Experiment 2, the performance of the amnesic patients was improved by presenting each study list eight times. With these additional presentations of the study list, the performance of the amnesic patients with damage to the hippocampal region matched the performance of the controls from Experiment 1 on both the single-item and associative memory tasks. In summary, in neither experiment did we find evidence for a differential impairment on the associative memory task. Thus, these results are inconsistent with the view that the hippocampal region is any more involved in memory for associations than single-item memory.
These results complement our report (Stark and Squire, in press) of similarly impaired memory for single items and memory for conjunctions in patients with damage to the hippocampal region. The present results extend our previous results in two ways. First, in the previous study, a continuous recognition memory task was used in which participants were asked to decide whether each item in a continuing stream of items had been encountered previously. In the task (based on one developed by Kroll et al. 1996), items could be entirely novel, novel with one previously encountered (repeated) component, novel but with both components repeated, or a true repetition. We observed similar levels of impairment for all three groups of novel items and found no evidence of differential impairment for the explicitly associative recombined stimuli. In the present study, we extended this finding to the case of a traditional recognition memory task in which separate study phases and test phases were presented. Second, while three of the patients tested in the prior work had damage thought to be limited primarily to the hippocampal region, two others had damage that extended into adjacent medial temporal lobe structures. Although the performance of these two groups of patients did not differ overall, in the present study we were able to examine four patients with damage thought to be limited primarily to the hippocampal region.
There have been a number of findings that have been taken as support for the view that the hippocampal region is particularly involved in associative forms of memory. For example, two neuroimaging studies reported greater hippocampal activity during an associative memory encoding task than during a nonassociative memory encoding task (Henke et al. 1997, 1999). Two studies reported similar results during memory retrieval (Eldridge et al. 2000; Yonelinas et al. 2001). Yet, in each of these cases, the associative versus nonassociative contrast revealed activity in both the hippocampal region and the parahippocampal gyrus (also see Stark and Squire 2001, for an example of similar levels of hippocampal activity during associative and nonassociative recognition tasks). Therefore, although these data support a role for the medial temporal lobe in associative memory, they do not differentiate between the hippocampus and parahippocampal gyrus.
The observation that neurons within the hippocampus often respond maximally to conjunctions of features (for reviews, see Eichenbaum 2000; Suzuki and Eichenbaum 2000; Brown and Aggleton 2001) has also been taken to support the hypothesis that the hippocampal region is especially important for associative memory. Yet, such conjunctive codes are present as well in the parahippocampal gyrus (Fried et al. 1997). In addition, what appear to be single-feature codes are also present in the hippocampus (Wood et al. 1999). When these findings are considered in the light of the neuroanatomical evidence for associational connections not only within the hippocampus, but also within the entorhinal, perirhinal, and parahippocampal cortices (for review, see Lavenex and Amaral 2000), support for a distinction between the hippocampal region and the parahippocampal gyrus with respect to associative memory is weakened.
From these and other studies (see Stark and Squire, in press, for additional discussion), we suggest that although there is likely functional specialization within the medial temporal lobe memory system, attempts to differentiate between the hippocampal region and the adjacent cortex based on simple dichotomies such as associative versus nonassociative memory are unlikely to be successful. That the hippocampal region is important for associative, recollective, episodic, conjunctive, and relational memory is quite clear. However, ascribing the capacity for associative (or recollective, episodic, conjunctive, or relational) memory only to the hippocampal region and the capacity for nonassociative (or familiarity, semantic, single-item, or nonrelational) memory only to structures in the parahippocampal gyrus does not account for the available neuropsychological, neuroimaging, neuroanatomical, or electrophysiological data. We suggest, as have others (e.g., Lavenex and Amaral 2000; Suzuki and Eichenbaum 2000; Norman and O'Reilly, in press), that whatever the different roles of individual structures in the medial temporal lobe may be, the division of labor among these structures is not absolute. The present data suggest that the hippocampal region is equally important for single-item recognition memory and for recognition memory tests that overtly assess memory for associations between two stimuli.
MATERIALS AND METHODS
Experiment 1
Participants
Four amnesic patients (A.B., G.W., L.J., and M.J.) with damage thought to be limited primarily to the hippocampal region (CA fields, dentate gyrus, and subiculum) participated in the study (Table 1). A.B. became amnesic in 1976 after an anoxic episode following cardiopulmonary arrest. G.W. became amnesic in September 2001, following an overdose of heroin and associated respiratory distress. L.J. became amnesic during a 6-mo period that began in 1998 with no known precipitating event. Her memory impairment has remained stable since that time. M.J. had a 10-y history of cardiovascular disease. On June 6, 1996, he awoke from a night's sleep complaining of memory difficulties. His impairment has remained stable since that time.
Table 1.
Characteristics of Amnesic Patients
| Patient
|
Age
|
Education
|
WAIS-III IQ
|
WMS-R
|
||||
|---|---|---|---|---|---|---|---|---|
| Attention
|
Verbal
|
Visual
|
General
|
Delay
|
||||
| A.B. | 64 | 20 | 107 | 87 | 62 | 72 | 54 | <50 |
| G.W. | 43 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| L.J. | 64 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| M.J. | 61 | 16 | 139 | 125 | 62 | 93 | 62 | <50 |
The Wechsler Adult Intelligence Scale-III (WAIS III) and the indices of the Wechsler Memory Scale-Revised (WMS-R) yield a mean score of 100 in the normal population with a standard deviation of 15. Three of the patients are male; L.J. is female.
For three of the patients (G.W., L.J, and M.J.), magnetic resonance imaging (MRI) has identified the damage within the medial temporal lobe by comparison with three to four age- and gender-matched healthy controls (for G.W. and M.J., volumes of hippocampal region and parahippocampal gyrus relative to intracranial volume; for L.J., areal measurements, see Manns and Squire 2001). All three patients have reduced hippocampal area bilaterally (reductions of 45%, 46%, and 10%, respectively) and substantially less damage to the parahippocampal gyrus (reductions of 15%, 6%, and 3%, respectively). Patient A.B. wears a pacemaker and is ineligible to participate in MRI studies. In CT scans obtained in 2001, temporal lobe volume appeared normal, and the temporal horns were symmetric and normal in size. The basal ganglia and thalamus also appeared normal. The only focal lesions detected were small bilateral foci in the white matter lateral to the head of the caudate nucleus, which appeared to be old lacunar infarctions. Neurological exam indicated well-circumscribed amnesia. These findings, together with reports that histologically confirmed damage limited to the hippocampal formation can occur after anoxia (Rempel-Clower et al. 1996), suggest that A.B.'s memory impairment is likely due to damage within the hippocampal region.
Ten healthy control volunteers (five men and five women) were also tested. The controls were matched to the patients with respect to age (mean = 62.8 years; range = 38–75; patient mean = 58.0), education (mean = 16.2 years; range = 12–20; patient mean = 15.0), and WAIS-III subtest scores for Information (23.3; patients = 20.5) and Vocabulary (57.3; patients = 52.3). Because one of the patients (G.W.) was substantially younger than the other three, two of the control volunteers were specifically matched to G.W.
Materials
The materials consisted of 80 color pictures of houses and 80 color portrait-style pictures of faces, presented on a computer screen (Fig. 2). A total of 40 houses and 40 faces were randomly assigned to the single-item condition. The remaining 40 houses and 40 faces were randomly paired and assigned to the associative condition.
Figure 2.
Examples of a house and a face stimulus. In the single-item condition, either a house or a face was presented in the middle of the screen. In the associative condition, the house and the face were presented side-by-side with instructions that encouraged associating the two stimuli.
Procedure
Each participant completed four single-item and four associative study/test sequences. Single-item and associative tests were alternated, counterbalancing the initial test across participants.
For each single-item test, participants were shown either 10 color pictures of houses or 10 color pictures of faces. Houses were used for two of the single-item tests, and faces were used for the other two (participants alternated between houses and faces, with the stimulus type for the initial single-item condition counterbalanced across participants). Participants were asked to make a judgment about each item within 4 sec. In the case of single-item stimuli, participants were asked to judge whether the house was built before or after 1960. In the case of faces, participants were asked to judge whether the person was a “cat person” or a “dog person”. After 4 sec, the screen was cleared, and participants were asked to press the space bar to begin the next trial. After all 10 study trials were presented, a 3-min delay was imposed before the test phase began. To test memory for the items presented in the single-item tests, 20 stimuli (houses or faces) were presented. Half of the stimuli were studied targets and half were novel foils. On each trial, participants were asked to make a yes/no recognition memory judgment using keys labeled “yes” and “no”. Test trials were self-paced.
In each test of the associative condition, participants first studied a set of 10 house-face pairs. On each trial, a picture of a house and a picture of a face were presented on the computer screen side-by-side, and participants were asked to judge whether that person might live in that house. Participants were given four seconds to indicate their decision using keyboard keys labeled “yes” and “no”. After four seconds, the screen was cleared, and participants were asked to press the space bar to begin the next trial. After all 10 study trials were presented, a 3-min delay was imposed, and 10 house-face pairs were presented. Participants were asked to judge (by pressing keys labeled “yes” and “no”) to indicate whether the house and face had been studied together previously or whether they had been studied separately (half of the pairs were intact pairs and half were recombined). Test trials were self-paced.
Experiment 2
Participants
The same four amnesic patients participated.
Materials
Two lists of 90 houses and 90 faces were constructed, similar to those used in Experiment 1. The two lists were used in separate test sessions. In each list, 60 houses and 60 faces were assigned to the single-item condition. The remaining 30 houses and 30 faces were randomly paired and assigned to the associative condition.
Procedure
The procedure was identical to that of Experiment 1 with three exceptions. First, each patient was tested in two sessions, separated by at least 6 mo. Second, in contrast to the single 4-sec presentation of the study list in Experiment 1, each study list was presented eight times in succession for 6 sec each prior to the test. Third, within each session, patients were given four associative study/test sequences, as in Experiment 1, but only three single-item sequences (vs. two of each type in Experiment 1). In each session, all three single-item tests involved either houses or faces, and the single-item stimulus type in the second session was the one that had not been tested in the first session (stimulus type in the first session was counterbalanced across participants).
Acknowledgments
We thank Shauna Stark for programming, data collection, and data analysis; and Jennifer Frascino, Joyce Zouzounis, and Jeffrey Gold for assistance. This research was supported by the Medical Research Service of the Department of Veterans Affairs, NIMH Grants MH24600 and MH12278, the National Alliance for Research on Schizophrenia and Depression (NARSAD), and the Metropolitan Life Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lsquire@ucsd.edu; FAX (858) 552-7457.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.51802.
REFERENCES
- Broadbent NJ, Clark RE, Zola SM, Squire LR. The medial temporal lobe and memory. In: Squire LR, Schacter DL, editors. The neuropsychology of memory. 3rd ed. New York, NY: Guilford Press; 2002. pp. 3–23. [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two component functions of the hippocampal memory system. Behav Brain Sci. 1994;17:449–517. [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fried I, MacDonald K, Wilson C. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M. The relationship between recall and recognition in amnesia: Effects of matching recognition between patients with amnesia and controls. Neuropsychology. 2001;15:444–451. doi: 10.1037//0894-4105.15.4.444. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci. 1999;96:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll NEA, Knight RT, Metcalfe J, Wolf ES, Tulving E. Cohesion failure as a source of memory illusions. Journal of Memory and Language. 1996;35:176–196. [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR. Perceptual learning, awareness, and the hippocampus. Hippocampus. 2001;11:776–782. doi: 10.1002/hipo.1093. [DOI] [PubMed] [Google Scholar]
- Norman, K.A. and O'Reilly, R.C. Modeling hippocampal and neocortical contributions to recognition memory: A complementary learning systems approach. Psychological Rev. (in press). [DOI] [PubMed]
- Reed JM, Squire LR. Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behav Neurosci. 1999;113:3–9. doi: 10.1037//0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. Simple and associative recognition memory in the hippocampal region. Learn Mem. 2001;8:190–197. doi: 10.1101/lm.40701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, C.E.L. and Squire, L.R. Hippocampal damage equally impairs memory for single-items and memory for conjunctions. Hippocampus (in press). [DOI] [PMC free article] [PubMed]
- Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann NY Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Praesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko P, Eichenbaum H. The global record of memory in hippocampal neural activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Yonelinas A, Hopfinger J, Buonocore M, Kroll N, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fMRI study. Neuroreport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]