Abstract
The studies reported here used an interference paradigm to determine whether a long-term consolidation process (i.e., one lasting from several hours to days) occurs in the learning of two implicit motor skills, learning of a movement sequence and learning of a visuo-motor mapping. Subjects learned one skill and were tested on that skill 48 h later. Between the learning session and test session, some subjects trained on a second skill. The amount of time between the learning of the two skills varied for different subjects. In both the learning of a movement sequence and the learning of a visuo-motor mapping, we found that remote memories were susceptible to interference, but the passage of time did not afford protection from interference. These results are inconsistent with the long-term consolidation of these motor skills. A possible difference between these tasks and those that do show long-term consolidation is that the present tasks are not dynamic motor skills.
The notion that memories initially exist in a labile state and with time become stable was first proposed in 1900 by Müller and Pilzecker (cited in Lechner et al. 1999). These researchers found that memory for a list of words was disrupted if new information was learned less than 1 min later, but not if learned 10 min later. They concluded that the memories had undergone a stabilization process, which they termed consolidation. In the current study, we will use the term consolidation to refer to the stabilization of memory (i.e., a decrease in susceptibility to forgetting via decay or interference) in the absence of further practice of that memory. There are two putative phases of consolidation processes, short-term consolidation processes, which operate over a period of seconds to hours post-training and are mediated by local cellular mechanisms resulting in long-term potentiation (LTP), and long-term consolidation processes, which operate over a period of hours to months post-training and are mediated by neural reorganization (for review, see McGaugh 2000; Dudai 2002). The focus of the current study is on long-term consolidation processes. Much of the research on long-term consolidation processes has focused on explicit memory tasks, tasks in which memory is demonstrated through conscious recollection of previously learned information (Schacter 1987). Although less consolidation research has focused on implicit memory tasks, tasks in which memory is revealed through performance and does not require conscious recollection of learned information, recent interest in the long-term consolidation of motor learning is one exception (Brashers-Krug et al. 1996). The experiments described here were designed to assess whether a long-term consolidation process is ubiquitous for different components of motor skill learning.
Although there is much evidence that a long-term process of consolidation is evident in performance on explicit memory tasks (e.g., Squire et al. 1989; Zola-Morgan and Squire 1990), it is not immediately apparent that a long-term consolidation process would be evident in the performance of implicit memory tasks. A critical difference between performance on explicit and implicit memory tasks is that performance on explicit tasks relies on the integrity of the medial temporal lobe (i.e., the declarative memory system), whereas performance on implicit tasks does not (Squire 1992; see Chun and Phelps 1999, for an exception). Performance on implicit tasks depends on the integrity of various anatomical structures outside of the medial temporal lobe (i.e., nondeclarative memory). One result of this difference is that patients with hippocampal damage are impaired on tests of explicit memory (e.g., Corkin 1984), but their performance is relatively spared on tests of motor skill learning (Corkin 1968; Brooks and Baddeley 1976; Squire et al. 1984). We will focus on the memory supporting motor skill learning as a particular type of nondeclarative memory, although the tasks discussed here are not meant to be representative of all forms of nondeclarative memory.
There is behavioral evidence of long-term motor skill consolidation in a force-field task requiring learning of dynamic transformations, that is, the learning of new muscle forces (Brashers-Krug et al. 1996). In this task, subjects must compensate for a perturbing force while moving a cursor to a target location on a computer screen. In one demonstration of temporally graded retroactive interference (RI) in the learning of the force-field task, all subjects practiced on one pattern of forces (force A). Some subjects practiced on a second, opposing pattern of forces (force B) at varying amounts of time after initial learning of the first force. All subjects were then tested on force A 24 h after the initial learning of A. Subjects who trained on force B either 5 min or 1 h after training on force A had impaired retention of A (i.e., greater RI) compared with groups that never trained on force B or that trained on force B 4 h after force A training. This pattern of temporally graded RI, in which only those subjects learning force B soon after having learned force A show an impairment in their memory for A, was interpreted as evidence that a long-term process of consolidation occurs for motor skill. Furthermore, it has been suggested that a reorganization of neural connectivity may be responsible for the consolidation seen in the force-field task (Shadmehr and Brashers-Krug 1997; Shadmehr and Holcomb 1997).
Recent work does indicate that the primary motor cortex (M1) may be responsible for the consolidation of dynamic information. Mullbacher et al. (2002) found that administering repetitive transcranial magnetic stimulation (rTMS), which interferes with synaptic activity, to M1 immediately following the learning of a finger movement task-disrupted retention of performance. Administering the rTMS to M1 6 h after completing practice on the task did not, however, disrupt retention of performance. These results provide a possible neuropyschological mechanism for the changes in RI witnessed in the force-field learning task (Brashers-Krug et al. 1996).
One problem with attempting to generalize the results that a process of consolidation exists for implicit motor skill learning beyond the force-field learning task is that different brain structures are responsible for the learning and execution of different components of motor skill (for review, see Jueptner and Weiller 1998; Willingham 1998). In particular, learning new patterns of muscles forces relies on M1 (Kakei et al. 1999), and their representation also relies on pools of interneurons in the spinal cord (see Bizzi and Mussa-Ivaldi 1999 for a review of recent evidence). On the other hand, learning and performing a sequence of actions relies on the basal ganglia and supplementary motor area (Grafton et al. 1994; Willingham et al. 1996); whereas learning kinematic transformations involving the integration of perceptual and motor information relies on the posterior parietal cortex (Clower et al. 1996; Ghilardi et al. 2000). Therefore, demonstrating consolidation in the learning of dynamic information, which is dependent on M1, is not necessarily informative about the existence of consolidation in other motor skills that do not rely upon the same brain structure.
The set of experiments reported here explored the existence of a long-term consolidation process in two different nondynamic components of motor skill whose anatomical basis is well understood, the learning of a movement sequence and the learning of a new visuo-motor mapping. We used an interference design to assess consolidation. Subjects learned a skill at one point in time (skill A) and their retention of that skill was tested 48 h later. Between the learning session and the test session, some subjects learned a second skill that was incompatible with the first (skill B). The appropriate response for skill B was as different as possible from that for skill A, given very similar retrieval cues. The amount of time between the learning of the first and second skills varied for different subjects.
EXPERIMENT 1: RESULTS AND DISCUSSION
To assess whether consolidation occurs in the learning of a movement sequence, we chose the serial response time (SRT) task. In this task, subjects see circles appear one at a time in one of four boxes arranged horizontally on the computer screen. The task of the subjects is to press a key on the computer keyboard that directly corresponds to the spatial location of the circle as quickly as possible. Unbeknownst to the subject, the circles appear in a repeating sequence of spatial locations. Subjects become faster at responding to the repeating sequence of circles. Additionally, if the experimenter later introduces randomly determined circle locations, the subjects' reaction time (RT) increases. These effects occur even if the subjects do not become aware that the circles are repeating in a sequence (Willingham et al. 1989). As such, learning in this task can be implicit.
SRT
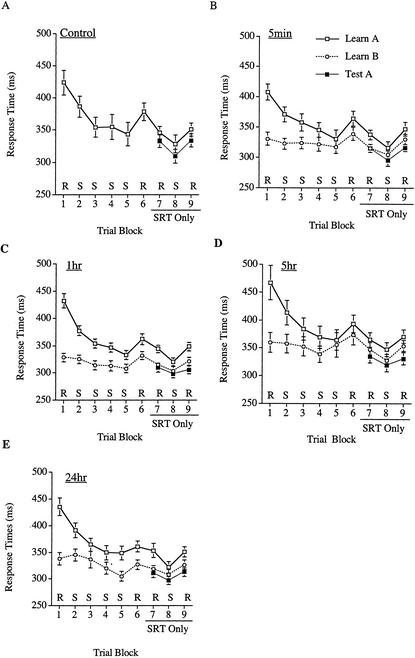
As learning in the SRT task is heavily dependent on making the correct series of spatial responses (Willingham 1999), six subjects who failed to get at least 90% correct on the SRT task during training were excluded from the analysis, leaving 24 subjects in the control condition, 24 in the 5-min, 24 in the 1-h, 21 in the 5-h, and 23 in the 24-h. For the remaining subjects, accuracy in the SRT task was uniformly high (95%–98% correct for each block in each condition). There were no reliable effects in analyses of the accuracy scores (all Fs <1.5, Ps >0.20). This result is unsurprising given that the subjects were instructed to respond as quickly and as accurately as possible. The remaining SRT analyses reported here are based on RT as recorded in msec. RTs were summarized by taking the median of each group of 12 trials and then finding the mean of the 8 medians for each block, yielding a single summary RT per trial block for each subject. RT across blocks for each of the conditions is depicted in Figure 1.
Figure 1.
Experiment 1 RT during training and testing sessions for each condition. (A) Control; (B) 5-min; (C) 1-h; (D) 5-h; (E) 24-h. (□) Training on Sequence A; (○) training on Sequence B; (▪) testing on Sequence A. (Rs) Random blocks; (Ss) sequenced blocks. Error bars, +/− 1 SE. The measures of learning were taken when subjects performed the SRT task alone by subtracting performance in the S block from the average of that of the two R blocks.
Learning of A
Although it is possible to assess learning during training on sequence A (with the concurrent memory task), any improvements during the sequenced training blocks may be attributable to one of three sources, learning of the movement sequence, general improvements on the button-pushing task, or learning to better coordinate simultaneous performance of both tasks. Additionally, the concurrent memory task may have influenced the expression of learning. Therefore, learning was assessed at transfer (without the concurrent memory task) only. A learning score for sequence A was created by subtracting the response time in the final sequenced block from the average of that in the final two random blocks. (Similar measures were created for the learning of B and testing of A.) All groups showed equivalent learning of sequence A, regardless of when they learned sequence B (see Table 1). An ANOVA with time-learned sequence B (control, 5 min, 1 h, 5 h, or 24 h) as a variable failed to reach significance, F<1. The learning scores for sequence A differed reliably from zero, t(115)=8.66, P<0.0001. Subjects responded faster to the sequenced than to the random blocks.
Table 1.
Means and SE of the Learning Scores for Skill A
|
|
Control
|
Learning score for skill A
|
|||
|---|---|---|---|---|---|
| 5 min
|
1 hr
|
5 hr
|
24 hr
|
||
| Experiment 1 | |||||
| SRT | 19.96 (8.3) | 26.42 (6.9) | 26.40 (4.5) | 20.5 (5.9) | 29.87 (6.0) |
| Experiment 2 | |||||
| Exposure | 2.04 (.2) | 2.24 (.2) | NA | 2.22 (.2) | 2.02 (.2) |
| Aftereffects | −5.11 (.7) | −6.54 (.7) | NA | −5.84 (.8) | −6.19 (.7) |
Even though there was not a between-group difference in sequence A learning, it is possible that subjects' learning of A was related to their ability to learn a second sequence, as well as to their ability to retain the initial learning. As proactive interference (PI) and RI depend on prior learning, those who learn more have a greater opportunity for RI and PI—at the extreme, no learning at all should leave minimal opportunity for RI or PI. To assess this possibility, we used subjects' sequence A learning scores to predict the interference measures (described below) in separate simple linear regressions. The extent of sequence A learning was a reliable predictor of both PI and RI (see Table 2 for regression statistics). Subjects who showed greater learning of sequence A also experienced greater PI and greater RI. As learning of sequence A was related to subsequent interference measures, all reported ANOVAs for the RT results are analyses of covariance (ANCOVA), with the learning score for sequence A as the covariate.4 These ANCOVA results for the PI and RI analyses did not differ qualitatively from those results obtained when an ANOVA was used.
Table 2.
Regression Results for Predicting Proactive and Retroactive Interference Scores from Initial Learning of Skill A
| B | SE B | R2 | ||
|---|---|---|---|---|
| Experiment 1 | ||||
| PI | −.85 | .10 | .45a | |
| RI | −.58 | .06 | .47a | |
| Experiment 2: Exposure | ||||
| PI | .58 | .11 | .05a | |
| RI | −.06 | .06 | .00 | |
| Experiment 2: Aftereffects | ||||
| PI | .54 | .10 | .13a | |
| RI | .48 | .06 | .14a |
p = .0001.
Proactive Interference
The nature of PI bears on the interpretation of the RI results. Namely, if a particular group shows no RI, but extreme PI, it may be that this group never learned the second skill well enough to interfere with their memory for the first skill. For example, if RI was temporally graded in the direction predicted by a consolidation account (i.e., the greatest amount of RI occurred when the two skills were learned close together in time), but PI was temporally graded in the opposite direction (i.e., the least amount of PI occurred when two skills were learned close together in time), then the temporal gradient of the RI may only indicate that some subjects never learned the second skill.
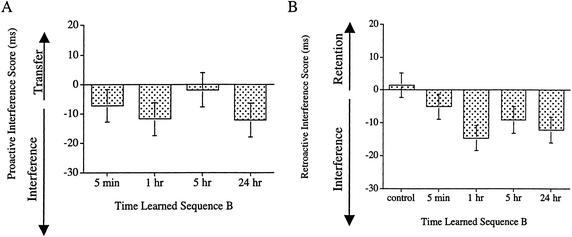
The PI measure was the difference between each subject's score when learning skills A and B. Positive scores on this measure indicate positive transfer; negative scores indicate PI. As would be expected from a visual inspection of the covariate-adjusted means of the PI scores (see Fig. 2A), there was PI in the learning of sequence B; however, there was no indication of a temporal gradient to the PI. Overall, the measure of PI was reliably less than zero (M = −8.37, SE=3.8), indicative of interference, t(91) = −2.282, P=0.0249 An ANCOVA on the PI scores with time learned sequence B as a variable yielded no significant effects, F<1. Two planned contrasts were performed to assess the temporal gradient of the PI scores, a planned linear contrast, and a planned comparison contrasting the 5 and 24 hr groups with the other groups learning sequence B. Both failed to reach significance, Fs<1. Even when the second sequence was learned 24 h after having learned the first, there was PI from having learned a different sequence previously. This pattern of proactive interference does not pose a problem for the interpretation of the RI results.
Figure 2.
Covariate-adjusted means of the (A) PI scores and (B) the RI scores for the RT measure in Experiment 1. PI scores were obtained by subtracting the Learning of A score from that for the Learning of B. RI scores were obtained by subtracting the Learning of A score from that for the Test of A. Negative values on these measures indicate interference. Positive values indicate positive transfer or retention.
Assessing Consolidation: Retroactive Interference Measure
The RI results are of critical importance in addressing the question of whether there is consolidation in sequence learning. If the memory for a movement sequence existed in a labile state for some period of time and slowly became stabilized (i.e., it was consolidated), then a person learning sequence B soon after having learned A should have an impaired memory for A. In particular, one would expect a temporally graded RI. That is, the memory impairment for A decreases as the temporal interval between the learning of the two skills increases. The RI measure is the difference between performance when subjects train on sequence A and when they test on A. Positive scores on this measure indicate an improvement in the memory for skill A. Negative scores indicate RI.
Statistical analyses confirmed the visual impression created by the covariate-adjusted means of the RI scores (see Fig. 2B); there was RI in the memory for sequence A. An ANCOVA on the RI scores with time learned sequence B (control, 5 min, 1 h, 5 h, or 24 h) as a variable failed to yield an overall effect, F<1.5, P<0.25. However, a planned comparison revealed that the groups that learned sequence B had greater RI than did the control group, F(1, 106) = 7.995, P=0.0056, MSE=335.662. This result indicates that all groups that learned a second sequence exhibited RI in their memory for the first sequence. However, the extent of RI did not depend on the amount of time that passed between the learning of the two sequences. To test for a temporal gradient in the RI, we performed two planned comparisons, a linear contrast among those groups that learned a second skill and a planned comparison contrasting the groups that learned skill B within 5 h of having learned skill A, with those groups that learned skill B 5 or more hours after having learned skill A. Neither contrast was reliable, Fs<1. Given the lack of temporal gradient in the RI scores, there is no evidence of consolidation in the learning of a movement sequence.
Explicit Memory Measures
Confidence Ratings
A repeated measures ANOVA with time-learned sequence B (control, 5 min, 1 h, 5 h, or 24 h) as a between-subjects variable and session (learn A, learn B, or test A) as a within-subjects variable yielded a reliable effect of session, F(2, 174) = 4.09, P=0.0184, MSE=1.236. Helmhert comparisons revealed that confidence ratings were greater for the second and third training sessions (M=4.22, SE=0.16 and M=4.20, SE=0.15, respectively) than for the first training session (M=3.92, SE=0.14), F(1, 174) = 6.909, P=0.0093. Subjects may have become more suspicious about the nature of the task after they had participated in at least one session. The effect of time-learned sequence B failed to reach significance, as did the interaction between time-learned sequence B and session, Fs<1.5, Ps>0.25. These results indicate that there were no differences between the groups in their confidence in having seen a sequence.
Free Recall
Each subject's free recall score reflected the number of positions in the sequence correctly recalled. A position was considered correct when it was included within a correctly recalled segment consisting of a minimum of three consecutive positions, but these recalled segments themselves need not be consecutive. For example, if a subject saw 314324123142 and recalled 123143, the score would be 6, because both 123 and 143 occurred in the sequence.
The time at which sequence B was learned had no influence on subjects' recall of sequences A and B. Overall, however, subjects had greater recall of sequence A (M=5.45, SE=0.19) than of sequence B (M=4.10, SE=0.22), indicating PI in the explicit memory for sequence B. A repeated measures ANOVA with time learned sequence B (control, 5 min, 1 h, 5 h, or 24 h) as a between-subjects variable and learning session (learn A, learn B, or test A) as a within-subjects variable yielded a significant effect of learning session, F(2, 174) = 11.48, P=0.0001, MSE=4.344. Helmhert comparisons revealed that recall of sequence A was greater than that of sequence B, F(1, 174) = 22.701, P=0.0001. Recall of A (learning or test) did not vary with the learning session, F<1. The effect of time-learned sequence B was not reliable, nor was the interaction, Fs<1.3, Ps>0.28.
Previous reports of explicit knowledge in SRT with similar amounts of sequence training have found that mean guessing performance is 4.6 (Willingham and GoedertEschmann 1999). The interference analyses on RT were rerun separately for those subjects who recalled five or more positions of sequence A, that is, more positions than would be expected by chance (n=79) and those subjects who recalled four or less positions (n=36). Splitting the analyses on the basis of recall did not change the qualitative nature of the results. There was ungraded PI and RI in both high and low recall groups.
Summary
In Experiment 1, interpolated sequence learning hurt the memory for a previously learned sequence, regardless of the amount of time that had passed between the original and interpolated learning. This pattern of RI is inconsistent with the notion that implicit sequence knowledge consolidates.
Why might there be consolidation in the learning of new muscle dynamics, but not in the learning of a new sequence of movements? One possibility is that the learning of a new sequence of movements and the learning of new muscles dynamics have different anatomic bases and, therefore, different mechanisms of long-term storage. A second possibility is that the sequence-learning task used in Experiment 1 is behaviorally different from the force-field learning task used to assess the learning of dynamic information. In the force field learning task, prior to executing any movements, all of the perceptual cues available to the subject are the same when learning force A and force B. This is not the case in the SRT task. The two sequences in Experiment 1 were chosen to be as different as possible from one another. Therefore, the perceptual cues for learning sequences A and B were not identical. Additionally, in the SRT task, a person does not have to interact with the environment before knowing the correct movement to make. Whereas in the force-field learning task, there is nothing in the environment that dictates the correct movement until the subject begins to interact with the robot arm. In the force-field learning task, the subject must gradually learn the correct movements on the basis of error feedback. In this way, the force-field learning task is an adaptation paradigm. Lastly, learning one sequence of movements is not necessarily incompatible with learning a second sequence of movements. In the force-field learning task, however, learning one force for interacting with the robot arm is incompatible with learning a different force for interacting with that robot arm.
In Experiment 2, we investigated this second possibility, that the failure to find consolidation in the learning of a movement sequence was due to the different behavioral requirements of that task, by testing whether there was temporally graded RI in a task that was behaviorally similar to the force-field learning task, yet which has a unique anatomic basis (Clower et al. 1996)—prism adaptation. The prism adaptation paradigm involves a kinematic transformation in which subjects must learn a new mapping between their vision and proprioception. It requires the subject to interact with the environment before knowing the correct movement to make. Likewise, the cues for the appropriate response are identical when learning two incompatible prism displacements.
EXPERIMENT 2: RESULTS AND DISCUSSION
Experiment 2 investigated consolidation in the learning of a new mapping between the perception and action systems (i.e., a kinematic transformation). We chose the prism adaptation paradigm to investigate the learning of a new visuo-motor mapping. In studies of prism adaptation, subjects don prism goggles that displace their vision laterally. During prism exposure, subjects are trained to point at targets with visual feedback. Before and after prism exposure, subjects' target-pointing accuracy is measured with normal vision. During the prism exposure period, the target-pointing performance of subjects is initially inaccurate, but with training, they learn to point accurately. A comparison of subjects' target-pointing performance with normal vision before and after the prism exposure period provides a measure of aftereffects. After prism exposure, subjects usually point inaccurately in the direction opposite of the prism displacement, that is, a negative aftereffect.
Performance was measured as the horizontal displacement from the target in inches (rounded to the nearest quarter inch). For throws made while wearing, or after wearing the leftward displacing goggles, the sign on all error was reversed so that the data could be averaged with that for the rightward displacing goggles. Positive values on this measure indicate error in the direction of the displacement; negative values indicate error in the direction opposite the displacement.
Exposure Performance
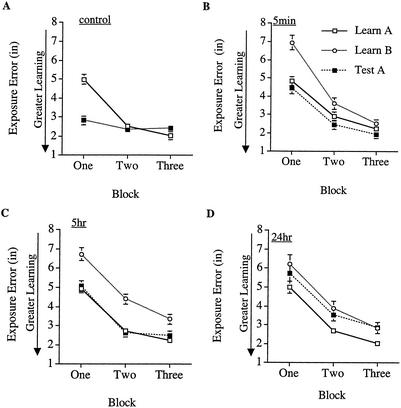
Exposure performance was assessed on trials in which the subjects wore the prism goggles and was summarized by averaging the error across the trials within a block separately for each of the target locations. This was done for each of the three sessions (learning of A, learning of B, and testing of A). Exposure error across sessions appears in Figure 3.
Figure 3.
Experiment 2 exposure performance across blocks during the training and testing sessions for each condition. (A) Control; (B) 5-min; (C) 5-h; (D) 24-h. (□) Training on Sequence A; (○) training on Sequence B; (▪) testing on Sequence A. Error bars, +/− 1 SE.
Learning of A
All groups of subjects learned the initial prism displacement equally well. Additionally, subjects were equally good at learning to throw with both right and left displacing prisms. In a repeated measures, ANOVA with trial block (one, two, or three) and target location (left, center, or right) as within-subjects variables and time learned displacement B (control, 5 min, 5 h, or 24 h) and initial prism displacement (leftward or rightward) as between subjects variables, there were no reliable effects of time-learned displacement B or of initial prism displacement, Fs<1. None of the effects involving target location were significant, Fs<1.5, P>0.25, which indicates that no target location was more difficult to learn than another. The only within-subjects variable to reach significance was that of trial block, F(2, 192) = 656.072, P=0.0001, MSE=3.062. As would be expected, subjects' error decreased across blocks of trials during their initial training on displacement A. Because error performance did not reliably vary with target location or initial prism displacement, these variables were excluded from further analyses.
Accuracy in the last block of trials while learning displacement A (see Table 1) reliably predicted PI scores (see Table 2 for regression statistics). Subjects with greater learning of displacement A had greater PI. Accuracy in the learning of A did not predict RI scores (see Table 2). Therefore, accuracy in the last block of trials of learning displacement A was used as a covariate in the PI analyses only. As in Experiment 1, ANCOVA results for the PI analyses did not qualitatively differ from those results obtained when an ANOVA was used on the PI scores.
Proactive Interference
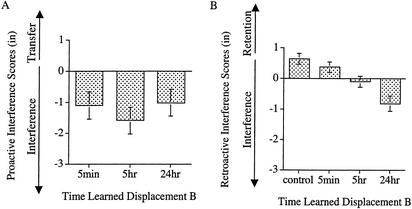
PI scores were created in the same manner as in Experiment 1. Covariate-adjusted means for the PI scores appear in Figure 4A. As is apparent in the figure, overall, there was PI in the learning of displacement B. Overall, the PI scores were reliably less than zero (M = −1.2, SE = 0.26), t(71) = −4.644, P<0.0001. However, the extent of PI did not depend on when subjects learned displacement B. In a repeated-measures ANCOVA with trial block (one, two, or three) as a within-subjects variable and time-learned displacement B (5 min, 5 h, or 24 h) as a between-subjects variable, there were no reliable effects, Fs<1.5, Ps>0.25. The planned contrast comparing the 5-min group with the 5- and 24-h group failed to reach significance, as did the linear contrast, Fs<1. All groups showed a similar impairment in their learning of displacement B regardless of the amount of time that had passed between the learning of the two displacements.
Figure 4.
(A) Covariate-adjusted means for the PI scores on the measure of exposure performance. PI scores were obtained by subtracting subjects' Learning of B score from their Learning of A score. (B) Raw means for the RI scores on the measure of exposure performance. Error bars, +/− 1 SE. RI scores were obtained by subtracting subjects' Test of A score from their Learning of A score. Negative values on both the PI and RI measures indicate interference; positive values indicate either positive transfer or retention.
Assessing Consolidation: Retroactive Interference Measure
Once again, the RI measure is of critical importance to the question of whether there was consolidation in the exposure performance of prism adaptation. RI scores were created in the same manner as in Experiment 1. Means for the RI scores appear Figure 4B. As is apparent in the figure, overall, there was RI; the groups that learned displacement B were impaired in their memory for displacement A relative to the control group. An ANOVA on the RI scores with trial block (one, two, or three) as a within-subjects variable and time-learned displacement B (control, 5 min, 5 h, or 24 h) as a between-subjects variable yielded a reliable effect of time learned displacement B, F(3, 100) = 6.799, P=0.0003, MSE=4.725.The planned comparison between the control group and the groups that learned a second displacement revealed that the control group had greater retention of displacement A, F(1,100) = 9.533, P=0.0026.
In the above ANOVA, there was a reliable effect of trial block, F(2, 200) = 7.721, P=0.0006, MSE=1.719, as well as a reliable interaction between time-learned displacement B and trial block, F(6, 200) = 7.548, P=0.0001. Helmhert comparisons revealed that the interaction was due to greater facilitation in the memory of displacement A for the control group in trial block one (M=2.159, SE=0.296 for block one, and M = −0.104, SE=0.196 for blocks two and three), F(2, 100) = 15.51, P=0.0001, whereas, for the remaining groups, there was no difference between trial block 1 and trial blocks 2 and 3, Fs<1.
As is apparent in Figure 4B, there was a temporal gradient to the RI scores. This gradient, however, was in the direction opposite from that which would be expected due to consolidation. The planned linear contrast revealed that impairment in the memory for displacement A increased as the amount of time between the learning of displacements A and B increased, F(1, 69) = 9.782, P=0.0026, MSE=5.175. There is, therefore, no evidence of consolidation in subjects' exposure performance during prism adaptation.
Aftereffects Performance
Exposure performance is thought to reflect the combination of an implicit adaptation between the perceptual and motor systems and the use of explicit strategies that allow for accurate pointing performance. For example, subjects may learn that when wearing the prism goggles, which displace their vision 12° to the right, that accurate performance can be obtained by consciously selecting a spatial target for pointing that is 12° to the left of where the target appears visually. Aftereffects performance, however, is thought to reflect only the implicit adaptation between the perception and action systems. As the subjects are not wearing the prism-displacing goggles during tests of aftereffects, it would not be reasonable for them to consciously draw on a strategy to correct the effects of a displacement. As such, the results central to the question of whether there is consolidation in the implicit learning of a new visuo-motor mapping are those for the aftereffects performance.
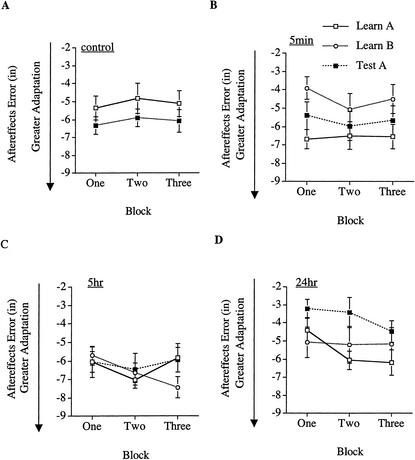
Aftereffects were assessed in trials during which subjects wore nondisplacing goggles. Only the first aftereffects trial in each block was used, as subjects may begin applying strategies to combat the aftereffects on subsequent throws within that block. To rid the aftereffects scores of any directional bias, albeit small (overall M=0.14, SE=0.14), that the subjects may have started out with prior to prism training, the average of each subject's baseline performance was subtracted from his or her error on each aftereffects trial. Positive aftereffects scores reflect error in the direction of the prism displacement; negative scores reflect error in the direction opposite the prism displacement. Once a subject takes the prism goggles off, error in the opposite direction of the displacement reflects the extent of the implicit adaptation between the perception and action systems. Therefore, aftereffects performance differs from exposure performance in that greater negative values are indicative of greater learning. Subjects' aftereffects performance across blocks for each of the training session is depicted in Figure 5.
Figure 5.
Experiment 2 aftereffects performance across blocks during the training and testing sessions for each condition: (A) Control; (B) 5-min; (C) 5-hr; (D) 24-hr. (□) Training on Sequence A; (○) training on Sequence B; (▪) testing on Sequence A. Negative values are indicative of a greater adaptation between the perception and action systems. Error bars, +/− 1 SE.
Learning of A
All groups of subjects acquired similar aftereffects during the learning of displacement A (see Table 1) and these aftereffects were reliably less than zero, t(103) = −16.373, P<0.0001, indicating an adaptation between the perceptual and motor systems. In a repeated-measures ANOVA with time-learned displacement B (control, 5 min, 5 h, 24 h) as a between-subjects variable and trial block (one, two, or three) as a within-subjects variable, there were no reliable effects, Fs<1.5, Ps>1.8. The lack of an effect of trial block suggests that the aftereffects were acquired fairly quickly—within the first block of trials.
The aftereffects for the last block of training on displacement A reliably predicted both PI and RI (see Table 2 for regression statistics). Those subjects who had greater adaptation during the learning of displacement A also experienced greater PI, as well as greater RI. Therefore, the aftereffects score on the last block during training on displacement A was used as a covariate in the subsequent analyses. These ANCOVA results for the PI and RI analyses did not differ qualitatively from those results obtained when an ANOVA was used.
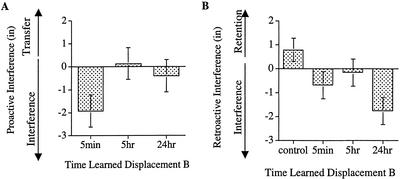
Proactive Interference
PI scores were created in the same manner as in Experiment 1 and appear in Figure 6A. As is apparent in the figure, there was PI when learning displacement B 5 min after having learned displacement A, but this PI disappeared once at least 5 h passed between the learning of the two displacements. In a repeated-measures ANCOVA with condition (5 min, 5 h, 24 h) as a between-subjects variable and trial block (one, two, three) as a within-subjects variable there was only a marginal effect of condition, F(2, 68) = 2.345, P= 0.1035, MSE=34.98. Whereas the linear contrast among those groups learning displacement B was not reliable, F(1, 68) = 2.3.85, P=0.1271, the planned comparison contrasting the 5-min group with the 5- and 24-h groups was reliable, F(1, 68) = 4.396, P=0.0397.
Figure 6.
(A) Covariate-adjusted means for the PI scores on the measure of aftereffects performance. PI scores were obtained by subtracting subjects' Learning of B score from their Learning of A score. (B) Means for the RI scores of aftereffects performance. Error bars, +/− 1 SE. RI scores were obtained by subtracting subjects' Test of A score from their Learning of A score. Negative values on both the PI and RI measures indicate interference; positive values indicate either positive transfer or retention.
In the above analysis, there was also a reliable effect of trial block, F(2, 136) = 8.034, P=0.0005, MSE=10.888. Post hoc analyses on the effect of trial block revealed that across all conditions, the PI scores were greater in blocks one and two (M = −0.812, SE=0.547, M = −0.888, SE=0.566, respectively) than those in block three (M = −0.479, SE=0.573). The interaction between condition and trial block did not reach significance, F<1. As one might expect, the extent of PI decreased with extensive practice on the second displacement; this decrease was present in all groups that learned displacement B.
Assessing Consolidation: Retroactive Interference
RI scores were created in the same manner as in Experiment 1 and appear in Figure 6B. Overall, those groups learning displacement B experienced RI in their memory of the aftereffects for displacement A. In a repeated-measures ANCOVA with time-learned displacement B (control, 5 min, 5 h, 24 h) as a between-subjects variable and trial block as a within-subjects variable (one, two, three), there was a significant effect of time-learned displacement B, F(3, 99) = 3.803, P=0.0126, MSE=22.775. A planned comparison revealed that the control group retained the aftereffects for displacement A, whereas those groups that learned a second displacement experienced an impairment, F(1, 99) = 7.308, P=0.0081. Although the RI scores in Figure 6B appear to be temporally graded in the direction opposite that predicted by a consolidation account, this effect was only marginally significant. In Fisher's PLSD post hoc analyses, the RI scores of the 5-min and 5-h groups did not differ from each other, F<1, but the 24-h group had marginally greater RI than did the 5-min and 5-h groups, F(1, 99) = 3.737, P=0.0561.
In the above ANCOVA, there was also an effect of trial block, F(2, 198)=6.714, P=0.0015, MSE=11.548. Post-hoc analyses on the effect of trial block revealed that the RI was greater in block three (M = −0.528, SE = 0.444) than in blocks one or two (M = −0.258, SE = 0.409, M = −0.272, SE = 0.404, respectively), F(1, 198) = 11.066, P = 0.001, MSE = 11.479. This result suggests that the interference produced by learning displacement B did not dissipate with training as one might expect. The interaction between trial block and time-learned displacement B did not reach significance, F <1.
Summary
When subjects learned a new mapping between their vision and their proprioception (i.e., a kinematic transformation) they experienced RI in their exposure performance, but the gradient to this interference was in the direction opposite that predicted by a consolidation account. The RI for the aftereffects when learning two displacements was qualitatively similar to that for the exposure performance. Whereas all groups that learned a second displacement were impaired in their memory for the aftereffects of the first displacement, there was a trend for the RI in the aftereffects to be temporally graded in the direction opposite that predicted by a consolidation account (although this trend was not reliable). Nevertheless, the pattern of RI in the aftereffects performance is inconsistent with the notion that memory for the implicit perceptual-motor adaptation consolidates.
GENERAL DISCUSSION
The current set of experiments tested the ubiquity of a long-term consolidation process for different components of motor-skill learning. A consolidation account predicts temporally graded RI in the learning of two skills, such that RI would decrease as the amount of time that passed between the learning of the two skills increased. Results of the current experiments are inconsistent with the notion that the learning of nondynamic motor information undergoes a long-term consolidation process.
Experiment 1 examined the existence of a long-term consolidation process in the learning of a sequence of movements. In this experiment, the learning of one movement sequence proactively interfered with the learning of a second movement sequence, and this PI was not temporally graded. Most notably, in Experiment 1, learning a second movement sequence caused an impairment in the memory for the first movement sequence (i.e., RI). This interference, however, was not temporally graded as would be predicted by a consolidation account.
Experiment 2 examined the existence of a long-term consolidation process in the learning of new mappings between the perception and action systems. In this experiment, subjects experienced PI in both their exposure performance (which is a combination of conscious strategies and of the implicit adaptation between the perceptual and motor systems) and in their aftereffects performance (which is thought to be a pure measure of the implicit adaptation between the perceptual and motor systems). The extent of this PI was temporally graded in the aftereffects performance, but not in the exposure performance. When attempting to learn a new displacement only 5 min after having trained on an opposing displacement, the adaptation between the perceptual and motor systems was impaired. However, whether 5 or 24 h passed between the attempts to learn the two displacements, the adaptation when learning the second displacement was not impaired. These results are congruent with results from an attempt to train monkeys to reach under two opposing visual displacements (via prism spectacles). When training sessions were separated by only 5 min, the monkeys were impaired at learning the second displacement. However, when the training sessions were separated by 24 h, the monkeys were not impaired at adapting to the second displacement (Flook and McGonigle 1977).
Consistent with other demonstrations of RI in the learning of kinematic transformations (Krakauer et al. 1999; Tong et al. 2002), in Experiment 2, learning of one prism displacement hurt the memory for a previously learned displacement (i.e., there was RI) in both the exposure and the aftereffects performance. In the exposure performance, this RI was temporally graded, but in the direction opposite that predicted by a consolidation account. As the amount of time between the learning of the two prism displacements increased, the amount of RI also increased. Subjects were influenced by what they had done most recently.
This pattern of RI, although inconsistent with a consolidation account, is consistent with a number of accounts of RI in paired-associate (i.e., explicit) memory. In one account, the retrieval-induced forgetting account, retrieving any particular target item involves a temporary, reversible, and active suppression of competing memories (Anderson et al. 1994). Having recently retrieved a competing response (e.g., displacement B) from memory hurts the retention of a previously learned response (e.g., displacement A) more so than if a longer period of time has passed between retrieving the competing response and the retention test for the original response (MacLeod and Macrae 2001). A second alternative is the unlearning account (Melton and Irwin 1940), which posits that learning a second response to the same cue (learning displacement B while wearing goggles) will weaken the association between the first cue and response (that between displacement A and the goggles). Unlearning is analogous to the extinction of conditioned responses in conditioning paradigms, in that it predicts spontaneous recovery of the original association when memory is tested at longer delays after the interpolated learning. A third alternative is that the more recently the competing memory is learned, the stronger it is and, therefore, the more likely it is to interfere with what has been learned previously.
Experiment 2 was not designed to distinguish between these explanations. It is therefore possible that any one may account for our results. Note that all of these explanations rely on the notion that the same cue becomes associated with two different responses that directly compete with one another. We would only expect to see this pattern of reversed temporally graded RI in a task in which the responses do directly compete with one another. This is exactly the condition of the experimental situation in the prism adaptation task of Experiment 2, but not the SRT task of Experiment 1. We do not see this reverse pattern of temporally graded RI in Experiment 1. Furthermore, a similar reversed pattern of temporally graded RI has been observed in the force-field learning task, in which responses do directly compete with one another (Shadmehr and Holcomb 1999). In this study, recent learning of a new force interfered retroactively with the memory for a previously learned force, even though 24 h had passed since the old force was learned (i.e., it should have been consolidated). It appears that even if a memory has consolidated, recently retrieving a different response for the same cue can interfere with the retrieval of this consolidated memory.
One might expect the RI scores of the exposure performance in our Experiment 2 to be temporally graded in the direction predicted by a consolidation account, because it is known that explicit knowledge consolidates. In this instance, however, any explicit knowledge subjects acquired for improving performance on one displacement could potentially be used for improving performance on the second displacement. For example, a subject could decide to throw to a visual target in the opposite direction of his or her error. This type of strategy would not generate RI.
The results of the current set of experiments appear to be inconsistent with demonstrations of RI that are thought to reflect consolidation (e.g., Krakauer et al. 1999; Tong et al. 2002). These studies have shown that learning a second kinematic transformation immediately after having learned a first transformation interferes with the memory for that first transformation. We argue, however, that these studies are not convincing demonstrations of a long-term consolidation process, as they have not tested whether the RI dissipates as predicted by a consolidation account. We demonstrated RI in the learning of two skills in the current set of experiments while failing to find evidence for a long-term consolidation process.
Why have there been sufficient demonstrations of long-term consolidation in the learning of dynamic information (e.g., Brashers-Krug et al. 1996) but not in the learning of a movement sequence or of a kinematic transformation? One possible reason for the discrepancy between our results and those with dynamic tasks is differences in long-term memory mechanisms due to the differences in the underlying anatomical bases of the tasks. Consolidation of motor skill has been shown for dynamic transformations that may rely largely on M1, but the evidence of a long-term consolidation process for other motor areas such as the basal ganglia, SMA, and posterior parietal cortex is less consistent. Cho and Kesner (1996) have found that parietal cortex lesions produce a retrograde amnesia that is not temporally graded, inconsistent with the notion that the parietal cortex is involved in the consolidation of a spatial discrimination problem in rats. In the striatum, there is plenty of evidence for short-term consolidation processes, but less consistent evidence for long-term consolidation processes. Various treatments have been found to mediate a striatal-dependent memory when injected into the striatum of rats immediately following training (e.g., Packard et al. 1994; Salado-Castillo et al. 1996; Packard and Teather 1998), indicating the presence of a short-term consolidation process. There is, however, conflicting evidence as to whether a long-term consolidation process that continues beyond 1.5 h exists in the striatum. For example, scopolamine injected into the anterior striatum produced deficits in the retention of a passive avoidance task at 2- and 8-min post-training delays, but not at a 15-min delay (Diaz del Guante et al. 1991), consistent with a short-term consolidation process. Furthermore, immediate inactivation of the NMDA receptors of the ventral striatum post-training produced retention deficits, but inactivation 120 min post-training did not (Roullet et al. 2001), again consistent with a short-term consolidation process. Whereas another study found that post-training injections of tetrodotoxin in the whole striatum produced deficits in a passive avoidance task at 15 min and 1.5 h, but not at 6 h (Lorenzini et al. 1995), consistent with a longer-term consolidation process.
Additionally, there is evidence that long-term memory mechanisms in the basal ganglia may rely on more immediate changes in the tuning of neural connectivity that occur with practice rather than a slower long-term consolidation process that occurs in the absence of practice. For example, Brainard and Dupe (2000) have found that the basal ganglia are important in the on-line modification, and continual tuning of birdsong with practice and feedback and are not responsible for the maintenance of birdsong in the absence of practice with feedback.
Whether or not the striatum is involved in consolidation also appears to depend on how well the task is learned or how strong the learning experience is. Functional inactivation studies of the striatum have found that positively rewarded tasks that are well mastered fail to show temporally graded retrograde amnesia (Tikhonravov et al. 1997), as do passive avoidance tasks that use a very strong footshock (Perez-Ruiz and Prado-Alcala 1989). These findings imply that overlearned striatal-dependent tasks may not rely on the striatum for consolidation. It may be that subjects in our sequence-learning experiment had overlearned the SRT task, and therefore, we failed to see a pattern of temporally graded retroactive interference consistent with a long-term consolidation account.
The goal of the current set of experiments was to assess whether a long-term consolidation process is ubiquitous for different components of motor skill learning – in particular, components of motor skill learning not involving dynamic information. Although it is possible that a short-term consolidation process (i.e., <5 min) may have been at work in the current tasks, in both the learning of a movement sequence and in the learning of a new perceptual-motor adaptation, we found that remote memories were not less susceptible to interference from new learning as predicted by a consolidation account. These results are inconsistent with the notion that a process of long-term consolidation is ubiquitous for different components of motor skill learning.
MATERIALS AND METHODS
Experiment 1
Subjects
A total of 122 University of Virginia students (43 male, mean age = 20.5 years) participated in the study either as partial fulfillment of a course requirement or for payment of $15–$20. A total of 25 students participated in the control condition, 26 in the 5-min group, 25 in the 1-h group, 22 in the 5-h group, and 24 in the 24-h group.
Design
All subjects trained on sequence A and were tested on their memory for that sequence 48 h later. The time at which training on the incompatible sequence (sequence B) took place was varied. Subjects trained on sequence B either 5 min, 1 h, 5 h, or 24 h after training on sequence A. A fifth group, the control group, did not train on sequence B.
Stimuli and Apparatus
Stimuli appeared on a video monitor and subjects made responses on a computer keyboard, both controlled by a Macintosh G3 computer. Four boxes (2-cm square, center-to-center distance 5 cm), arranged horizontally on the screen, appeared continuously throughout the SRT task. On each trial, a black filled circle (.3 cm diameter) appeared in the center of one of the boxes.
Procedure
SRT Task: Subjects rested the index and middle fingers of each hand on the z, c, b, and m keys of the computer keyboard. Subjects responded to the circle by pressing the key that corresponded to the circle's location. The keys corresponded to the circle locations such that z, the leftmost key, corresponded to the leftmost box and likewise for the remaining locations. Once the subject made the correct response, the circle disappeared and after 250 msec, appeared in a different location. Upon an incorrect response, a tone sounded for 120 msec, and the circle remained on screen until the subject made the correct response.
In the first experimental session, subjects trained on sequence A in the SRT task. For each subject, sequence A was selected randomly from a corpus of 563 12-unit sequences that met the following criteria: a stimulus could not repeat itself (e.g., 1332); each stimulus appeared an equal number of times in the sequence; the sequence could not contain runs of four units (e.g., 1234) or trills of four units (e.g., 2424). For each subject, sequence B was selected randomly from the corpus of sequences with the constraint that it did not share any triplets (i.e., did not share three stimulus positions in a row) with that subject's sequence A. Sequenced blocks were created by appending the stimulus sequence to itself 8 times (for a total of 96 trials). Random blocks were created by pseudorandomly selecting eight different sequences from the corpus and appending them, with the stipulation that the entire random block met the same criteria detailed above.
Sequence Training: Training on sequence A and sequence B proceeded identically. To minimize the acquisition of explicit sequence knowledge during training, subjects performed a concurrent task. Prior to each block of SRT trials, a series of seven letters appeared on the screen for 5 sec. Subjects were instructed to commit the letters to memory and retain them throughout the block of SRT trials. Subjects were asked to report back the letter string at the end of the block. Subjects performed six blocks of SRT with the concurrent task. The first of these was a random block, followed by four sequenced blocks and another random block. Immediately following, the subjects performed three blocks of SRT without the concurrent task, one random, one sequenced, one random. It has been shown that a concurrent memory task may disrupt performance in SRT, but has only a small effect, if any, on learning (Frensch et al. 1999). The measure of learning was taken during these last three blocks when the subjects were not performing the concurrent task. Subjects were not informed of the repeating sequence in the SRT task, but were told to respond on each trial as quickly and as accurately as possible. Subjects were told that the memory task and the SRT task were equally important.
A 48-h test of sequence A: Forty-eight hours following training on sequence A, subjects were tested on their retention of that sequence. Subjects performed three blocks of SRT without a concurrent task: random, sequenced, random.
Measures of explicit knowledge: To assess whether subjects became explicitly aware of the sequence, after the 48-h test of sequence A, we asked subjects to rate their confidence in having seen a sequence during each of the three sessions. All subjects were told that they may or may not have been in a group that saw a repeating sequence of stimuli during initial training. Subjects rated their confidence regarding which condition they were in on a scale from 1 (confident in random group) to 7 (confident in sequenced group). Subjects first made a confidence rating for the session they had just completed and then made similar confidence ratings for their second and first training sessions. Subjects rated their confidence on the basis of how they felt while performing the SRT task in the relevant session.
A free recall task was administered to assess explicit knowledge of the sequence. Subjects were told that during the last training session the stimuli had sometimes appeared in a 12-unit repeating sequence and were asked to recall as much of the sequence as possible. On a sheet of paper containing 12 rows and 4 columns, with each row corresponding to a unit of the sequence and each column corresponding to a screen location, subjects indicated where a stimulus had appeared by putting an X in the corresponding column. Subjects then made similar recall judgments for their second and initial training session.
Experiment 2
Subjects
A total of 104 University of Virginia students (42 male, mean age = 18.8 years) participated in the experiment either in partial fulfillment of a course requirement or for a payment of $15–$20. A total of 32 subjects participated in the control condition, and 24 subjects participated in each of the other conditions. All subjects were right-handed and had normal or corrected-to-normal vision.
Design
The design of the current experiment was identical to that of Experiment 1, with the exception that there were only four levels of the between-subjects variable, the time at which the incompatible prism displacement, displacement B, was learned. The 1-h group was excluded in the current study.
Stimuli and Apparatus
Subjects threw balls of clay (1-in diameter) at three targets. Targets were three black strips of tape (1.25 in x 7 in), placed vertically on a wall 8-in apart and 72 in from where the subject stood. The center target corresponded to the subject's body midline. Subjects' vision was displaced 12.4° to the left or to the right with prism goggles. The prism goggles were created by adhering 3M Press-On Optics fresnel prism lenses of 20 diopters to clear plastic safety goggles.
Procedure
Learning of Displacement A: Subjects were tested individually. They made underhanded throws with their right hand. Each subject made 15 practice throws to the center target without wearing goggles. During these practice throws, no measurements were taken. For all subsequent throws, the experimenter handed the subject a clay ball and called out the target they should throw to on that trial. The clay balls were of various colors and when thrown against the wall, they left a small, colored grease mark. After each throw, the experimenter measured and recorded the horizontal distance (in inches) between the center of this grease mark and the closest edge of the target. After every five throws, the experimenter erased the grease marks.
After practice, subjects made three throws to the center target while wearing clear goggles (no prism). These first three measured throws were used as a baseline to assess aftereffects. Subjects then put on the prism goggles and performed three blocks of exposure trials. One 12-trial exposure block consisted of 4 sets of trials. Each set consisted of one throw to each of the three targets. Within each set, the order of the targets was pseudorandomly determined. After each block of exposure trials, subjects took off the prism goggles and made three throws to the center target while wearing clear, nondisplacing goggles, allowing for assessment of aftereffects across the learning period.
Learning of Displacement B and Testing on A
Training on the second displacement (displacement B) and the 48-h test of displacement A proceeded identically to training on displacement A, with the exception that subjects did not repeat the initial practice throws. During training on displacement B, those subjects who had trained with the rightward displacing goggles during initial training now trained with the leftward displacing goggles and vice versa. At the 48-h test, all subjects trained with the same set of goggles they initially trained with.
Acknowledgments
We thank Kelly M. Goedert, Department of Psychology, Pacific Lutheran University, and Daniel B. Willingham, Department of Psychology, University of Virginia. This research was supported by a NSF grant to D.B.W. A portion of these results were presented at the Annual Meeting of the Society for Neurosciences (New Orleans, 2000). We thank Barbara Spellman, Dennis Proffitt, and Linda Bunker for helpful comments throughout the completion of this project, and Emily Hartle, Katy Jett, Brandon Jordan, Erin Kelleher, and Laura McMullen for help with data collection.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL goedert@plu.edu; FAX (253) 535-8305.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.50102.
Use of the covariate was an attempt to eliminate variability in interference scores attributable to differences in original learning. For cases in which the covariate was used only the covariate-adjusted means are reported. Covariate-adjusted group means were obtained in the following manner: Adj YA = YA – bS/A(XA – XT) where Adj YA = covariate-adjusted treatment mean for level AYA = unadjusted treatment mean for level AbS/A = average within-groups regression coefficientXA = group mean on the covariate for level AXT = grand mean on the covariate (mean of group means)
REFERENCES
- Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: Retrieval dynamics in long-term memory. J Exper Psychol: Learn, Mem, and Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Mussa-Ivaldi A. Toward a neurobiology of coordinate transformations. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: The MIT Press; 1999. pp. 489–500. [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;13:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Brooks DN, Baddeley AD. What can amnesic patients learn? Neuropsychologia. 1976;14:111–122. doi: 10.1016/0028-3932(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Cho YH, Kesner KP. Involvement of the entorhinal cortex or parietal cortex in long-term spatial discrimination memory in rats: Retrograde amnesia. Behav Neurosci. 1996;110:436–442. doi: 10.1037//0735-7044.110.3.436. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander G. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature. 1996;383:618–621. doi: 10.1038/383618a0. [DOI] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–265. [Google Scholar]
- ————— Lasting consequences of bilateral medial temporal lobectomy: Clinical course and experimental findings in H.M. Sem Neurol. 1984;4:252–262. [Google Scholar]
- Diaz del Guante MA, Cruz-Morales SE, Prado-Alcalá RA. Time-dependent effects of cholinergic blockade of the striatum on memory. Neurosci Lett. 1991;122:79–82. doi: 10.1016/0304-3940(91)90198-3. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Molecular bases of long-term memories: A question of persistence. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- Flook JP, McGonigle BO. Serial adaptation to conflicting prismatic rearrangement effects in monkey and man. Perception. 1977;6:15–29. doi: 10.1068/p060015. [DOI] [PubMed] [Google Scholar]
- Frensch PA, Wenke D, Runger D. A secondary tone-counting task suppresses expression of knowledge in the serial reaction task. J Exper Psychol: Learn, Mem, Cogn. 1999;25:260–274. [Google Scholar]
- Ghilardi M-F, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Grafton SR, Woods RP, Tyska M. Functional imaging of procedural motor learning relating cerebral blood flow with individual subject performance. Hum Brain Mapp. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121:1437–1449. doi: 10.1093/brain/121.8.1437. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi M-F, Ghex C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci. 1999;2:1026–1031. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Squire LR, Byrne JH. 100 years of consolidation–remembering Müller and Pilzecker. Learn Mem. 1999;6:77–87. [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Tassoni G. Time-dependent deficits of rat's memory consolidation induced by tetrodotoxin injections into the caudate-putamen, nucleus accumbens, and globus pallidus. Neurobiol Learn Mem. 1995;63:87–93. doi: 10.1006/nlme.1995.1008. [DOI] [PubMed] [Google Scholar]
- MacLeod MD, Macrae CN. Gone but not forgotten: The transient nature of retrieval-induced forgetting. Psychol Sci. 2001;121:148–152. doi: 10.1111/1467-9280.00325. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory – A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Melton AW, Irwin JM. The influence of the degree of interpolated learning on retroactive inhibition and the overt transfer of specific responses. Amer J Psychol. 1940;3:173–203. [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallet M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Amygdala modulation of multiple memory systems: Hippocampus and caudate-putamen. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Ruiz C, Prado-Alcalá RA. Retrograde amnesia induced by lidocaine injection into the striatum: Protective effect of the negative reinforcer. Brain Res Bull. 1989;22:599–603. doi: 10.1016/0361-9230(89)90076-2. [DOI] [PubMed] [Google Scholar]
- Roullet P, Sargolini F, Oliverio A, Mele A. NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a nonassociative task in mice. J Neurosci. 2001;21:2143–2149. doi: 10.1523/JNEUROSCI.21-06-02143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salado-Castillo R, Diaz del Guante MA, Alvarado R, Quirarte GL, Prado-Alcalá RA. Effects of regional GABAergic blockade of the striatum on memory consolidation. Neurobiol Learn Mem. 1996;66:102–108. doi: 10.1006/nlme.1996.0051. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit memory: History and current status. J Exper Psychol: Learn, Mem, and Cogn. 1987;13:501–518. [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb H. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- ————— Inhibitory control of competing motor memories. Exper Brain Res. 1999;126:235–251. doi: 10.1007/s002210050733. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ, Zouzounis JA. Preserved memory in retrograde amnesia: Sparing of a recently acquired skill. Neuropsychologia. 1984;22:145–152. doi: 10.1016/0028-3932(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Squire LR, Haist F, Shimamura AP. The neurology of memory: Quantitative assessment of retrograde amnesia in two groups of amnesiac patients. J Neurosci. 1989;9:828–839. doi: 10.1523/JNEUROSCI.09-03-00828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonravov DL, Shapovalova KB, Dyubkacheva TA. Effects of microinjection of scopolamine into the neostriatum of rats on performance of a food conditioned reflex at different levels of fixation. Neurosci Behavi Physiol. 1997;27:312–317. doi: 10.1007/BF02462901. [DOI] [PubMed] [Google Scholar]
- Tong C, Wolpert DM, Flannagan JR. Kinematics and dynamics are not represented independently in motor working memory: Evidence from an interference study. J Neurosci. 2002;22:1108–1113. doi: 10.1523/JNEUROSCI.22-03-01108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB. A neuropsychological theory of motor skill learning. Psychol Rev. 1998;105:558–584. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- ————— Implicit motor sequence learning is not purely perceptual. Mem & Cogn. 1999;27:561–572. doi: 10.3758/bf03211549. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Goedert-Eschmann K. The relation between implicit and explicit learning: Evidence for parallel development. Psychol Sci. 1999;10:531–534. [Google Scholar]
- Willingham D B, Nissen MJ, Bullemer P. On the development of procedural knowledge. J Exper Psychol: Learn, Mem, Cogn. 1989;15:1047–1060. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Koroshetz WJ, Peterson E. Motor skills have diverse neural bases: Spare and impaired skill acquisition in Huntington's disease. Neuropsychology. 1996;10:315–321. [Google Scholar]
- Zola-Morgan S, Squire LR. The primate hippocampal formation: Evidence for a time-limited role in storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]