Summary
Nuclear transfer to eggs or oocytes provides a potential route for cell-replacement therapies [1] because oocytes directly reprogram transplanted mammalian somatic-cell nuclei such that they have an embryo-like pattern of gene expression. This includes a large increase in the mRNA level of the stem-cell marker gene oct4[2]. We have developed a novel procedure to identify new proteins that greatly increase the level of oct4 mRNA upon nuclear transfer. We have isolated Xenopus oocyte proteins that bind to the regulatory region of the mouse oct4 gene and identified these by mass spectrometry. The proteins include the retinoic-acid-receptor γ, a known repressor of oct4 transcription, and Tpt1, a cancer-associated factor [3]. The depletion of transcripts of retinoic-acid receptor γ from oocytes increases oct4 and nanog transcription as expected, and depletion of tpt1 transcripts in oocytes reduces oct4 and nanog transcription in injected HeLa nuclei. An elevation of tpt1 transcripts in oocytes results in an earlier activation of oct4 transcription. Therefore, we identify a novel role for tpt1 in activating pluripotency genes upon nuclear transfer. Our results help to elucidate the mechanism by which somatic-cell nuclei are reprogrammed to have an embryo-like pattern of gene expression.
Keywords: DNA, STEMCELL
Results and Discussion
Apart from global chromatin decondensation [4–7], the mechanisms and molecules involved in nuclear reprogramming and in establishing pluripotency are not well understood. oct4 is a gene that is normally expressed only in pluripotent, embryo-like cells. It is not expressed in somatic cells but is activated upon nuclear transfer into oocytes and in embryonic stem (ES) cells [2, 8]. When development and differentiation proceed [9–12], or when retinoic acid is present [13], oct4 transcription is downregulated. Pluripotency proteins such as Oct4 and Nanog are transcription factors with essential roles in permitting the proliferation of ES cells [14–16]. Even though several proteins, such as GCNF [17], SF1 [18], LRH1 [19], retinoic acid [18], and Sall4 [20], are already known to regulate the oct4 promoter region in cultured cells or in later development, it is still unknown what is responsible for the activation of oct4 transcription, which correlates with cloning efficiency [21, 22]. Xenopus oocytes provide abundant material and contain proteins that cause a large increase in the level of oct4 mRNA. The identification of such proteins is important for a better understanding of generating pluripotency and of the molecular events by which eggs or oocytes reprogram somatic-cell nuclei.
To identify proteins that bind to the oct4 regulatory region, we have used radioactively labeled DNA oligos corresponding to regions of the mouse oct4 promoter (Figures 1A and 1B). These oligos were incubated in oocyte extract and DNA-protein complexes analyzed on gels. Many of the oocyte-derived proteins showed sequence specificity for the oligos representing the oct4 regulatory region, as shown by the addition of increasing concentrations of unlabeled competitor DNA (Figure 1C). Competition experiments with CpG methylated versus unmethylated oligos revealed that the proteins prefer to bind to the unmethylated oligos (Figure 1D). To identify which proteins bind to the different oligos, we recovered the proteins from gels and identified them by mass spectrometry. We have identified proteins already known to have a function in nuclear reprogramming and have thereby validated our procedure. These proteins include nucleoplasmin [4, 7, 23] and the histone-binding protein N1/N2 [24]. We identified FRGY2, which is involved in disassembling somatic nucleoli in egg cytoplasm; this disassembly process accompanies nuclear reprogramming [25]. The retinoic-acid receptor γ was also identified. Retinoic acid induces retinoic-acid receptors in cultured cells, and an increase in both retinoic acid and its receptors causes differentiation of pluripotent cells [13, 18]. A retinoic-acid receptor has already been shown to bind to the oct4 regulatory region [18]. Most intriguingly, we have identified Tpt1 by using mass spectrometry with oligo Sf1 (data not shown); Tpt1 has not so far been implicated in regulating oct4 transcription.
Figure 1.
Xenopus laevis Oocyte Proteins Bind Specifically to the Mouse oct4 Promoter
(A) Diagram of the 2.3 kb regulatory region of the mouse oct4 gene (not to scale) [30]. The region contains a distal enhancer (DE), a proximal enhancer (PE), and a promoter (P). Translation is initiated at nucleotide +1. Four methyl-sensitive restriction sites are shown (M). Double-stranded DNA oligo fragments were designed to represent fragments of the oct4 regulatory region, as aligned below the diagram.
(B) oct4 promoter fragments (oligos) and their corresponding DNA sequences.
(C) Specificity of protein binding to oligos was determined by competition experiments, as shown here for the −166 oligo. Oocyte extract was added to P32-labeled oligos in the presence of an increasing amount (12.5–50 μM) of competitor. The addition of the same but unlabeled oligo resulted in a stronger decrease of the gel-shift signal than the addition of an unspecific oligo of the same length that is not represented in the oct4 upstream region. This illustrates specificity for protein-oligo complex formation, which was found in all oligos.
(D) Xenopus oocyte proteins are competed out less efficiently with methylated oligos compared to the same oligos that are unmethylated. Competition with increasing concentrations (12.5–50 μM) of unlabeled oligos (unmethylated or methylated) revealed a protein binding preference for unmethylated oligos (only shown for the −166 oligo). The following abbreviation is used: methylated (Met).
Tpt1 stands for tumor translationally controlled protein 1. It is widely expressed and conserved throughout vertebrates, and was first identified in a screen for genes involved in tumor reversion. tpt1 is the gene that shows the strongest differential expression between tumor and tumor-reversed states in human leukemia and breast-cancer cells [3]. tpt1 is strongly expressed in tumor cells and is downregulated upon tumor reversal. Moreover, when Tpt1 is inhibited in tumor cells, suppression of the malignant phenotype is observed [3]. oct4 is expressed in tumor cells [26], and we recovered Tpt1 with our oligos. Hence, we decided to focus on Tpt1.
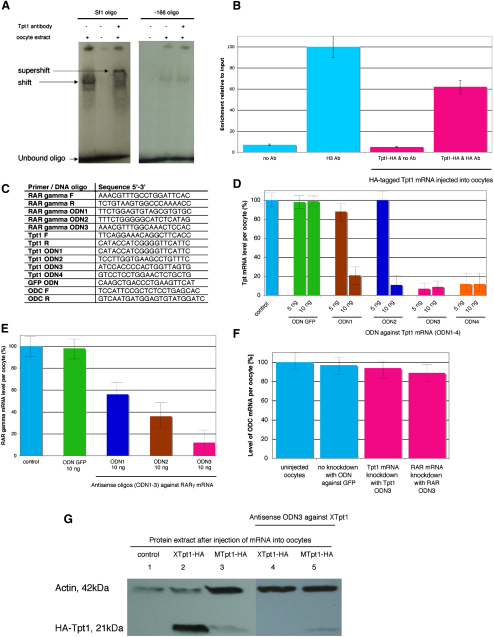
We tested whether Tpt1 binding to the Sf1 oligo could be confirmed. We repeated the gel shift, but this time in the presence of a Tpt1 antibody (Figure 2A). This resulted in a supershift, indicating that Tpt1 in fact binds to the Sf1 oligo and is not an artifact of the mass-spectrometry results. In addition, Tpt1 was also found to bind in vivo, as determined by chromatin immunoprecipitation (ChIP) analysis with Xenopus oocytes (Figure 2B).
Figure 2.
Tpt1 Binds to oct4 DNA, and Its mRNA Can Be Downregulated in Xenopus laevis Oocytes
(A) The Tpt1 protein binds to the Sf1 oligo. An antibody bound to the Tpt1-Sf1 protein-oligo complex resulted in a supershift, confirming that Tpt1 binds the Sf1 oligo. As a control, the −166 oligo, which did not bind Tpt1 according to the mass spectrometry results, was used, and the antibody did not shift the extract-oligo complex under these conditions.
(B) To confirm Tpt1 binding to the mouse oct4 promoter in vivo, we injected oocytes with HA-tagged tpt1-HA mRNA and an oct4 promoter-containing plasmid, pOct4. A ChIP analysis was performed with an HA antibody and verified Tpt1 binding to the oct4 promoter. An H3 antibody was used as a positive control, and the absence of antibodies served as a negative control. Error bars indicate the standard error of the mean (SEM). The following abbreviation is used: antibody (Ab).
(C) Short antisense oligo deoxynucleotides (ODNs) were designed to target Xenopus laevis mRNA-encoding proteins identified by mass spectrometry (only Tpt1, RAR, and GFP control shown). Primers used for monitoring mRNA downregulation are indicated, together with the primers used for detecting the Xenopus laevis housekeeping gene ornithine decarboxylase (ODC). The following abbreviations are used: forward (F), reverse (R).
(D) We injected 5 or 10 ng of different ODN oligos (Tpt1 ODN1–4) into oocytes to establish which ODNs are the most successful in downregulating tpt1 mRNA. As a negative control, an ODN was used against gfp (ODN GFP). After 24 hr, the level of tpt1 mRNA was established. Injection of 10 ng Tpt1 ODN3 resulted in the most significant decrease of tpt1 mRNA, down to 9% in comparison to the tpt1-mRNA level of uninjected oocytes (100%). Error bars indicate the SEM.
(E) We injected 10 ng of different ODNs (anti-RAR ODN1–3) and the negative ODN GFP into oocytes to establish which ODNs downregulate RAR mRNA most successfully. After 24 hr, the level of RAR mRNA was most downregulated by RAR ODN3, down to 12% in comparison to the RAR mRNA level in control oocytes (100%). Error bars indicate the SEM.
(F) ODNs against tpt1, RAR, and gfp do not significantly alter the transcriptional level of the Xenopus housekeeping gene ornithine decarboxylase, suggesting specificity for mRNA downregulation. Error bars indicate the SEM.
(G) Antisense oligo against Xenopus tpt1 mRNA results in downregulation of endogenous oocyte Tpt1 protein and mouse-tpt1 (Mtpt1)-mRNA injection in Tpt1 overexpression. Extracts from uninjected oocytes (track 1), oocytes injected with HA-tagged Xenopus tpt1 mRNA (XTpt1-HA, track 2), HA-tagged mouse tpt1 mRNA (MTpt1-HA, track 3), and antisense oligos against Xenopus tpt1 (tracks 4 and 5) were run on a protein gel in the presence of Xtpt1-HA (track 4) and Mtpt1-HA mRNA (track 5). Tpt1 was detected with an HA antibody. Mouse and Xenopus tpt1-HA-mRNA-injected oocytes resulted in an increase of Tpt1 protein (tracks 2 and 3) in comparison to the control oocytes (track 1). XTpt1-HA was successfully depleted by antisense oligos against tpt1 (track 4), but MTpt1-HA was not (track 5). This illustrates that the endogenous Tpt1 protein can be successfully downregulated with antisense oligos but that Mtpt1-HA cannot.
To determine the developmental function of Tpt1, we carried out a test that depends on the downregulation of the tpt1 mRNA. We wanted to find out whether oct4 activation is inhibited in reduced abundance of tpt1. Antisense DNA oligos (ODN, oligo deoxynucleotides) that successfully degrade endogenous tpt1 mRNA in living oocytes were designed (Figures 2C and 2D). Antisense oligos were also prepared for the identified retinoic-acid receptor γ (RAR), which serves as a control because of its known function in downregulating oct4 transcription [18] (Figures 2C and 2E). After downregulation of both tpt1 and RAR mRNAs, the levels of other oocyte mRNAs were unchanged (Figure 2F). As a control, antisense oligos against gfp did not cause downregulation of any other genes tested (Figures 2C–2F). This suggests that the antisense oligos against RAR and tpt1 act specifically. Moreover, antisense oligos against Xenopus tpt1 mRNA resulted in specific downregulation of Xenopus Tpt1 protein but not of mouse Tpt1 protein, which is not targeted by these antisense oligos (Figure 2G).
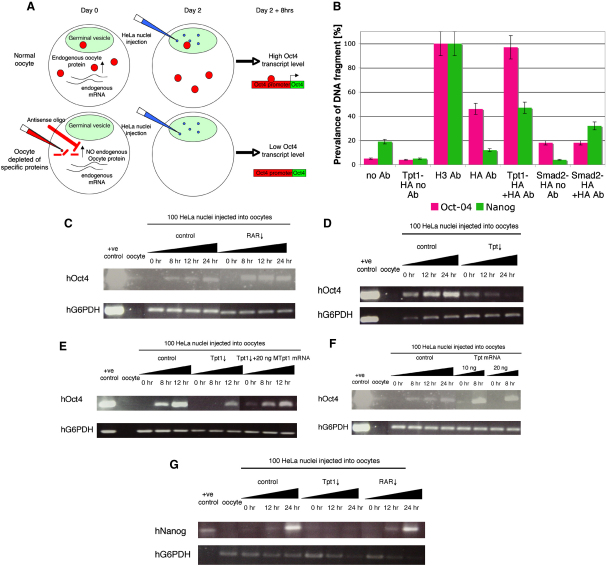
To reinforce our evidence for the importance of tpt1 for oct4 activation, we have asked whether tpt1 is required for an increase in oct4 mRNA in nuclear-transfer experiments. Forty hours after oocyte injection of antisense oligos, approximately 100 HeLa nuclei were injected into the germinal vesicles of oocytes, which were cultured for up to 24 hr. We took different time points and analyzed them by RT-PCR in order to observe the activation of oct4 (Figure 3A). Because our method is very sensitive, we found that oct4 and nanog were expressed in nonpluripotent cells, such as human HeLa cells, but at a very low background level. However, if a large number (several thousand) of nonpluripotent HeLa cells was analyzed, the signal was strong and was used as a positive control for the RT-PCR. The time course revealed a gradual increase of oct4 mRNA (Figure 3C). In control samples that were only injected with HeLa nuclei and not antisense oligos, we observed a 100-fold increase in oct4 mRNA within several hours of nuclear transfer (Yen-Hsi Kuo, personal communication). We find that oocytes depleted of RAR mRNA activate human oct4 transcription earlier and more strongly than do oocytes with a normal endogenous level of RAR (Figure 3C). This increased level of oct4 transcription due to RAR mRNA downregulation agrees with previous findings that RAR is an inhibitor of pluripotency and induces differentiation [13, 18] and thus validates our functional test. When antisense oligos against tpt1 were injected into oocytes, the opposite result was obtained. We found that a reduction in the oocyte content of tpt1 greatly reduced oct4 transcription (Figure 3D). This suggests that tpt1 is involved in oct4 transcriptional activation.
Figure 3.
A Functional Assay for Proteins Regulating the Level of oct4 mRNA in Xenopus Oocytes, Illustrating That Tpt1 is Required for oct4 Activation upon Nuclear Transfer
(A) Assay identifying proteins that regulate oct4 transcription. Antisense ODN oligos injected into living oocytes result in degradation of their target mRNAs. This is associated with a decrease in the level of the corresponding protein. If this protein is involved in regulating oct4 transcription, HeLa nuclei injected into the living oocytes should show a decrease in the oct4 expression level.
(B) Tpt1 binds to the oct4 gene in mouse ES cells, as confirmed by ChIP analysis. Mouse ES cells were transfected with a plasmid containing HA-tagged mouse tpt1 or HA-tagged smad2 (negative control). A cytomegalovirus (CMV) promoter ensured expression of the mRNA in the ES cells. Antibodies against H3 (positive control) or HA were used for determining whether Tpt1-HA binds to the mouse Oct4 or Nanog promoter regions. oct4 was enriched when Tpt1-HA was overexpressed and the HA antibody was used, in comparison to when Tpt1 was not overexpressed or when no or a different antibody was used. In contrast, the nanog signal was not significantly increased, suggesting very weak or no binding of Tpt1 to the nanog promoter region.
(C) We injected human HeLa nuclei into oocytes and aimed for their germinal vesicles. Human oct4 reactivation was monitored over time by RT-PCR. An increase of oct4 transcripts was observed with time. When the RAR was downregulated with antisense oligos and HeLa nuclei were injected into the living oocytes, the increase in oct4 mRNA level resulted in accelerated transcriptional activation in comparison to the control. The level of human oct4 mRNA was already higher at 8 hr than the mRNA level detected in control oocytes after 24 hr.
(D) tpt1 depletion inhibited the increase of oct4 transcription after nuclear transfer. tpt1 mRNA was depleted with antisense oligos. As a result, the oct4 mRNA level did not increase over time as observed in the control samples. The oct4 mRNA level was much reduced in oocytes depleted of tpt1, showing that tpt1 is required for activation of the pluripotency gene oct4.
(E) To show that tpt1 mRNA downregulation is specific, we carried out a rescue experiment. Twenty nanograms of mouse tpt1 mRNA could restore and increase human oct4 expression in HeLa nuclei transplated into oocytes that have been depleted of their endogenous (Xenopus) tpt1 mRNA.
(F) Overexpression of tpt1 mRNA resulted in accelerated activation of oct4 transcription. We injected 10 ng and 20 ng of tpt1 mRNA into oocytes before injecting HeLa nuclei. After 8 hr, a significant level of oct4 transcripts was detected, and such a level was not reached even after 24 hr in control oocytes.
(G) Tpt1 is required for nanog activation. Upon transfer of HeLa nuclei, nanog is activated, and its level increases with time. Depletion of tpt1 resulted in the lack of nanog activation, as also observed with oct4. RAR depletion did not seem to have a significant effect on nanog, in contrast to oct4. Error bars indicate the SEM.
To test the specificity of our antisense oligo against tpt1 mRNA, we have rescued Xenopus tpt1 depletion by injecting mouse tpt1 mRNA into oocytes previously injected with antisense oligos against tpt1 (Figure 3E). Importantly, mouse tpt1 mRNA does not contain the sequence that is targeted by the Xenopus antisense oligos. Hence, mouse tpt1 mRNA was not degraded and rescued the depletion of Xenopus tpt1 mRNA and, subsequently, oct4 expression (Figure 3E). This result confirms that the downregulation of tpt1 mRNA is specific.
To determine more precisely how the level of tpt1 influences oct4 transcription, we injected different concentrations of mouse tpt1 mRNA into oocyte cytoplasm. An incubation period of 16 hr allowed for the mRNA to be translated, and HeLa nuclei were then injected into the oocyte germinal vesicle. After 8 hr of incubation, the oct4 signal was already enhanced in comparison to control oocytes (Figure 3F). As a result, mouse tpt1 mRNA injected into Xenopus oocytes was effective at enhancing oct4 transcription. These observations were independent of the mRNA concentrations used (Figure 3E). Hence, in our nuclear-transfer experiments, tpt1 overexpression enhanced oct4. This suggests that Tpt1 might have an important role in regulating oct4; namely, it might ensure a level required for pluripotency and normal development.
We were interested in whether Tpt1 is a transcription factor specific for oct4 or whether it is a more generally important component that might act on other pluripotency genes. Using the same up- and down-regulation procedure just described for oct4, we have tested the transcriptional activation of the human pluripotency gene nanog by using nuclear transfer to oocytes (Figure 3G) [27, 28]. nanog seems to be an important pluripotency gene because its overexpression increases the efficiency of nuclear reprogramming by 200-fold in cell-fusion experiments [29]. Overall, we found that a reduction in the RAR did not significantly affect nanog expression. However, we had expected some effect on nanog expression because retinoic acid is known to downregulate pluripotency genes [13, 18]. One explanation for our finding is that Nanog or Nanog orthologs do not exist in amphibians (NCBI blast). As a result, some aspects of transcriptional activation upon nuclear transfer might not be conserved between mammals and amphibians. However, we saw a reduction in nanog transcription after downregulation of tpt1 in oocytes (Figure 3G). To test for binding of Tpt1 to nanog, we carried out ChiP analysis in mouse ES cells and found that Tpt1 bound to oct4, but not significantly to the immediate promoter of nanog (Figure 3B). This suggests that Tpt1 regulates oct4 by binding to its DNA directly, whereas Tpt1 might regulate nanog in an indirect way or by binding to a distant site.
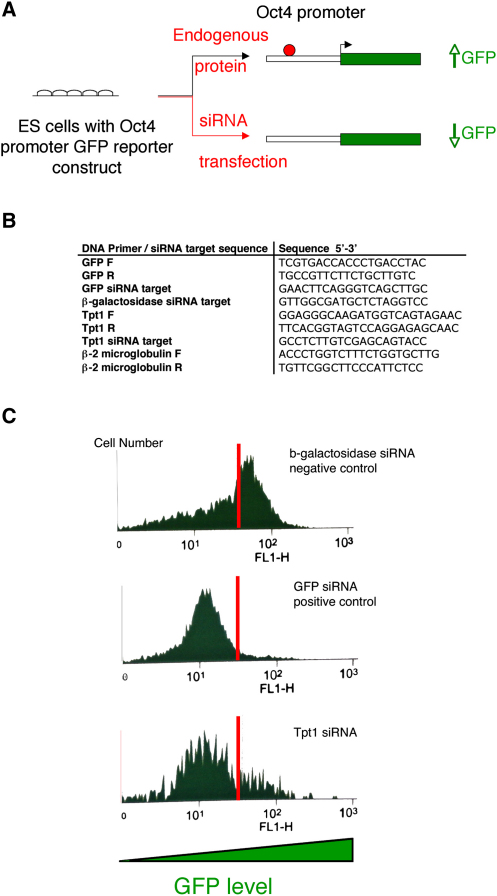
Overall, Tpt1 seems to represent a global regulator of pluripotency. We suggest that Tpt1 might have a somewhat general role in establishing an embryonic pattern of gene expression because it controls transcription of oct4 and nanog. In support of this view, we have also found that the transfection of tpt1 antisense RNA into mouse ES cells can reduce the transcription from the oct4 promoter (Figures 4A–4C), suggesting a conserved mechanism between mammals and amphibians.
Figure 4.
Knockdown of tpt1 mRNA in Mouse ES Cells Is Associated with a Decrease in the Transcriptional Activity of the oct4 Promoter
(A) Illustration of a functional assay for Tpt1 in ES cells. Mouse ES cells containing an additional integrated oct4 promoter driving an egfp reporter were transfected with siRNA against tpt1. Upregulation of egfp mRNA indicates that the targeted mRNA is required for oct4 repression. Conversely, egfp-mRNA downregulation shows that the mRNA is responsible for oct4 activation or maintenance.
(B) DNA primers and siRNA target sequences for knockdown are shown. DNA primers were designed to detect reduction of the mRNA of choice. The mouse housekeeping gene β-2-microglobulin was used as a control and reference. siRNAs were designed to target specifically gfp, β-galactosidase, and tpt1. siRNA against tpt1 mRNA resulted in decrease down to 17% of its endogenous level (not shown).
(C) FACS analysis of the siRNA experiments shows that Tpt1 siRNA causes a decrease in oct4 transcription in mouse ES cells. Mouse ES cells were transfected with siRNAs against β-galactosidase, gfp, and tpt1, and the GFP level was monitored by FACS analysis after 48 hr, as expected. siRNA targeting β-galactosidase, which is usually not present in mouse ES cells, did not reduce the GFP level (negative control). siRNA against gfp resulted in a decrease of GFP, showing that siRNA can downregulate a message and that this can be detected by FACS analysis. siRNA targeting tpt1 gave rise to a decrease in the GFP level, indicating that tpt1 is also an activator of oct4 transcription in mouse ES cells. The corresponding downregulation of the endogenous oct4 mRNA itself was also confirmed by RT-PCR (data not shown). No differentiation was seen, because the siRNA transfection was transient. The effect of the decrease was restored within 3 days, which is not long enough for ES cells to be differentiated. Finally, we found that the RNAi knockdown experiment was very variable, and different batches of ES cells produced different results.
The molecular events occurring after nuclear transfer and the initiation of pluripotency are not well understood. We describe here the first protein that has been discovered to be required for activating transcription of pluriptoency genes such as oct4 and nanog after nuclear transfer into oocytes. In the future, this type of work might provide a generally applicable experimental design, which in combination with the advantages of Xenopus oocytes, might lead to the identification of other proteins that activate pluripotency genes and thereby further elucidate mechanisms involved in nuclear reprogramming.
Experimental Procedures
Oligo Preparation for Gel Shifts
Complementary single-stranded 30 bp oligos that represent DNA fragments of the oct4 promoter [30] were annealed to double-stranded (DS) DNA. Longer DS oligo fragments were made with PCR amplification. Oligos were end labeled with γ-P32 ATP.
Gel Shifts
The phosphate binding buffer consisted of 20 mM phosphate (pH 7.4), 10% glycerol, 3 mM MgCl2, 17 mM KCl, 2.5 μM DTT, and 50 mM–2 M NaCl. We used 8 μl oocyte extract per sample and prepared it by mixing freshly obtained oocytes in presence of protease inhibitors. Centrifuging the mixture overnight eliminated debris and lipids. For the gel shift, 8 μl oocyte extract, 2 μg BSA, and 10 μl phosphate binding buffer were mixed with approximately 0.1 pmol radioactively labeled oligos and run on a native gel. To confirm binding of Tpt1 to any one oligo, we added 1 μl of Tpt1 antibody (monoclonal antibody anti-HRF/TCTP, Medical & Biological Laboratories) to the sample before was running it on a gel.
Protein Recovery and Identification
Proteins were recovered directly from the gels. The samples were washed, concentrated, and analyzed with MALDI-TOF mass spectrometry.
Acknowledgments
We thank S. Maslen and E. Stephens for help with the mass spectrometry, A. Surani for Oct4-GFP ES cells, Stina Simonsson for initial guidance, and A. Bannister for discussion and advice. This work was supported by the Wellcome Trust.
References
- 1.Gurdon J.B., Byrne J.A., Simonsson S. Nuclear reprogramming and stem cell creation. Proc. Natl. Acad. Sci. USA. 2003;100:11819–11822. doi: 10.1073/pnas.1834207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne J.A., Simonsson S., Western P.S., Gurdon J.B. Nuclei of adult mammalian somatic cells are directly reprogrammed to Oct-4 stem cell gene expression by amphibian oocytes. Curr. Biol. 2003;13:1206–1213. doi: 10.1016/s0960-9822(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 3.Tuynder M., Susini L., Prieur S., Besse S., Fiucci G., Amson R., Telerman A. Biological models and genes of tumor reversion: Cellular reprogramming though tpt1/TCTP and SIAH-1. Proc. Natl. Acad. Sci. USA. 2002;23:14976–14981. doi: 10.1073/pnas.222470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laskey R.A., Honda B.M., Mills A.D., Finch J.T. Nucleosomes are assembled by an acidic protein which bind histones and transfers them to DNA. Nature. 1978;275:416–420. doi: 10.1038/275416a0. [DOI] [PubMed] [Google Scholar]
- 5.Kikyo N., Wade P.A., Guschin D., Ge H., Wolffe A.P. Active remodelling of somatic nuclei in egg cytoplasm by the nucleosomal ATPase ISWI. Science. 2000;5488:2360–2362. doi: 10.1126/science.289.5488.2360. [DOI] [PubMed] [Google Scholar]
- 6.Hansis C., Barreto G., Maltry N., Niehrs C. Nuclear reprogramming of human somatic cells by Xenopus egg extract requires BRG1. Curr. Biol. 2004;16:1475–1480. doi: 10.1016/j.cub.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Tamada H., Van Thuan N., Reed P., Nelson D., Katoku-Kikyo N., Wudel J., Wakayama T., Kikyo N. Chromatin decondensation and nuclear reprogramming by nucleoplasmin. Mol. Cell. Biol. 2006;4:1259–1271. doi: 10.1128/MCB.26.4.1259-1271.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tada M., Takahama Y., Abe K., Nakatsuji N., Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr. Biol. 2001;11:1553–1558. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 9.Schoeler H.R., Ruppert S., Suzuki N., Chowdhury K., Gruss P. New type of POU domain in germ line-specific protein Oct4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri S.L., Peter W., Hess H., Schoeler H.R. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev. Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 11.Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schoeler H.R., Smith A.G. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 12.Fuhrmann G., Chung A.C.K., Jackson K.J., Hummelke G., Baniahmad A., Suttler J., Sylvester I., Schoeler H.R., Cooney A.J. Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Dev. Cell. 2001;3:377–387. doi: 10.1016/s1534-5807(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 13.De Luca L.M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991;5:2924–2933. [PubMed] [Google Scholar]
- 14.Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee T.I., Jenner R.G., Boyer L.A., Guenther M.G., Levine S.S., Kumar R.M., Chevalier B., Johnstone S.E., Cole M.F., Isono K. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loh Y.H., Wu Q., Chew J.L., Vega V.B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;4:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 17.Hummelke G.C., Cooney A.J. Germ cell nuclear factor is a transcriptional repressor essential for embryonic development. Front. Biosci. 2001;6:1186–1191. doi: 10.2741/hummelke. [DOI] [PubMed] [Google Scholar]
- 18.Barnea E., Bergman Y. Synergy of SF1 and RAR in activation of Oct-3/4 promoter. J. Biol. Chem. 2000;275:6608–6619. doi: 10.1074/jbc.275.9.6608. [DOI] [PubMed] [Google Scholar]
- 19.Gu P., LeMenuet D., Chung A.C., Mancini M., Wheeler D.A., Cooney A.J. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol. Cell. Biol. 2005;25:3492–3505. doi: 10.1128/MCB.25.9.3492-3505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Tam W.L., Tong G.Q., Wu Q., Chan H.Y., Soh B.S., Lou Y., Yang J., Ma Y., Chai L. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 21.Bortvin A., Eggan K., Skaletsky H., Akutsu H., Berry D.L., Yanagimachi R., Page D.C., Jaenisch R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130:1673–1680. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- 22.Boiani M., Eckardt S., Schoeler H.R., McLaughlin K. Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 2002;16:1209–1219. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betthauser J.M., Pfister-Genskow M., Xu H., Golueke P.J., Lacson J.C., Koppang R.W., Myers C., Liu B., Hoeschele I., Eilertsen K.J. Nucleoplasmin facilitates reprogramming and in vivo development of bovine nuclear transfer embryos. Mol. Reprod. Dev. 2006;73:977–986. doi: 10.1002/mrd.20493. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt J.A., Dingwal C., Maier G., Franke W.W. Molecular characterization of a karyophilic, histone-binding protein: cDNA cloning, amino acid sequence and expression of nuclear protein N1/N2 of Xenopus laevis. EMBO J. 1986;13:3547–3552. doi: 10.1002/j.1460-2075.1986.tb04681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonda K., Fowler J., Katoku-Kikyo N., Haroldson J., Wudel J., Kikyo N. Reversible disassembly of somatic nucleoli by the germ cell proteins FRGY2a and FRGY2b. Nat. Cell Biol. 2003;3:205–210. doi: 10.1038/ncb939. [DOI] [PubMed] [Google Scholar]
- 26.Gidekel S., Pizov G., Bergman Y., Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 27.Chambers I., Colby D., Roberston M., Nichols J., Lee S., Tweedie S., Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 28.Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. The homeoprotein nanog is required for maintenance of pluripotency in mouse emiplast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 29.Silva J., Chambers I., Pollard S., Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- 30.Nordhoff V., Hubner K., Bauer A., Orlova I., Malapetsa A., Schoeler H.R. Comparative analysis of human, bovine, and muringe Oct4 upstream promoter sequences. Mamm. Genome. 2001;12:309–317. doi: 10.1007/s003350010279. [DOI] [PubMed] [Google Scholar]