Abstract
Radiotherapy has been the primary therapy for managing metastatic spinal disease; however, surgery that decompresses the spinal cord circumferentially, followed by reconstruction and immediate stabilization, has also proven effective. We provide a quantitative comparison between the “new” surgery and radiotherapy, based on articles that report on ambulatory status before and after treatment, age, sex, primary neoplasm pathology, and spinal disease distribution. Ambulation was categorized as “success” or “rescue” (proportion of patients ambulatory after treatment and proportion regaining ambulatory function, respectively). Secondary outcomes were also analyzed. We calculated cumulative success and rescue rates for our ambulatory measurements and quantified heterogeneity using a mixed-effects model. We investigated the source of the heterogeneity in both a univariate and multivariate manner with a meta-regression model. Our analysis included data from 24 surgical articles (999 patients) and 4 radiation articles (543 patients), mostly uncontrolled cohort studies (Class III). Surgical patients were 1.3 times more likely to be ambulatory after treatment and twice as likely to regain ambulatory function. Overall ambulatory success rates for surgery and radiation were 85% and 64%, respectively. Primary pathology was the principal factor determining survival. We present the first known formal meta-analysis using data from nonrandomized clinical studies. Although we attempted to control for imbalances between the surgical and radiation groups, significant heterogeneity undoubtedly still exists. Nonetheless, we believe the differences in the outcomes indicate a true difference resulting from treatment. We conclude that surgery should usually be the primary treatment with radiation given as adjuvant therapy. Neurologic status, overall health, extent of disease (spinal and extraspinal), and primary pathology all impact proper treatment selection.
The spine is the most common osseous site for metastatic disease and may be involved in up to 40% of patients with cancer (Bohm and Huber, 2002; Wong et al., 1990). Metastatic spinal disease can arise from one of three locations (Fig. 1): the osseus components of the vertebral column (85%), the paravertebral region (10%–15%), and, rarely, the epidural or subarachnoid/intramedullary space (<5%), where it remains isolated (Byrne, 1992; Gerszten and Welch, 2000; Gilbert et al., 1978). Ten to twenty percent of those patients with preexisting spinal disease and 5% to 10% of all cancer patients will develop epidural spinal cord compression (Barron et al., 1959; Bilsky et al., 1999; Byrne, 1992; Gerszten and Welch, 2000; Healey and Brown, 2000; Wong et al., 1990). This results in more than 25,000 cases per year, with the number expected to grow (Gerszten and Welch, 2000; Lada et al., 1997; Schaberg and Gainor, 1985). Although the treatment of metastatic spinal disease has remained somewhat static for the last 30 years, a growing literature supports surgery having a greater role.
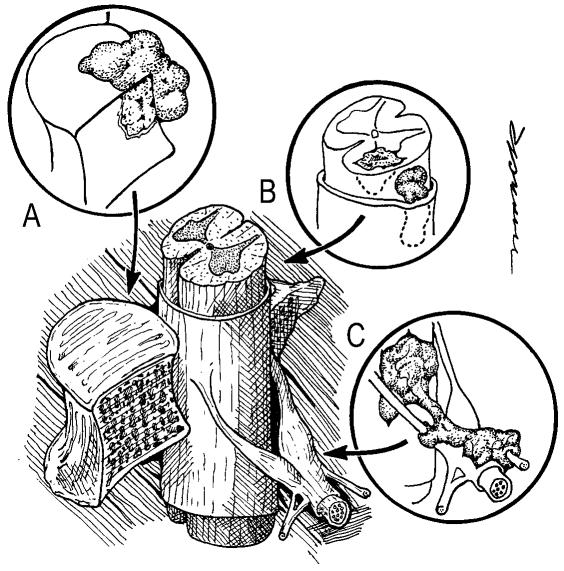
Fig. 1.
Locations of metastases to the spine. Most tumor emboli seed the vertebral column surrounding the spinal cord, with the posterior half of the vertebral body being the most common initial focus (A). Tumor can also originate in a paravertebral location and track along the spinal nerves to enter the spinal column by way of the neural foramina (C). Both of these mechanisms can lead to epidural spinal cord compression. Intramedullary, subdural/leptomeningeal, and isolated epidural metastatic deposits are rarely encountered (B).
Until the mid-1980s, posterior decompressive laminectomy was viewed as the only surgical option for these patients. A number of articles, including controlled cohort studies (Class II evidence), compared the efficacy of laminectomy alone versus radiation alone versus laminectomy followed by radiation (Black, 1979; Constans et al., 1983; Findlay, 1984; Gilbert et al., 1978; Martenson et al., 1985; Sørensen et al., 1990; Stark et al., 1982; Young et al., 1980). These studies collectively showed that decompressive laminectomy offered no additional benefit compared with conventional radiotherapy in terms of maintaining and recovering neurologic function and pain control. In addition, laminectomies were associated with significant complications, most significantly, wound infections, and new or worsening preexisting spinal instability. Indiscriminate use of decompressive laminectomy was prone to failure because, in 70% of cases, the metastatic emboli seed the vertebral body, causing ventral spinal cord compression. This makes it impossible to accomplish a meaningful decompression or tumor resection with a laminectomy without significant retraction on the thecal sac. As a result of these studies, conventional radiotherapy assumed the primary treatment modality for patients with metastatic spinal disease.
In the early to mid-1980s, surgeons began to use approaches, primarily anterior, that allowed them to directly decompress the spinal cord (Harrington, 1981, 1984; Siegal and Siegal, 1985). In his 1984 article, Findlay reviewed the existing data on the use of anterior vertebrectomy and found “dramatic results” with regard to neurologic recovery but cautioned that it is “unclear as to how often such success could be achieved” (Findlay, 1984). This marked the beginning of a “new era” in the surgical management of this disease. Applying surgical approaches commensurate with the location and extent of the disease, the goals of surgery today are to circumferentially relieve the spinal cord of compression (from tumor, bone fragments, or both), to perform maximal cytoreductive resection to prevent local recurrence, and to reconstruct and immediately stabilize the spinal column with internal stabilization devices. Approaches can broadly be classified as anterior (e.g., transthoracic, retroperitoneal) or posterior, including posterolateral trajectories (e.g., laminectomy, transpedicular, costotransversectomy, lateral extracavitary) (Fig. 2). Reflecting the use of this new philosophy, many published surgical reports seem to indicate a superior rate of preserving and restoring neurologic function compared with conventional radiotherapy articles from the same period. Nonetheless, radiation continues to be the primary treatment for the majority of patients. We refer readers to two of our recently published articles for a more detailed review of the metastatic spinal disease literature (Klimo et al., 2003; Klimo and Schmidt, 2004).
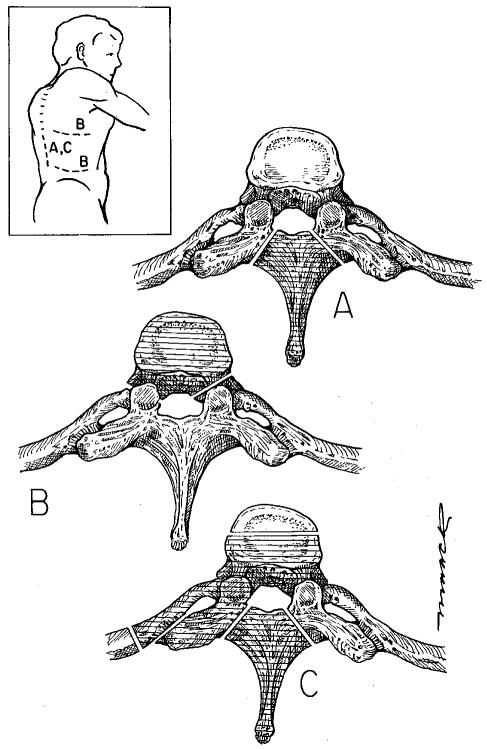
Fig. 2.
Surgical approaches to the spine. The shaded areas indicate the bone removed in each of the approaches. A. Laminectomy. The spinous process and the adjacent lamina are removed up to the junction of the pedicles. This was the standard surgical procedure for many years regardless of where the tumor was actually located within the vertebra. It can still be used for disease isolated to the posterior elements. B. Transthoracic or retroperitoneal. These anterior approaches provide direct access to the vertebral body in the thoracic (transthoracic) and thoracolumbar/lumbar regions (retroperitoneal). C. Posterolateral. For patients who cannot tolerate an anterior approach or have significant posterior extension of their disease, a posterolateral approach provides excellent access to both the anterior and posterior elements. Inset. Skin incisions for each of the approaches. The laminectomy and posterolateral approaches can be taken through a midline incision. The transthoracic (upper B line) and retroperitoneal (lower B line) approaches require flank incisions.
The goal of this meta-analysis was to critically and analytically review and compare the existing surgical and radiotherapy literature as it pertains to the treatment of metastatic epidural spinal cord compression. It is hoped that the results of this meta-analysis will allow physicians (surgeons, radiation oncologists, oncologists, primary care physicians) to make better evidence-based decisions for their patients.
Clinical Materials and Methods
Search Strategy
The goal of the search strategy was to identify articles that assessed the effectiveness of surgery in the new era and standard external beam radiation therapy in the treatment of metastatic spinal disease. The search strategy employed an electronic database search, a manual search of journals, and examination of bibliographies of relevant review papers. The electronic search on Medline (PubMed) targeted English-language articles published from 1980 to August 2003 and used the following terms in various combinations: “spine,” “metastases,” “radiation,” “surgery,” “treatment,” “cancer,” “decompression,” and “vertebrectomy.” Articles were also found by using the Related Articles function on PubMed. Articles were reviewed and data were abstracted by the primary and senior authors (PK, MHS).
Inclusion Criteria, Data Extraction, End Points, and Definitions
The focus of all papers included in this meta-analysis was a group of adults with symptomatic metastatic spinal disease who were treated either with surgery (with radiation given either preoperatively or postoperatively or not at all) or with radiation alone and who were followed in time for the development of certain end points (i.e., retrospective or prospective cohort studies). The surgical papers had to employ the goals as described previously: circumferential spinal cord decompression, reconstruction, and stabilization. Any approach could be used to achieve these goals. Although the meta-analysis would ideally have been limited to papers where surgery (with or without adjuvant radiation) was the sole treatment, many of the patients within the surgical papers had previously received radiation therapy, and it was impossible to isolate and analyze these patients separately. All patients within the radiation series received radiation as their primary treatment. The radiation papers had to state the cumulative radiation dose and schedule clearly.
In an attempt to control for as many potential confounders as possible, all papers used in this meta-analysis had to contain certain demographic information, namely, age, sex, site of primary disease, and site of disease within the spine. For the surgical papers, we also recorded the approach used (anterior, posterior, or combined), whether the patients had previously received radiation or surgery, and whether they received any adjuvant therapy. For the radiation papers, we recorded whether any other treatments, such as surgery, were required.
The primary outcome we considered was ambulatory status. This was the outcome most consistently reported in the literature reviewed and was one of our entry criteria. Patients were considered ambulatory if they could walk with or without assistance. In some papers, the number of patients ambulatory before and after treatment was explicitly stated, whereas in others it was calculated from various neurologic grading schemes used by the authors, such as the modified Frankel score (Frankel et al., 1969), the Tomita scale (Tomita et al., 1983), the Cooper scale (Cooper et al., 1993), and the Brice and McKissock scale (Brice and McKissock, 1965) (Table 1). The ambulatory status was analyzed in two ways. The success rate was defined as the proportion of patients that maintained or regained the ability to ambulate after their treatment (surgery or radiation). The rescue rate was the proportion of patients who regained ambulatory function. If available within the articles, other secondary end points, such as pain control, sphincter function, survival, local recurrences (defined as recurrence of disease within the area treated by either surgery or radiation), and treatment-related complications, were recorded.
Table 1.
Ambulatory grading scales*
| Grade | Description |
|---|---|
| Frankel Score | |
| A | No motor or sensory function |
| B | Preserved sensation only, no motor function |
| C | Nonambulatory, wheelchair bound, some motor function |
| D | Ambulatory but with neurological symptoms |
| E | Normal neurological functions |
| Tomita Scale | |
| I | Able to walk without support |
| II | Able to walk with support |
| III | Unable to walk |
| IV | Paraplegia |
| Cooper Scale | |
| 0 | Intact |
| 1 | Walks independently but not normally |
| 2 | Walks with cane or walker |
| 3 | Stands but is not ambulatory |
| 4 | Slight movement but cannot walk or stand |
| 5 | No movement |
| Brice and McKissock Scale | |
| I | Mild weakness, but able to walk |
| II | Moderate weakness, able to move legs, but not against gravity |
| III | Severe weakness, slight residual motor and sensory function |
| IV | No motor, sensory, or sphincter function below the level of the lesion |
The scales included are the Frankel Score (Frankel et al., 1969), the Tomita Scale (Tomita et al., 1983), the Cooper Scale (Cooper et al., 1993), and Brice and McKissock Scale (Brice and McKissock, 1965).
Mortality and morbidity were defined as death or complication within 30 days of the operation, respectively. The morbidity rate was calculated as the number of complications divided by the number of patients in the study. In this way, an inflated rate might arise if a patient suffered more than one complication. Morbidity was further classified as medical, neurologic, hardware-related, or surgical. The number of patients in each complication category was recorded for each study that met our criteria. Surgical complications include wound infection, hematomas, and cerebrospinal fluid fistulas. Examples of hardware complications include broken or misplaced screws and graft migration/dislodgement. Medical complications are those that affect various physiologic systems not directly affected by the surgery, such as pneumonia, myocardial infarction, and deep venous thrombosis/pulmonary embolism. Finally, patients who suffered new neurologic deficits were considered to have neurologic complications.
Data Analysis
For the analysis of the primary outcome, ambulation, we calculated the success and rescue rates for each of the papers, otherwise known as “effect sizes.” In addition to the study success and rescue rate, a standard error and 95% confidence interval (CI),2 which are functions of the number of subjects in each study, were also calculated. Studies with more subjects generate narrower CIs and are weighted more heavily than papers with fewer subjects (Figs. 3 and 4). A mixed-effects model was then used to calculate cumulative (pooled) success and rescue rates for the surgical literature, the radiation literature, and all papers combined. The mixed-effects model contains both fixed and random effects and was used because we assumed that we could explain some but not all of the heterogeneity within the papers (surgery vs. radiation). In addition, the observational nature of the papers employed in this meta-analysis precludes the assumption of a fixed-effects model, because substantial heterogeneity (and bias) is suspected to exist among the studies (Egger et al., 2001). Furthermore, the random component of the mixed-effects model is intended to capture and adjust for the between-study variation (Whitehead, 2002). Ultimately, this statistical model allowed us to test whether a statistical difference, known as heterogeneity or variance, existed among the papers with respect to the success and rescue rates. A mixed-effects model was used because we assumed that we could explain some of the heterogeneity among the papers (surgery vs. radiation), but not all. This assumption was tested for both ambulatory success and rescue rates, and it supported our decision to use the mixed-effects model to detect and mitigate the between-study heterogeneity.
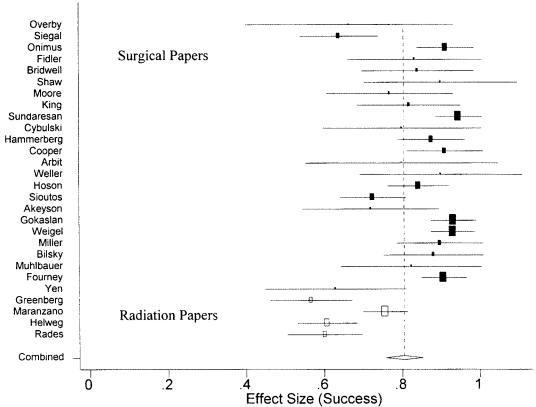
Fig. 3.
Overall (vertical dotted line) and individual standardized effect sizes with their respective confidence intervals for ambulatory “success” in the surgical (filled rectangles) and radiation (empty rectangles) papers.
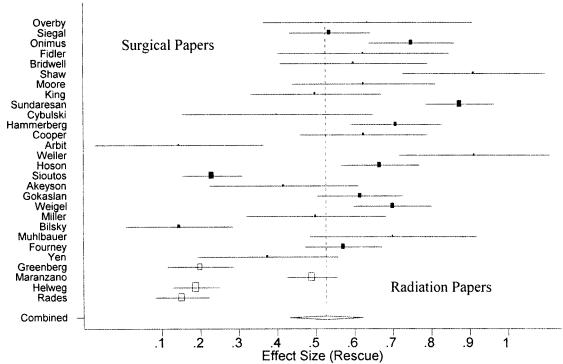
Fig. 4.
Overall (vertical dotted line) and individual standardized effect sizes with their respective confidence intervals for ambulatory “rescue” in the surgical (filled rectangles) and radiation (empty rectangles) papers.
We encountered a problem with our model for papers that had a success or rescue rate of 0.0 or 1.0. Two surgical papers with small patient populations (9 and 8) had success rates and therefore rescue rates of 1.0 (Shaw et al., 1989; Weller and Rossitch, 1995). Two surgical papers had rescue rates of 0.0 (Arbit and Galicich, 1995; Bilsky et al., 2000). These numbers would not fit into our model because they lacked individual study variation (i.e., their standard error equaled zero). These studies, nonetheless, met our original study criteria. In order to keep them in our study, we assigned more conservative effect sizes. For the four papers that had a rescue effect size of 0.0 or 1.0, we recoded them with an effect size set to the value of 2 standard deviations from the pooled effect size of the papers, minus the aforementioned papers (0.5287 ± 0.1914). Therefore, papers with a rescue rate of 1.0 were reassigned a rate of 0.9115, and those with a rate of 0.0 were given a rate of 0.1459. This procedure is sometimes referred to as Windsorizing (Lipsey and Wilson, 2001). We were unable to perform this technique to correct for the 1.0 success rates because the pooled success rate for the surgical papers was already so high. The two papers that had 100% success rates were arbitrarily given a rate of 0.9 because this was the effect size closest to 1.0 that would not significantly affect the weights of the other papers. We felt that these statistical manipulations were justified in keeping with the search criteria and did not introduce any additional significant systematic bias.
When significant heterogeneity was identified (indicating a real difference among the success and rescue rates), a meta-regression was employed to formally investigate the cause. The meta-regression technique allows for the relation of study-level covariates to the outcome (e.g., success rate), resulting in a quantity referred to as τ2, that is, the residual heterogeneity after adjusting for select covariate(s) (Egger et al., 2001). We used meta-regression to test our hypothesis that treatment mode (radiation versus surgery) had a marked effect on ambulatory status. A univariate analysis was performed first with ambulatory outcome (success and rescue) as the dependent variable and the mode of treatment (surgery vs. radiation) as the variable tested. A multivariate analysis was then undertaken by adding the other covariates that we extracted from each article: age, sex, primary pathology, and level of involvement within the spine. These covariates are at the study level as opposed to the individual level because not all the papers provided each of the covariates on an individual level. As a means of quantifying the effectiveness of the different treatment modes for the success and rescue parameters, crude risk ratios (cRRs) were calculated.
Because secondary outcomes were not consistently reported within each of the articles, no inferential statistical analysis was performed. Rather, we simply present the data with reference to the specific articles. We used Stata version 8 (StatCorp, College Station, Tex.) for all statistical analyses.
Results
Demographics—Surgical Literature
A total of 24 articles published between 1984 and 2002 were identified that met our entry criteria (Akeyson and McCutcheon, 1996; Arbit and Galicich, 1995; Bilsky et al., 2000; Bridwell et al., 1988; Cooper et al., 1993; Cybulski et al., 1991; Fidler, 1986; Fourney et al., 2001; Gokaslan et al., 1998; Hammerberg, 1992; Hosono et al., 1995; King et al., 1991; Miller et al., 2000; Moore and Uttley, 1989; Muhlbauer et al., 2000; Onimus et al., 1986; Overby and Rothman, 1985; Shaw et al., 1989; Siegal and Siegal, 1985; Sioutos et al., 1995; Sundaresan et al., 1991; Weigel et al., 1999; Weller and Rossitch, 1995; Yen et al., 2002). All of them, except one, represented uncontrolled, nonrandomized, prospective or retrospective cohort studies (Class III evidence). The paper by Siegal and Siegal (1985) was a prospective cohort study with internal controls (Class II evidence). The demographic data for these studies are shown in Table 2. There were 999 patients with 1020 treated spinal lesions in the 24 studies. The average age was 56.4, and 52% were male. The location of the lesions was primarily the thoracic spine (68%), with the cervical and lumbosacral spine having 11% and 21% of the lesions, respectively. Three primary sites accounted for more than 50% of the tumors: breast, renal, and lung (all histologic subtypes of lung cancer combined). Other primary sites (number of patients in parentheses) not shown in the table included sarcoma (58), multiple myeloma/plasmacytoma (55), unknown primary (46), melanoma (28), gastrointestinal (35), thyroid (27), lymphoma (25), head and neck (11), gynecological (17), and other locations (86). Twenty patients who were described in six articles had primary spinal bone tumors rather than metastatic disease (Cooper et al., 1993; Miller et al., 2000; Moore and Uttley, 1989; Shaw et al., 1989; Sundaresan et al., 1991; Siegal and Siegal, 1985). Although this technically violated our entry criteria, we could not separate out these individual patients in the data analysis, and we felt that, as they constitute only 2% of our total population, they would have a minimal impact on our results.
Table 2.
Demographic and outcome data (ambulation and pain only) for all surgical and radiation papers
| Location | Pathology2 | Ambulatory Patients3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper | Year | M | F | Age (avg) | N1 | C | T | LS | L | P | B | R | Pre | Post | Success (%) | Rescue (%) | Pain (%) |
| Surgical | |||||||||||||||||
| Overby | 1985 | 7 | 5 | 52 | 12 | 12 | 1 | 2 | 3 | 1 | 8 | 67 | 64 | NA | |||
| Siegal | 1985 | 54 | 32 | 50 | 86 | 4 | 63 | 19 | 11 | 2 | 10 | 8 | 19 | 55 | 64 | 59 | NA |
| Onimus | 1986 | 27 | 30 | 60 | 60 | 11 | 39 | 10 | 12 | 2 | 21 | 3 | 37 | 52 | 87 | 65 | 93 |
| Fidler | 1986 | 8 | 10 | 53 | 18 | 0 | 16 | 2 | 2 | 7 | 3 | 10 | 15 | 83 | 63 | 92 | |
| Bridwell | 1988 | 11 | 14 | 63 | 26 | 18 | 8 | 3 | 2 | 5 | 3 | 15 | 21 | 84 | 60 | 76 | |
| Shaw | 1989 | 4 | 5 | 55 | 9 | 8 | 1 | 1 | 3 | 2 | 6 | 9 | 100 | 100 | 100 | ||
| Moore | 1989 | 12 | 14 | 55 | 26 | 9 | 12 | 5 | 3 | 1 | 8 | 10 | 20 | 77 | 63 | 71 | |
| King | 1991 | 18 | 15 | 55 | 33 | 8 | 6 | 19 | 33 | 21 | 27 | 82 | 50 | 88 | |||
| Sundaresan | 1991 | 36 | 18 | 57 | 54 | 15 | 23 | 16 | 5 | 4 | 5 | 6 | 30 | 51 | 94 | 88 | 90 |
| Cybulski | 1991 | 8 | 7 | 45 | 15 | 15 | 5 | 3 | 1 | 10 | 12 | 80 | 40 | 85 | |||
| Hammerberg | 1992 | 25 | 31 | 58 | 58 | 9 | 31 | 18 | 10 | 1 | 21 | 4 | 32 | 49 | 88 | 71 | 91 |
| Cooper | 1993 | 23 | 10 | 58 | 33 | 28 | 5 | 3 | 5 | 6 | 3 | 25 | 30 | 88 | 25 | 97 | |
| Arbit | 1995 | 7 | 3 | 55 | 10 | 8 | 2 | 4 | 2 | 1 | 8 | 8 | 80 | 0 | NA | ||
| Weller | 1995 | 6 | 2 | 61 | 8 | 7 | 1 | 4 | 1 | 3 | 8 | 100 | 100 | 100 | |||
| Hosono | 1995 | 40 | 42 | 54 | 82 | 27 | 37 | 18 | 8 | 4 | 12 | 8 | 43 | 69 | 84 | 67 | 94 |
| Sioutos | 1995 | 61 | 48 | 59 | 109 | 109 | 45 | 21 | 19 | 15 | 70 | 79 | 72 | 23 | NA | ||
| Akeyson | 1996 | 13 | 12 | 54 | 25 | 20 | 5 | 8 | 4 | 5 | 13 | 18 | 72 | 42 | 80 | ||
| Gokaslan | 1998 | 24 | 48 | 56 | 72 | 72 | 9 | 2 | 10 | 19 | 59 | 67 | 93 | 61 | 92 | ||
| Weigel | 1999 | 38 | 38 | 59 | 86 | 6 | 49 | 31 | 8 | 5 | 16 | 16 | 66 | 80 | 93 | 70 | 89 |
| Miller | 2000 | 16 | 13 | 55 | 29 | 19 | 10 | 6 | 2 | 8 | 6 | 23 | 26 | 90 | 50 | 92 | |
| Bilsky | 2000 | 13 | 12 | 59 | 25 | 21 | 4 | 3 | 3 | 4 | 4 | 22 | 22 | 88 | 0 | 100 | |
| Muhlbauer | 2000 | 3 | 14 | 64 | 17 | 1 | 12 | 4 | 4 | 6 | 7 | 14 | 82 | 57 | NA | ||
| Fourney | 2001 | 55 | 40 | 54 | 100 | 52 | 48 | 4 | 3 | 12 | 27 | 74 | 86 | 86 | 46 | 87 | |
| Yen | 2002 | 12 | 15 | 62 | 27 | 22 | 5 | 8 | 2 | 5 | 11 | 17 | 63 | 38 | NA | ||
| Total or average | 521 | 478 | 56.4 (avg) | 1020 | 109 | 690 | 221 | 162 | 68 | 189 | 167 | 615 | 843 | 83 (avg) | 54 (avg) | 90 (avg) | |
| Radiation | |||||||||||||||||
| Greenberg | 1980 | 39 | 44 | 56 | 83 | 12 | 59 | 12 | 11 | 12 | 21 | 3 | 38 | 47 | 57 | 20 | 73 |
| Maranzano | 1995 | 99 | 110 | 62 | 244 | 16 | 131 | 97 | 38 | 24 | 103 | 7 | 109 | 158 | 76 | 49 | 54 |
| Helweg | 1996 | 78 | 75 | 68 | 153 | 7 | 102 | 44 | 27 | 43 | 56 | 6 | 79 | 93 | 61 | 19 | 83 |
| Rades | 2002 | 52 | 46 | 64 | 98 | 62 | 36 | 7 | 12 | 31 | 52 | 59 | 60 | 15 | NA | ||
| Total or average | 268 | 275 | 62.5 (avg) | 578 | 35 | 354 | 189 | 83 | 91 | 211 | 16 | 278 | 357 | 64 (avg) | 26 (avg) | 70 | |
Abbreviations: avg, average; B, breast; C, cervical location; L, lung; LS, lumbosacral location; NA, not available; P, prostate; R, renal; T, thoracic location.
N represents the number of lesions treated. This may not necessarily equal the number of patients within the study.
Only the number of patients with each of the four pathologies is listed. Please refer to the individual papers for the other primaries.
The numbers of patients ambulatory before and after treatment are listed.
Most articles provided information regarding prior treatments. Among all of the papers, 17 contained a total of 360 patients who had previously undergone conventional external beam radiotherapy (Arbit and Galicich, 1995; Bilsky et al., 2000; Bridwell et al., 1988; Cooper et al., 1993; Fidler, 1986; Fourney et al., 2001; Gokaslan et al., 1998; King et al., 1991; Moore and Uttley, 1989; Muhlbauer et al., 2000; Overby and Rothman, 1985; Shaw et al., 1989; Siegal and Siegal, 1985; Sioutos et al., 1995; Weigel et al., 1999; Weller and Rossitch, 1995; Yen et al., 2002). Four papers reported a total of 28 patients that had previously received a decompressive laminectomy (Bridwell et al., 1988; Fourney et al., 2001; Muhlbauer et al., 2000; Shaw et al., 1989). A total of 206 patients from 10 papers were reported to have undergone postoperative radiation therapy, although we believe that this significantly under-reported the totals (Bilsky et al., 2000; Fidler, 1986; Fourney et al., 2001; King et al., 1991; Moore and Uttley, 1989; Onimus et al., 1986; Shaw et al., 1989; Sioutos et al., 1995; Sundaresan et al., 1991; Weigel et al., 1999). Surgical approaches to the spine were recorded. These were classified as anterior (e.g., transthoracic, retroperitoneal), posterior, including posterolateral trajectories (e.g., laminectomy, transpedicular, costotransversectomy, lateral extracavitary), and combined. Anterior approaches were used 556 times (55%); posterior, 395 times (39%); and combined, 68 times (6%).
Demographics—Radiation Literature
Four articles published between 1980 and 2002 met our inclusion criteria (Greenberg et al., 1980; Maranzano and Latini, 1995; Helweg-Larsen, 1996; Rades et al., 2002). All of them represented uncontrolled non-randomized prospective or retrospective cohort studies (Class III evidence). The demographic data for these studies are shown in Table 2. There were 543 patients with 578 treated spinal lesions. The average age was 62.5, and 49% were male. The locations of the lesions were thoracic spine (68%), lumbosacral (33%), and cervical (6%). The three main primary sites that accounted for almost 70% of the tumors were breast, lung, and prostate. Only 16 patients had renal metastases (3%). Other primary sites not shown in the table included (number of patients in parentheses) sarcoma (8), multiple myeloma/plasmacytoma (27), unknown primary (25), melanoma (8), gastrointestinal (26), thyroid (1), lymphoma (20), head and neck (6), gynecological (4), and other locations (52). No patients were reported with primary bone tumors.
Radiation was delivered in a standard regimen in three of the papers, with a total dose ranging from 2800 to 3200 cGy divided over 7 to 12 days (Greenberg et al., 1980; Helweg-Larsen, 1996; Rades et al., 2002). Maranzano and Latini (1995) used different protocols according to the radiosensitivity of the tumor. Those with favorable histology (lymphomas, seminomas, and myelomas) were given an accelerated treatment of 3 to 30 Gy in 10 fractions over two weeks. Patients with less radiosensitive tumors were given a course of 5 Gy daily fractions for three days followed by four days of rest, and patients who seemed to respond were then given five daily doses of 3 Gy. A total of nine patients from the studies by Maranzano and Latini (1995) and Helweg-Larsen (1996) underwent a decompressive laminectomy for failure to respond or progression of neurologic deficits during treatment.
Primary Outcome — Ambulation
“Success.”
The reported ambulatory success was markedly higher in the surgical papers than in the radiation papers. Out of 999 surgical patients, 615 were ambulatory before treatment and 843 after treatment. In the radiation literature, out of 543 patients, 278 and 357 patients were ambulatory before and after treatment, respectively. Thus, surgical patients had a 1.3 greater chance of being ambulatory than those patients described in the radiation literature (cRR = 1.28; 95% CI, 1.20–1.37; P < 0.001). The mixed-effect model results show that the cumulative success rates for the surgical and radiation literature were 0.85 and 0.64, respectively (Fig. 3). The overall success rate, calculated via the mixed-effect model, for all papers was 0.805 (95% CI, 0.758–0.852). The model also detected a highly statistically significant amount of heterogeneity (Q) between studies (Q = 164.592, df = 27, P < 0.001).
To delineate the source of this heterogeneity, a meta-regression analysis was conducted. The estimated variance (τ2), or the amount of heterogeneity, between studies was 0.012 prior to any meta-regression on the studies. When treatment mode was inserted into a meta-regression model as a univariate independent variable, the estimated variance was reduced to 0.0052 and was significantly associated with a negative direction in cumulative success rate (−0.205; 95% CI, −0.296 to −0.113; P < 0.001). In other words, if radiation was the treatment mode, the overall success rate significantly declined. As a further method of examining the clinical implication of treatment mode, we also conducted a multivariate meta-regression analysis. The multivariate meta-regression slightly reduced the estimated variance from 0.0052 in the univariate model to 0.0045. Moreover, treatment mode maintained its significant negative direction (−0.243; 95% CI, −0.365 to −0.121; P < 0.001) when controlling for the aforementioned factors. None of the variables in the model (age, sex, primary pathology, lesion distribution within the spine) were significant predictors of ambulatory success.
“Rescue.”
As was the case for ambulatory success, the rescue rates for the surgical papers were generally greater than those calculated in the radiation papers. Of 384 surgical patients who were non-ambulatory before treatment, 228 regained the ability to walk. In the radiation literature, of 265 patients who were nonambulatory before treatment, 79 became ambulant. Thus, surgical patients were twice as likely to regain ambulatory function compared with patients in the radiation series (cRR = 1.99; 95% CI, 1.63–2.44; P < 0.001). The cumulative rescue rates for the surgical and radiation literature using the mixed-effects model were 0.58 and 0.26, respectively (Fig. 4). The overall rescue rate for all papers was 0.53 (95% CI, 0.431–0.621). As with ambulatory success, significant heterogeneity was detected (Q = 480.896, df = 27, P < 0.001).
To investigate the cause of this heterogeneity, we performed a meta-regression. Prior to a meta-regression, the estimated between-studies variance (τ2) was 0.059. Again, as with ambulatory success, procedure type was significantly associated with a decline in the rescue rate (−0.318; 95% CI, −0.523 to −0.113; P = 0.002) and the between-study variance declined approximately 40% to 0.0353. A multivariate meta-regression was then conducted in a fashion similar to that described for the multivariate model specified for ambulatory success. The between-study variance reduced from 0.0353 to 0.0184; however, treatment mode was no longer significantly associated with a decline in rescue rate, although it was still suggestive (–0.353; 95% CI, –0.805 to 0.100; P = 0.127).
Secondary Outcomes
Pain
Pain control was the variable most consistently reported after ambulatory function in both the surgical and radiation papers. All papers except for six surgical and one radiation paper analyzed pain before and after treatment (Arbit and Galicich, 1995; Muhlbauer et al., 2000; Overby and Rothman, 1985; Rades et al., 2002; Siegal and Siegal, 1985; Sioutos et al., 1995; Yen et al., 2002). This assessment was quite crude in that no distinction could usually be made between the type of pain (axial vs. radicular), and improvement was simply a dichotomous variable (i.e., yes/no). Gokaslan et al. (1998) provided probably the most comprehensive analysis of pain. Out of the 72 patients who underwent a thoracotomy for vertebral metastases, 65 presented with pain. Complete resolution was achieved post-treatment in 15 patients (23%), significant improvement in 45 (69%), and no change or worsening in five (8%). Gokaslan et al. also recorded and classified the type of analgesics used by patients both preoperatively and postoperatively. They found that 28 patients were able to decrease their class of analgesic use by at least one category. We recorded the percentage of patients within each study that had any improvement in pain after their primary treatment. Within the surgical literature, the average percentage of patients that experienced an improvement in pain was 90% (71%–100%) compared with 70% (54%–83%) within the radiation literature.
Sphincter function
Very few papers that met our entry criteria recorded the sphincter function of patients before and after treatment, and most focused on bladder function. One reason for this may be that no scale accurately assesses sphincter function (both bladder and bowel), as the Frankel scale does for ambulation. Function was usually categorized as “continent” or “incontinent,” with some papers adding a “dysfunctional” category. Three surgical and two radiation papers assessed sphincter function (Helweg-Larsen, 1996; King et al., 1991; Maranzano and Latini, 1995; Overby and Rothman, 1985; Siegal and Siegal, 1985). Of the 131 patients within the surgical articles, 65 (50%) were incontinent preoperatively compared with 22 (17%) postoperatively. Within the radiation literature, 82 out of 397 patients (21%) were incontinent prior to radiation compared with 61 patients postradiation (15%). Thus, the sphincter rescue rate with surgery was 66% compared with 26% with radiation.
Survival
Survival was difficult to assess within this literature because it was inconsistently and variously reported. The most consistent means of presenting survival data is the 12-month mortality rate, which was readily available in nine surgical articles and two radiation articles. The one-year survival in the surgical literature (n = 502) ranged from 12% to 62%, with an average of 41% (Akeyson and McCutcheon, 1996; Arbit and Galicich, 1995; Cybulski et al., 1991; Fourney et al., 2001; Gokaslan et al., 1998; Hammerberg, 1992; Miller et al., 2000; Sioutos et al., 1995; Weigel et al., 1999). For the radiation articles (n = 397), the rate was 20% to 28%, with an average of 24% (Helweg-Larsen, 1996; Maranzano and Latini, 1995). The most significant factor that determined post-treatment survival was the primary histology. In the surgical article by Gokaslan et al. (1998), the one-year survival rates for renal, breast, lung, and melanoma/sarcoma were 65%, 63%, 55%, and 52%, respectively. The radiotherapy study by Maranzano and Latini (1995) reported one-year survival rates as well for various histologies: breast, 45%; myeloma, 45%; prostate, 27%; renal, 14%; and lung, 8%. Weigel et al. (1999) reported the mean survival (months) for various histologies in their surgical cohort study: breast, 21.2; multiple myeloma, 35.2; renal cell, 13.1; prostate, 7.3; lung, 2.1; and melanoma, 1.5. Although the survival statistics vary among the papers, in general, patients with breast and renal cancer have a more favorable survival prognosis than those with lung cancer and sarcoma. Survival has also been correlated, although not consistently within the literature, with more than one site of metastatic disease within the spine (Fourney et al., 2001; Sioutos et al., 1995), preoperative neurologic status (Maranzano and Latini, 1995; Sioutos et al., 1995), age (Weigel et al., 1999), and presence of extra-spinal metastases (Weigel et al., 1999).
Complications and local recurrences
No significant treatment-related complications were reported in the radiation literature. Within the surgical papers, 63 patients died within 30 days of their operation (6.3%). Two hundred thirty-three complications (23%) occurred within the following categories defined previously: medical, 100; neurologic, 19; hardware, 18; and surgical, 96. Again, it is impossible to determine how many patients actually suffered complications because undoubtedly there were instances where one patient suffered more than one complication.
Only one radiation article described patients that developed local recurrences. Maranzano and Latini (1995) reported that five patients out of their cohort of 209 patients with 244 treated compressive lesions developed local recurrences. Eighty-one patients described in nine surgical papers also developed local recurrences for an incidence of at least 8% (Bilsky et al., 2000; Fourney et al., 2001; Hammerberg, 1992; Hosono et al., 1995; King et al., 1991; Miller et al., 2000; Muhlbauer et al., 2000; Siegal and Siegal, 1985; Weigel et al., 1999).
Discussion
The management of patients with epidural spinal cord compression from metastatic disease is an increasingly challenging endeavor. With the failure of indiscriminate use of posterior decompressive laminectomy as virtually the sole surgical option, conventional external beam radiotherapy emerged as the primary therapy for these patients. The revival of surgery as a consideration for primary therapy for metastatic spinal disease required the incorporation of more sophisticated and individualized surgical approaches as well as the development of internal fixation devices. In the last 20 years, advances in these areas have resulted in a large body of literature evaluating the effectiveness of surgery in which the goal is to circumferentially decompress the spinal cord and immediately reconstruct and stabilize the spine.
Traditional indications for surgery include radioresistant tumors (sarcoma, lung, colon, renal cell), obvious spinal instability, clinically significant neural compression secondary to retropulsed bone or from spinal deformity, intractable pain unresponsive to nonoperative measures, and radiation failure (progression of deficit during treatment or spinal cord tolerance reached). Indications for radiotherapy are radiosensitive tumors (lymphoma, multiple myeloma, small-cell lung carcinoma, seminoma of testes, neuroblastoma, Ewing’s sarcoma), expected survival of less than three to four months, inability of the patient to tolerate an operation, total neurological deficit below the level of compression for more than 24 to 48 h, and multilevel or diffuse spinal involvement. Despite the literature suggesting a marked improvement in the outcomes with new-era surgery compared with the days of decompressive laminectomy, the vast majority of patients with newly diagnosed metastatic spinal disease continue to receive radiation as their primary treatment, and spine surgeons are often not consulted initially. Loblaw et al. (2003) recently conducted a population-based study and found that of patients with newly diagnosed metastatic spinal disease, at least 60.2% were initially treated with radiotherapy. Among the surgical papers that met our study criteria, at least 360 patients had also previously undergone radiation treatment (36%). A number of factors may explain the bias toward radiotherapy. Radiation can be administered easily and quickly, a powerful attribute when dealing with patients who present with neurologic deficits from mass effect. Additionally, its effectiveness has been repeatedly demonstrated through time. The majority of the literature supporting surgery is Class III evidence and is probably unknown to many oncologists, the front-line physicians for this disease. Nonetheless, radiation carries certain disadvantages. A tumor’s response to radiation is directly dependent on the tumor’s inherent radiosensitivity. Radiotherapy does not address any deformity (most commonly kyphosis) and is ineffective at relieving spinal cord compression if it is due to bone. Therefore, despite the bias toward radiation, the question of which treatment is optimal still needs to be addressed, even after all these years.
Our meta-analysis found a statistically significant difference between surgery and radiation in the ambulatory success rates. Overall, the surgical patients were 1.3 times more likely to be ambulatory after treatment compared with the radiation patients. The cumulative rate for success was 85% in the surgical papers compared with 64% in the radiation papers. This difference remained statistically significant even when placed into our meta-regression model, in which we could control for the other covariates (age, sex, primary pathology, lesion distribution within the spine). More important from a clinical standpoint was the difference in the rescue rate. This was defined as the proportion of patients who regained the ability to ambulate after treatment. Here, patients within the surgical series were twice as likely to regain the ability to ambulate. The cumulative effect sizes for surgery and radiation were 58% and 26%, respectively. When placed into our meta-regression model, the difference in treatment was responsible for a statistically significant portion of the heterogeneity that was present. However, on multivariate analysis, it did not reach statistical significance. Nonetheless, a trend towards a more favorable rescue rate with surgery is apparent.
A number of secondary outcomes were also retrieved from the literature. Both treatment modes improved pain, but surgery seemed to have a better chance of improving pain. On average, 90% of patients in the surgical literature experienced some degree of pain improvement compared with 70% with radiation. Although limited data were available, surgery also appeared to be superior at regaining sphincter function. The sphincter rescue rate for surgery was 66%, compared with 26% for radiation. The average one-year survival rates also seemed to be better for surgery than for radiation (41% vs. 24%); however, this discrepancy most likely results from the difference in the composition of primary tumor pathology rather than the treatment itself. Radiation appeared to have a superior local control rate. Finally, a large discrepancy in the incidence of treatment-related complications (morbidity and mortality) was obvious. Virtually no immediate treatment-related complications related to radiation were reported. Specifically, radiation-induced myelopathy was not reported in any cases. Conversely, surgery with its more aggressive approach was associated with a significant 30-day mortality rate of 6.3% and many potential complications, most notably surgical and medical. It is interesting to note that surgical and medical is interesting that of the 543 patients reported within the radiation literature, only nine (1.6%) required a decompressive laminectomy for treatment failure. However, in the surgical papers, 360 patients (36%) had received radiation prior to their decompression. Although not formally considered a complication, this discrepancy is a consequence of inadequate follow-up within the radiation literature and highlights the fact that for too many patients, surgery is offered as a second-line therapy.
Our study had significant limitations. A meta-analysis is an attempt to review the cumulative data from studies involving similar participants and similar intervention strategies to reach a consensus on the overall results. The most common reason to pursue a meta-analysis is to strengthen the power to observe small, but clinically important differences between interventions. As such, the quality of the meta-analysis is primarily limited by the quality and content of the existing literature. Most meta-analyses published to date use randomized clinical trials (Whitehead, 2002). The power of a randomized clinical trial lies in its ability to distribute all known and unknown confounders equally among the treatment arms. As such, it is the best way of assuring that any difference between the treatment groups is most likely due to the treatment itself. Applying a meta-analysis to the metastatic spinal literature, which contains primarily uncontrolled cohort studies, required us to develop a somewhat unique although valid statistical methodology.
The articles were selected to control for as many potential covariates as possible and to try to correct for any imbalances that might arise. Nonetheless, significant heterogeneity for which we could not account remained between the patients described within the surgical papers, as compared with those within the radiation papers. The composition of the two patient populations differed, most notably in the distribution of primary tumor types and in the high incidence of prior radiation therapy in the surgical patients (i.e., surgery was a secondary treatment). Ambulation was the most consistently reported outcome, and it was therefore chosen as our primary outcome. However, the papers inconsistently reported the secondary outcomes. Our conclusions made with regard to the secondary outcomes are thus weaker than those made for the primary outcome but do serve as a rough indicator of treatment-related differences. We can only speculate on further potential differences between the two groups. For example, other important data, such as the time between presentation and treatment and how the various outcome measures are affected by time after treatment, were simply not available. Although we assume that, at most high-volume, multidisciplinary oncological centers, patients with newly diagnosed metastatic spinal disease are evaluated by all parties (oncologists, radiation therapists, spinal surgeons) to determine the most appropriate treatment, we believe that many of the patients in the radiation articles would also be surgical candidates. At our own institution, patients with newly diagnosed disease are still routinely sent to radiation therapists without a surgical consultation. Furthermore, the fact that many of the patients in the surgical papers had failed radiation therapy and still demonstrated better outcomes raises the possibility that even better outcomes could have been achieved if the patient had received surgery first followed by adjuvant therapy.
As this paper was being prepared, the preliminary results of the first randomized clinical trial in the metastatic spine literature were presented at several meetings, including the 2003 meeting of the American Society of Clinical Oncology. Patchell et al. (2003) randomized 101 patients to direct decompressive surgical resection followed by adjuvant radiation (n = 50) versus conventional radiation alone (n = 51). Both groups were treated with the same steroid protocol, and both received the same total radiation dose (30 Gy). Patients treated with surgery were more likely to retain or regain ambulatory or sphincter function than patients in the radiation group. Survival was not significantly different for the two groups. At this time, the results of this trial have not been published. Nonetheless, we firmly believe that our meta-analysis, along with these preliminary results, support a major change in the current management of metastatic epidural spinal disease. Surgery should be considered the primary treatment modality in all patients with newly diagnosed metastatic disease (who do not harbor any of the indications for radiotherapy mentioned previously) followed by adjuvant conventional radiation therapy.
A meta-analysis may produce some degree of order and cohesiveness from the confusion created by multiple small, uncontrolled studies, but it should not be used as an excuse to continue propagating such research. Future studies in the area of metastatic spine disease should accurately capture the effects of new interventions such as minimally invasive spine surgery and stereotactic radiosurgery. Such studies should be conducted on a multicenter basis. Outcomes should be expanded, properly defined, and quantifiable. For example, few articles use quality-of-life measures in patients with metastatic spinal disease (Wai et al., 2003). Ambulatory status should be quantified by using known mobility measures such as the 10-min timed walk and the 2-min walk test rather than kept as a categorical variable (Rossier and Wade, 2001). All outcome measures should be determined at various intervals after the treatment is rendered. We are currently in the process of designing a multicenter trial that will compare the effects of stereotactic radiosurgery with surgery followed by conventional radiotherapy using these and other outcomes. We believe this to be the next question that should be addressed. Whatever the design or outcome of any trial, a multidisciplinary approach, involving spine surgeons, radiation oncologists, oncologists, physiatrists, etc., must form the foundation on which appropriate treatment can be rendered.
Conclusions
Our meta-analysis was derived from uncontrolled cohort studies in the surgical and radiation oncology literature. This presented a unique methodological challenge and several significant limitations. Our results indicate that all patients with newly diagnosed metastatic spinal disease should be carefully evaluated for surgery as a primary treatment modality. Ambulatory function seems to be preserved and regained at a greater rate with surgery than with radiation. It also appears that surgery is superior at relieving pain and recovering sphincter function. However, the decision to pursue surgery must be tempered with the realization that significant morbidity and mortality exist. Patient selection is of utmost importance.
Acknowledgement
The authors thank Kristin Kraus for her editorial assistance in reviewing this paper.
Footnotes
Abbreviations used are as follows: CI, confidence interval; cRR, crude risk ratios; Q, heterogeneity.
References
- Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg. 1996;85:211–220. doi: 10.3171/jns.1996.85.2.0211. [DOI] [PubMed] [Google Scholar]
- Arbit E, Galicich JH. Vertebral body reconstruction with a modified Harrington rod distraction system for stabilization of the spine affected with metastatic disease. J Neurosurg. 1995;83:617–620. doi: 10.3171/jns.1995.83.4.0617. [DOI] [PubMed] [Google Scholar]
- Barron KD, Hirano A, Araki S, Terry RD. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91–106. doi: 10.1212/wnl.9.2.91. [DOI] [PubMed] [Google Scholar]
- Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459–469. [PubMed] [Google Scholar]
- Bilsky MH, Boland P, Lis E, Raizer JJ, Healey JH. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine. 2000;25:2240–2249. doi: 10.1097/00007632-200009010-00016. [DOI] [PubMed] [Google Scholar]
- Black P. Spinal metastasis: Current status and recommended guidelines for management. Neurosurgery. 1979;5:726–746. doi: 10.1227/00006123-197912000-00016. [DOI] [PubMed] [Google Scholar]
- Bohm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84:521–529. doi: 10.1302/0301-620x.84b4.12495. [DOI] [PubMed] [Google Scholar]
- Brice J, McKissock W. Surgical treatment of malignant extradural spinal tumours. Br Med J. 1965;5446:1341–1344. doi: 10.1136/bmj.1.5446.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridwell KH, Jenny AB, Saul T, Rich KM, Grubb RL. Posterior segmental spinal instrumentation (PSSI) with posterolateral decompression and debulking for metastatic thoracic and lumbar spine disease. Limitations of the technique. Spine. 1988;13:1383–1394. doi: 10.1097/00007632-198812000-00010. [DOI] [PubMed] [Google Scholar]
- Byrne TN. Spinal cord compression from epidural metastases. New Engl J Med. 1992;327:614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- Constans JP, de Divitiis E, Donzelli R, Spanziante R, Meder JF, Haye C. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59:111–118. doi: 10.3171/jns.1983.59.1.0111. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Errico TJ, Martin R, Crawford B, DiBartolo T. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1–8. doi: 10.1227/00006123-199301000-00001. [DOI] [PubMed] [Google Scholar]
- Cybulski GR, Stone JL, Opesanmi O. Spinal cord decompression via a modified costotransversectomy approach combined with posterior instrumentation for management of metastatic neoplasms of the thoracic spine. Surg Neurol. 1991;35:280–285. doi: 10.1016/0090-3019(91)90005-t. [DOI] [PubMed] [Google Scholar]
- Egger, M., Davey Smith, G., and Altman, D.G. (2001) Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed London: BMJ Publishing Group, pp. 211–224, 347–364.
- Fidler MW. Anterior decompression and stabilisation of metastatic spinal fractures. J Bone Joint Surg Br. 1986;68:83–90. doi: 10.1302/0301-620X.68B1.3941146. [DOI] [PubMed] [Google Scholar]
- Findlay GF. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry. 1984;47:761–768. doi: 10.1136/jnnp.47.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourney DR, Abi-Said D, Lang FF, McCutcheon IE, Gokaslan ZL. Use of pedicle screw fixation in the management of malignant spinal disease: Experience in 100 consecutive procedures. J Neurosurg Spine. 2001;94:25–37. doi: 10.3171/spi.2001.94.1.0025. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Huntingt) 2000;14:1013–1024. [PubMed] [Google Scholar]
- Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: Diagnosis and treatment. Ann Neurol. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- Gokaslan ZL, York JE, Walsh GL, McCutcheon IE, Lang FF, Putnam JB, Jr, Wildrick DM, Swisher SG, Abi-Said D, Sawaya R. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599–609. doi: 10.3171/jns.1998.89.4.0599. [DOI] [PubMed] [Google Scholar]
- Greenberg HS, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: Results with a new treatment protocol. Ann Neurol. 1980;8:361–366. doi: 10.1002/ana.410080404. [DOI] [PubMed] [Google Scholar]
- Hammerberg KW. Surgical treatment of metastatic spine disease. Spine. 1992;17:1148–1153. doi: 10.1097/00007632-199210000-00004. [DOI] [PubMed] [Google Scholar]
- Harrington KD. The use of methylmethacrylate for vertebral-body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J Bone Joint Surg Am. 1981;63:36–46. [PubMed] [Google Scholar]
- Harrington KD. Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg. 1984;61:107–117. doi: 10.3171/jns.1984.61.1.0107. [DOI] [PubMed] [Google Scholar]
- Healey JH, Brown HK. Complications of bone metastases: Surgical management. Cancer. 2000;88 (suppl. 12):2940–2951. doi: 10.1002/1097-0142(20000615)88:12+<2940::aid-cncr10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269–275. doi: 10.1111/j.1600-0404.1996.tb07064.x. [DOI] [PubMed] [Google Scholar]
- Hosono N, Yonenobu K, Fuji T, Ebara S, Yamashita K, Ono K. Vertebral body replacement with a ceramic prosthesis for metastatic spinal tumors. Spine. 1995;20:2454–2462. doi: 10.1097/00007632-199511001-00015. [DOI] [PubMed] [Google Scholar]
- King GJ, Kostuik JP, McBroom RJ, Richardson W. Surgical management of metastatic renal carcinoma of the spine. Spine. 1991;16:265–271. doi: 10.1097/00007632-199103000-00003. [DOI] [PubMed] [Google Scholar]
- Klimo P, Jr, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9:188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- Klimo P, Kestle JRW, Schmidt M. Treatment of metastatic spinal epidural disease: A review of the literature. Neurosurg Focus. 2003;15(5):1–9. doi: 10.3171/foc.2003.15.5.1. Article 1. [DOI] [PubMed] [Google Scholar]
- Lada, R., Kaminski, H.J., and Ruff, R. (1997) Metastatic spinal cord compression. Chapter 11 in: Vecht, C.J. (Ed.), Neuro-Oncology, Part III. Neurological Disorders in Systemic Cancer (Handbook of Clinical Neurology, Vol. 25 [69]). Amsterdam: Elsevier Biomedical Publishers, pp. 167–189.
- Lipsey, M.W., and Wilson, D.B. (2001) Analysis Issues and Strategies. In: Lipsey, M.W. (Ed.), Practical Meta-Analysis Thousand Oaks, Calif.: SAGE Publications, Inc., pp. 105–128.
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15:211–217. doi: 10.1016/s0936-6555(02)00400-4. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: Final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- Martenson JA, Jr, Evans RG, Lie MR, Ilstrup DM, Dinapoli RP, Ebersold MJ, Earle JD. Treatment outcome and complications in patients treated for malignant epidural spinal cord compression (SCC) J Neurooncol. 1985;3:77–84. doi: 10.1007/BF00165175. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Lang FF, Walsh GL, Abi-Said D, Wildrick DM, Gokaslan ZL. Coaxial double-lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J Neurosurg Spine. 2000;92:181–190. doi: 10.3171/spi.2000.92.2.0181. [DOI] [PubMed] [Google Scholar]
- Mones RJ, Dozier D, Berrett A. Analysis of medical treatment of malignant extradural spinal cord tumors. Cancer. 1966;19:1842–1853. doi: 10.1002/1097-0142(196612)19:12<1842::aid-cncr2820191212>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Moore AJ, Uttley D. Anterior decompression and stabilization of the spine in malignant disease. Neurosurgery. 1989;24:713–717. doi: 10.1227/00006123-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Muhlbauer M, Pfisterer W, Eyb R, Knosp E. Noncontiguous spinal metastases and plasmocytomas should be operated on through a single posterior midline approach, and circumferential decompression should be performed with individualized reconstruction. Acta Neurochir (Wien) 2000;142:1219–1230. doi: 10.1007/s007010070018. [DOI] [PubMed] [Google Scholar]
- Onimus M, Schraub S, Bertin D, Bosset JF, Guidet M. Surgical treatment of vertebral metastasis. Spine. 1986;11:883–891. doi: 10.1097/00007632-198611000-00007. [DOI] [PubMed] [Google Scholar]
- Overby MC, Rothman AS. Anterolateral decompression for metastatic epidural spinal cord tumors. Results of a modified costotransversectomy approach. J Neurosurg. 1985;62:344–348. doi: 10.3171/jns.1985.62.3.0344. [DOI] [PubMed] [Google Scholar]
- Patchell R, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Young B. A randomized trial of direct decompressive surgical resection in the treatment of spinal cord compression caused by metastasis. J Clin Oncol. 2003;21:237. doi: 10.1016/S0140-6736(05)66954-1. (abstract no. 2) [DOI] [PubMed] [Google Scholar]
- Rades D, Heidenreich F, Karstens JH. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2002;53:975–979. doi: 10.1016/s0360-3016(02)02819-5. [DOI] [PubMed] [Google Scholar]
- Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine. 1985;10:19–20. doi: 10.1097/00007632-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Shaw B, Mansfield FL, Borges L. One-stage posterolateral decompression and stabilization for primary and metastatic vertebral tumors in the thoracic and lumbar spine. J Neurosurg. 1989;70:405–410. doi: 10.3171/jns.1989.70.3.0405. [DOI] [PubMed] [Google Scholar]
- Siegal T, Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: A prospective study. Neurosurgery. 1985;17:424–432. doi: 10.1227/00006123-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::aid-cncr2820760824>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Sørensen S, Børgesen SE, Rohde K, Rasmusson B, Bach F, Bøge-Rasmussen T, Stjernholm P, Larsen BH, Agerlin N, Gjerris F. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1990;65:1502–1508. doi: 10.1002/1097-0142(19900401)65:7<1502::aid-cncr2820650709>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Stark RJ, Henson RA, Evans SJ. Spinal metastases: A retrospective survey from a general hospital. Brain. 1982;105:189–213. doi: 10.1093/brain/105.1.189. [DOI] [PubMed] [Google Scholar]
- Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: Results of a prospective study. Neurosurgery. 1991;29:645–650. doi: 10.1097/00006123-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Tomita T, Galich JH, Sundaresan H. Radiation therapy for epidural metastases with complete block. Acta Radiol Oncol. 1983;22:135–143. doi: 10.3109/02841868309134353. [DOI] [PubMed] [Google Scholar]
- Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M, Yee A. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/01.BRS.0000048646.26222.FA. [DOI] [PubMed] [Google Scholar]
- Weigel B, Maghsudi M, Neumann C, Kretschmer R, Muller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine. 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- Weller SJ, Rossitch E., Jr Unilateral posterolateral decompression without stabilization for neurological palliation of symptomatic spinal metastasis in debilitated patients. J Neurosurg. 1995;82:739–744. doi: 10.3171/jns.1995.82.5.0739. [DOI] [PubMed] [Google Scholar]
- Whitehead, A. (2002) Meta-Analysis of Controlled Clinical Trials. Chichester, West Sussex, England: John Wiley & Sons Ltd.
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: The obvious, the occult, and the impostors. Spine. 1990;15:1–4. [PubMed] [Google Scholar]
- Yen D, Kuriachan V, Yach J, Howard A. Long-term outcome of anterior decompression and spinal fixation after placement of the Wellesley Wedge for thoracic and lumbar spinal metastasis. J Neurosurg Spine. 2002;96:6–9. doi: 10.3171/spi.2002.96.1.0006. [DOI] [PubMed] [Google Scholar]
- Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]