Abstract
Ependymomas are glial cell–derived tumors characterized by varying degrees of chromosomal abnormalities and variability in clinical behavior. Cytogenetic analysis of pediatric ependymoma has failed to identify consistent patterns of abnormalities, with the exception of monosomy of 22 or structural abnormalities of 22q. In this study, a total of 19 pediatric ependymoma samples were used in a series of expression profiling, quantitative real-time PCR (Q-PCR), and loss of heterozygosity experiments to identify candidate genes involved in the development of this type of pediatric malignancy. Of the 12,627 genes analyzed, a subset of 112 genes emerged as being abnormally expressed when compared to three normal brain controls. Genes with increased expression included the oncogene WNT5A; the p53 homologue p63 and several cell cycle, cell adhesion, and proliferation genes. Underexpressed genes comprised the NF2 interacting gene SCHIP-1 and the adenomatous polyposis coli (APC)-associated gene EB1 among others. We validated the abnormal expression of six of these genes by Q-PCR. The subset of differentially expressed genes also included four underexpressed transcripts mapping to 22q12.3-13.3. By Q-PCR we show that one of these genes, CBX7 (22q13.1), was deleted in 55% of cases. Other genes mapping to cytogenetic hot spots included two overexpressed and three underexpressed genes mapping to 1q31-41 and 6q21-q24.3, respectively. These genes represent candidate genes involved in ependymoma tumorigenesis. To the authors’ knowledge, this is the first time microarray analysis and Q-PCR have been linked to identify heterozygous/homozygous deletions.
Ependymomas are glial cell–derived tumors that arise from the ependymal lining of the ventricular system of the central nervous system and manifest preferentially in childhood. They are the third most common primary brain tumor in childhood (following low-grade astrocytomas and medulloblastomas) and account for 6% to 12% of all intracranial neoplasms in the pediatric population (Heideman, 1979). These tumors may occur at any site in the ventricular system, although they most commonly develop in the posterior fossa (Pollack et al., 1995). There are four variants of ependymoma in the WHO classification, myxopapillary (WHO grade I), subependymoma (WHO grade I), classic ependymoma (WHO grade II), and anaplastic ependymoma (WHO grade III) (Kleihues et al., 2002). Ependymomas have a propensity to recur. The five-year survival rates in children are 34% to 45%, with local relapse being the major source of therapeutic failure (Pollack et al., 1995).
At present, the genetic events that contribute to the pathogenesis of pediatric ependymoma are essentially unknown. Comparatively few cytogenetic analyses have been carried out, and many of these have been single case reports or small series of tumors (Bhattacharjee et al., 1997; Bigner et al., 1997). Furthermore, up to 50% of tumors appear karyotypically normal. To date, the most common chromosomal aberrations reported are deletions or rearrangements of 6q, 17, and 22, although all are present in only <30% of cases (Kramer et al., 1998; Mazewski et al., 1999; Reardon et al., 1999). Recent comparative genomic hybridization (CGH)4 studies have also demonstrated gain of 1q to be present in a subset of ependymomas, and our laboratory has also reported high-copy-number amplification at 1q24-31 in three cases (Carter et al., 2002; Dyer et al., 2002; Ward et al., 2001). However, candidate genes mapping to these areas have not yet been identified. Although the NF2 gene maps to 22q12, and individuals affected by NF2 have increased susceptibility to ependymomas, NF2 is rarely mutated in sporadic tumors (Rubio et al., 1994; Slavc et al., 1995). Similarly, mutations of p53 are extremely rare in ependymoma (Tong et al., 1999).
At the molecular level, a small number of studies have linked ependymoma with abnormal expression of the ERBB2 and ERBB4 receptors (Gilbertson et al., 2002), the vascular endothelial growth factor protein (VEGF) (Korshunov et al., 2002), p73 (Kamiya and Nakazato, 2002), and MDM2 (Suzuki and Iwaki, 2000) in a subset of samples. However, to date, no consistent molecular alteration has been found in these tumors.
In recent years, global expression technologies such as oligonucleotide microarrays have been successfully used in the development of statistical algorithm-based classifications in several types of tumors (Dyrskjot et al., 2003; Luo, 2002; Perou et al., 2000; Watson et al., 2002). In this study we have used the Affymetrix GeneChip system U95Av2 (Affymetrix, Santa Clara, Calif.) validated by quantitative real-time polymerase chain reaction analysis (Q-PCR) to investigate abnormally expressed genes in ependymoma. We have identified 112 abnormally expressed genes, 10 of which map to regions of genomic imbalance identified by CGH at 1q, 6q, and 22q. We also report deletion of CBX7 at 22q13 in more than 50% of ependymomas as validated by Q-PCR. Real-time PCR provides a means for continuous detection of product throughout the amplification process, and this technique has been used to detect deletions and duplications in cancer (M’soka et al., 2000; Senchenko et al., 2003). Unlike loss of heterozygosity (LOH) analysis, it is not necessary to identify polymorphic markers. The subset of genes identified in this study may represent candidate genes involved in the genesis and development of intracranial pediatric ependymoma.
Materials and Methods
Tumor Samples and RNA Preparation
Tumor specimens were obtained with informed consent from 19 patients (Table 1). The mean age was 7.8 years (range, 6 months to 15 years). All tumors were graded according to WHO criteria and comprised two subependymomas, 13 ependymomas, and four anaplastic ependymomas (Kleihues et al., 2002). Twelve samples were fresh-frozen biopsies collected directly from the operating theatre and stored in liquid nitrogen until ready for use. In our validation experiments, we included seven short-term cell cultures, which were prepared as described previously (Lewandowicz et al., 2000). Samples were directly adjacent to tumor tissue processed for routine histologic evaluation and were first examined macroscopically to ensure that no frankly normal tissue was included in either culture preparation or DNA extraction.
Table 1.
Ependymoma case information. Summary of clinical information of tumor samples used in the (A) microarray and (B) Q-PCR sections of this study
| Sample | Agea | Sex | Histology | Location | Sourceb | LOHc | Sequencing analysisd |
|---|---|---|---|---|---|---|---|
| A. Microarray samples | |||||||
| IN2931 | 1.3 | F | E | Posterior fossa | FF | yes | yes |
| IN2935 | 9.3 | M | E | Posterior fossa | FF | yes | no |
| IN2939 | 0.6 | F | E | Posterior fossa | FF | yes | no |
| IN3087 | 3.8 | M | E | Posterior fossa | FF | yes | yes |
| IN3037 | 2 | M | AE | Posterior fossa | FF | no | no |
| IN3108 | 4 | M | AE | Posterior fossa | FF | yes | yes |
| B. Q-PCR samples | |||||||
| IN772 | 4 | M | SE | Supratentorial | CC | no | no |
| IN2242 | 2 | F | E | Posterior fossa | CC | no | no |
| IN2376 | 15 | M | E | Posterior fossa | FF | yes | no |
| IN2767 | 1.8 | F | AE | Posterior fossa | FF | no | no |
| IN3008 | 10.5 | M | E | Posterior fossa | FF | no | no |
| IN3071 | 4 | M | E | Posterior fossa | FF | no | no |
| IN3121 | 8 | M | E | Posterior fossa | FF | yes | no |
| IN3125 | 6 | F | E | Posterior fossa | FF | yes | yes |
| IN1134 | 5.5 | M | SE | Supratentorial | CC | yes | yes |
| IN1231 | 7 | F | E | Supratentorial | CC | no | no |
| IN1258 | 2.5 | F | E | Posterior fossa | CC | yes | yes |
| IN1759 | 10 | M | E | Posterior fossa | CC | yes | yes |
| IN2443 | 4.5 | M | AE | Posterior fossa | CC | no | no |
Abbreviations used: AE; anaplastic ependymoma; CC, short-term cultures, E, benign ependymoma; F, female; FF, fresh-frozen material; M, male; Q-PCR, quantitative real-time PCR; SE, subependymoma
Age at diagnosis in years
Source
Samples used in LOH based on Q-PCR
Samples used in sequencing analysis of the CBX7 gene
Two normal brain postmortem samples derived from the ventricular region of the corpus callosum were obtained from the Queen Square Brain Bank, Institute of Neurology. A third sample of normal corpus callosum (pooled RNA enriched for glial cells from tissues of 70 individuals) was purchased from Clontech (Clontech Laboratories Inc., Palo Alto, Calif.) as previously described (Ljubimova et al., 2001).
Total RNA was isolated from 12 biopsy samples by guanidine isothiocyanate buffer extraction (Life Technologies Inc., Rockville, Md.), phenol/chloroform extraction, and ethanol precipitation, followed by a second cleanup step (Qiagen Ltd., Crawley, UK). RNA was extracted from seven short-term cultures by using an anion exchange method on a resin column in the presence of a highly denaturing cell-lysis buffer containing guanidine isothiocyanate (Qiagen). Each RNA sample was quantified and quality assessed with an Agilent 2100 bioanalyzer machine (Agilent Technologies UK Ltd., West Lothian, Scotland).
cRNA Synthesis, Microarray Hybridization, and Data Collection
Total RNA from six biopsy samples and three normal brain controls was used to prepare biotinylated target RNA, with minor modifications from the manufacturer’s recommendations (Affymetrix, 2004). Briefly, 10 μg of total RNA was used to generate first-strand cDNA by using a T7-linked oligo(dT) primer. After second-strand synthesis, in vitro transcription was performed with biotinylated UTP and CTP (Enzo Diagnostics Inc., Farmingdale, N.Y.), resulting in approximately 100-fold amplification of RNA. Target cDNA generated from each sample was then processed according to manufacturer’s recommendation using an Affymetrix GeneChip Instrument System (Affymetrix, 2004). Briefly, spike controls were added to 10 μg of fragmented cRNA before overnight hybridization to U95Av2 GeneChip arrays. Arrays were then washed and stained with streptavidin-phycoerythrin (Molecular Probes Europe BV, Leiden, The Netherlands), before being scanned on an Affymetrix GeneChip scanner. After scanning, array images were assessed to confirm scanner alignment and the absence of significant bubbles or scratches. BioB spike controls were found to be present on all chips, with BioC, BioD, and CreX also present in increasing intensity. When scaled to a target intensity of 600 (with Affymetrix MAS 5.0 array analysis software), scaling factors for all arrays were within acceptable limits (0.3–1.5), as were background, Q values, and mean intensities.
All data and details of quality control measures were stored in a format compliant with MIAME (Minimum Information About a Microarray Experiment; Brazma et al., 2001) standards in an ArrayExpress database at the BioMap consortium at University College London and can be made available from the UCL ArrayExpress on request to the authors.
Data Analysis
The U95Av2 chip, which contains 12,627 transcripts including bacterial control spikes, was used in this study. To obtain a list of informative genes we used the Gene-Spring software version 4.2.1 (Silicon Genetics, Redwood City, Calif.). The global error model option of GeneSpring based on replicates was applied (which allows for standard deviation values and P values to be computed). Genes whose signal did not significantly exceed background strength (control signal filter was set at a minimum of 17) and genes whose expression did not reach a threshold value for reliable detection (based on Affymetrix MAS 5.0 software) were filtered out. The remaining genes were considered informative and were subjected to a t test between two conditions (samples and controls), with the variance statistic derived from replicates. Finally, to remove false differential gene expression, a Benjamini-Hochberg multiple correction test (Benjamini and Hochberg, 1995) was applied to the list of genes generated above.
Q-PCR
A total of 13 RNA samples were incubated for 30 min at 37° C with 2 units of RNase-free DNase (Ambion, Austin, Tex.) to allow for DNA degradation. Total RNA (1 μg) was processed to cDNA by reverse transcription with Superscript II (Invitrogen Ltd., Paisley, UK) following manufacturer’s instructions in a total volume of 20 μl. Q-PCR was performed by using an ABI Prism 7000 Sequence Detection System (PE Applied Biosystems, Foster City, Calif.). We performed Q-PCR of p63 FN1 WNT5A SCHIP-1, and EB1 in eight samples (Table 2). Gene-specific oligonucleotide probes with 5′ fluorescent and 3′ quencher dyes (TaqMan probes, Applied Biosystems), and primers were obtained from the Assays on Demand choice of Applied Biosystems (www.appliedbiosystems.com). The TaqMan Universal PCR Master Mix with AmpErase UNG was used in a 20-μl reaction volume following cycling conditions recommended by the manufacturer (Applied Biosystems). Each reaction was carried out in duplicate, together with a negative (H2O as template) and a reverse-transcriptase control (each cDNA synthesis reaction was carried out in the absence of reverse transcriptase). To compensate for RNA degradation and variability in the starting amounts of RNA, the β-actin gene was used as an endogenous control (Applied Biosystems; www.appliedbiosystems.com). The amplification values obtained from each of the five genes used in this analysis were divided by the corresponding amplification values for β-actin to produce an expression index.
Table 2.
Fold change expression ratios derived from microarray analysis and Q-PCR of six genes found to be abnormally expressed by microarray analysisa
| Gene Sample | p63 | FN1 | WNT5A | SCHIP-1 | EB1 | CBX7 |
|---|---|---|---|---|---|---|
| A. Microarray Analysis | ||||||
| IN2931 | 4.3 | 5.2 | 4.9 | −5.9 | −8.6 | −7.8 |
| IN2935 | 4.2 | 3.43 | 9.4 | −4.4 | −4.7 | −3.7 |
| IN2939 | 2.5 | 3.2 | 4.2 | −3.9 | −7.2 | −2.7 |
| IN3037 | 3.8 | 14.0 | 7.0 | −6.0 | −8.7 | −6.4 |
| IN3087 | 2.1 | 4.5 | 8.3 | −3.7 | −5.1 | −2.9 |
| IN3108 | 4.8 | 7.1 | 13.9 | −7.7 | −9.9 | −10.5 |
| B. Q-PCR Analysis | ||||||
| IN772 | 37.1 | 30.2 | 345.9 | −15.2 | −4.0 | −19.3 |
| IN2242 | 21.3 | 27.7 | 262.0 | −15.3 | −6.3 | −37.6 |
| IN2376 | 17.0 | 14.5 | 6.5 | −14.4 | −2.8 | −4.4 |
| IN2767 | 8.6 | 23.3 | 24.8 | −14.7 | −3.2 | −56.0 |
| IN3008 | 12.7 | 43.4 | 5.4 | −26.3 | −4.6 | −5.6 |
| IN3071 | 13.1 | 40.5 | 14.6 | −23.2 | −2.4 | −22.0 |
| IN3121 | 24.5 | 46.3 | 20.1 | −7.1 | −2.0 | −5.8 |
| IN3125 | 4.3 | 2.0 | 69.0 | −8.0 | −3.1 | −35.2 |
| IN1134 | ND | ND | ND | ND | ND | −11.0 |
| IN1231 | ND | ND | ND | ND | ND | −4.0 |
| IN1258 | ND | ND | ND | ND | ND | −20.4 |
| IN1759 | ND | ND | ND | ND | ND | −3.7 |
| IN2443 | ND | ND | ND | ND | ND | −17.0 |
Abbreviations: ND, reaction not done; Q-PCR, quantitative real-time polymerase reaction analysis.
Fold change expression ratios were obtained as follows: (A) By microarray analysis. Hybridization intensities normalized by GeneSpring v. 4.2.1 (Silicon Genetics) from each tumor sample were divided by the mean normalized hybridization intensity of all three controls. (B) By Q-PCR on a different set of ependymoma samples. Each Q-PCR value obtained per gene per neoplastic sample was first normalized with the corresponding mean (β-actin value obtained per sample and subsequently divided by the mean value obtained from the normalized control brain Q-PCR reaction for each of the six genes listed).
Expression analysis of the CBX7 gene was carried out for 13 samples in multiplex reactions. Selection of PCR primers and MGB probe sequences was performed with the ABI Primer Express software (version 5.1, Applied Biosystems). Primers (MWG-Biotech UK Ltd., Milton Keynes, UK) were as follows: CBX7 forward 5′-CCG ACC CCT CCC AGA TAC A-3′, CBX7 reverse 5′-CTT CCT TTG CAC AGA ATG AGC TT-3′, and the MGB probe 5′-AGT CTG AAC AAA GCT C-3′, and they were labeled with FAM. The sequences of primers and the MGB probe for the β-actin gene were as follows: β-actin forward 5′-ACG AGG CCC AGA GCA AGA G-3′, β-actin reverse 5′-GAC GAT GCC GTG CTC GAT-3′ and the MGB probe 5′-CAC CCT GAA GTA CCC-3′ and was labeled with VIC. Q-PCR reactions were carried out in triplicate in 25-μl volumes consisting of 1× Quantitect probe PCR mix (Qiagen), 75 nM β-actin primers, 900 nM CBX7 primers, 25 ng DNA, and 200 nM MGB probe. The thermal cycling conditions were as follows: 15 min at 95°C, followed by 40 cycles at two temperatures, 95°C for 10 s and 60°C for 1 min. Prior to expression quantification, primer limitation experiments were carried out in a matrix of forward and reverse primer concentrations (12.5–100 nM) to check that the two independent reactions did not compete. To determine changes in expression levels of CBX7 in relation to β-actin we used the comparative CT method (ΔΔCT method), where target sequence copy number of the CBX7 gene (target) in the tumor samples is compared to the relative amount of CBX7 in normal cDNA from pooled corpus callosum samples (calibrator) and relative to expression of the β-actin gene, which was used as an endogenous control (reference). Relative copy number of CBX7 in tumor and the corpus callosum samples is given by 2ΔΔCT, where
and each ΔCT = CTtarget − CTreference (Applied Biosystems, 1997).
LOH Analysis by Q-PCR
High-molecular-weight DNA was extracted from a total of eight biopsies, three short-term cell cultures, and 20 blood samples with the Qiagen genomic DNA kit following manufacturer’s instructions (Qiagen).
LOH analysis of the CBX7 gene was performed with the ABI Prism 7000 Sequence Detector System (Applied Biosystems). Reactions were carried out in quadruplets following the ΔΔCT method (see Q-PCR section, above) to determine CBX7 copy number in 11 neoplastic samples relative to normal DNA obtained from 20 pooled blood samples. Prior to carrying out this Q-PCR experiment, DNA quality was assessed by analyzing the PCR amplification in 2.5% agarose gel electrophoresis stained with ethidium bromide (results not shown).
Mutation Screening
The CBX7 gene was sequenced in seven samples previously shown to have both copies or LOH for the CBX7 gene (Table 1). Three pairs of primers were designed to amplify the coding region of CBX7 using the Primer3 program developed by the Whitehead Institute for Biomedical Research (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) (Table 3). Amplifications were performed in a 20-μl reaction volume containing 20 ng cDNA (see above), 10 mM each of dNTPs, 10-pmol primers, 2 μl 10 × Qiagen PCR buffer containing 15 mM MgCl2 and 1 unit of Qiagen HotStarTaq DNA polymerase (Qiagen). An initial denaturation step of 15 min at 95°C was followed by a sequence of 15 s at 95°C, 30 s at 56°C/58°C/60°C (see Table 3), and 45 s at 72°C for 35 cycles, with a final extension step at 72°C for 7 min. Amplification products were run in 1.5% agarose gels and purified with the QIAquick PCR purification kit. Sequencing reactions were performed with the Bigdye terminator v1.1 cycle sequencing kit following manufacturer’s instructions (PE Applied Biosystems, Foster City, Calif.). Reactions were run on a 377 ABI Prism bioanalyzer (PE Applied Biosystems), and sequences were analyzed with Sequencher 3.0 sequence analysis (Gene Codes Corporation, Ann Arbor, Mich.).
Table 3.
PCR primers primers used used in mutation screening of of thethe CBX7 gene (gi: 46852393) coding region
| Primer | Sequence | 3′ end of primer position | PCR fragment length | Ann.temp. (°C) |
|---|---|---|---|---|
| CBX7F1 | cccgcatggagctgtca | −5 (upstream) | 413 | 56 |
| CBX7R1 | agggcagggtgggcac | +393 | 56 | |
| CBX7F2 | gaagctctgcttctccctgac | +296 | 412 | 60 |
| CBX7R2 | atggagttggcggtgatgt | +688 | 60 | |
| CBX7F3 | ccctgaagaggaggcagat | +584 | 249 | 58 |
| CBX7R3 | cccccaacccatccctat | +833 | 58 |
Abbreviation: Ann. temp., annealing temperature.
Results
Gene Expression Profiles in Ependymoma
Using the GeneChip Affymetrix U95Av2 array technology, we studied differences in expression of >12,000 genes from six pediatric ependymoma samples and three samples derived from normal brain. Filtering was performed to remove genes whose expression in ependymoma samples did not differ from that in controls and genes whose hybridization signals fell below the threshold for reliable detection using GeneSpring v. 4.2.1. (Silicon Genetics). Further analysis was performed using a t test with P value cutoff at 0.05. Finally, to reduce false positives, a Benjamini-Hochberg multiple correction test (Benjamini and Hochberg, 1995) was applied to generate a final list of genes consisting of 112 transcripts. To establish fold change in expression levels, we compared the mean normalized value of each gene in the ependymoma cohort to the mean normalized value of each gene in the control cohort. A total of 26 genes were overexpressed ⩾3-fold P < 0.05) in ependymoma. Genes with increased expression included many encoding adhesion and extracellular matrix proteins such as FN1 and p63 (Korshunov et al., 2003) and the transcription factors Zic1. Overexpressed genes previously associated with brain tumors included the angiogenesis factor VEGF (Korshunov et al., 2002) and the oncogene WNT5A (Howng et al., 2002).
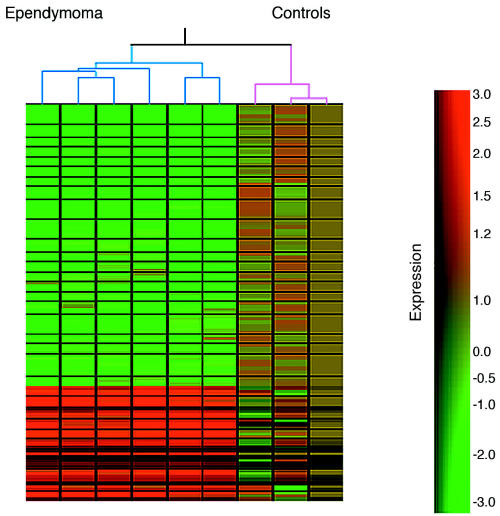
A total of 84 genes were underexpressed ⩾2-fold in the neoplastic group (P < 0.05). These comprised the NF2 interacting protein coding gene SCHIP-1 and several genes involved in vesicle trafficking and recycling, such as NPC1, RAB40B, TJ2, SH3GL3, and EB1. A full list of the genes described above with associated statistical values is available (http://www.ion.ucl.ac.uk/molpat/neuro-oncology/secured/microarray.html). To visualize the relationship between gene expression and sample identity we performed unsupervised hierarchical clustering per gene and per experiment with the 112 genes described above using GeneSpring v.4.2.1 (Silicon Genetics). Ependymoma and control groups clustered into two different branches of the tree, suggesting the data set identified in this study is likely to be involved in ependymoma genesis and progression (Fig. 1).
Fig. 1.
Unsupervised hierarchical clustering of six ependymoma samples (indicated with blue brackets at top of figure) and three controls (indicated with pink brackets). Cluster analysis was performed with the 112 genes that comprised the group of differentially expressed genes in neoplastic and nonneoplastic samples as computed by the predictive software. Color saturation is proportional to magnitude of the difference from the mean, and ranging from green (underexpressed) to red (overexpressed). See bar at right of figure.
Identification of Differentially Expressed Genes Located in CGH Hot Spots
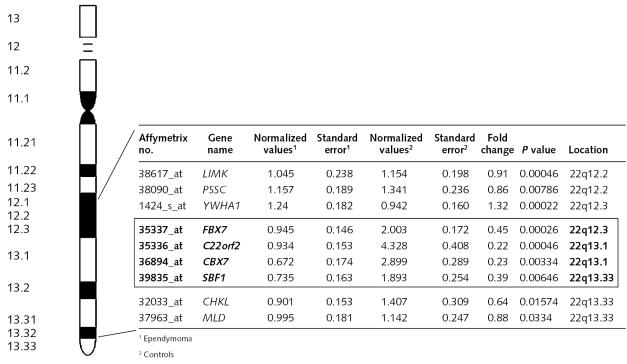
By CGH analysis, we and others have identified gains at 1q and losses at 6q and 22q as the most common genomic imbalances in ependymoma (Mazewski et al., 1999; Reardon et al., 1999; Ward et al., 2001). Our subset of 112 genes included a group of four underexpressed genes mapping to 22q12.3-22q13.3, which comprised the F-box protein coding gene FBX7, the uncharacterized transcript C22orf2, the chromobox protein coding gene CBX7, and the SET domain-binding protein coding gene SBF1. Expression analysis of other genes mapping to 22q12.3-22q13.3 was, in most cases, uninformative, as transcripts were either not present in U95Av2 or not expressed in neoplastic and control samples. However, we observed normal expression of a group of five genes flanking the set FBX7-C22orf2-CBX7-SBF1, suggesting the presence of a microdeletion (Fig. 2). Within the 112 subset, we also identified three underexpressed genes mapping to 6q, namely, the polyamine biosynthesis gene AMD1 (6q21), the cyclin-dependent kinase CDK11 (6q21), and the tumor suppressor gene SASH1 (6q24.3), and two overexpressed genes mapping to 1q, namely, laminin (1q31) and the glioma amplified gene GAC1, mapping to the 1q32-q41 amplicon (Bhattacharjee et al., 1997; Kramer et al., 1998; Ward et al., 2001).
Fig. 2.
Expression pattern of a subset of four genes found to be underexpressed in ependymoma as compared to normal brain (shown in bold) next to a group of five flanking genes. Values are based on hybridization signals normalized as explained in Materials and Methods. Normalized values are shown as an average of 1ependymoma and 2controls. Standard error values refer to those obtained from normalized values from 1ependymoma and 2controls. Detected P values shown refer to those computed by the Affymetrix MAS 5.0 array analysis software on chip intensities. P values are represented as an average of all samples and controls. The cutoff P value to reliably detect a transcript was set at <0.05 per individual sample. Other genes mapping to 22q12.2-q13.33 were either not present in U95Av2 or had P values >0.05 by MAS 5.0 in all control and neoplastic samples and were flagged absent by the computer program. The distance of the potential micro-deletion was obtained from Project Ensembl on the Internet (http://www.ensembl.org) and is shown above the chromosome figure.
Corroboration of Gene Expression by Q-PCR
To validate the data obtained by microarray profiling, and to extend the analysis to a larger set of clinical samples, we have chosen five genes from our 112 gene list to evaluate in a further set of eight ependymoma samples (six biopsies and two short-term cell cultures) by Q-PCR (Table 2). These genes were chosen by biological relevance and chromosomal location and included the oncogene WNT5A; p63, a p53 homologue; FN1, which has been previously associated with overexpression of ERBB2 (Mackay et al., 2003); the adenomatous polyposis coli (APC)-associated gene EB1; and the NF2 interacting gene SCHIP-1. The expression of a sixth gene, CBX7, which maps to 22q13.1, was studied in an additional 13 samples. To establish the concordance between microarray and Q-PCR we produced tumor to normal ratios of the values obtained by Q-PCR for WNT5A, FN1, p63, EB1, and SCHIP-1. When these ratios were depicted together with ratios calculated from the fold changes by the GeneSpring software (Silicon Genetics), the results that were obtained followed the same trend observed in our microarray data in five out of five cases. A comparison of fold change values collected from microarray analysis and Q-PCR is shown in Table 2.
We were able to perform multiplex reactions with the CBX7 and β-actin genes and chose to carry out the expression analysis using the ΔΔCT method. First, to check that the two independent reactions do not compete, primer limitation experiments were performed using a matrix of forward and reverse primer concentrations (12.5–100 nM) for the β-actin gene. The chosen concentration was 75 nM, which produced the lowest ΔRn (magnitude of the signal generated by the given set of PCR conditions), but had little effect on CT (results not shown). Second, for the ΔΔCT calculation to be valid, both reactions should have equal efficiencies. We therefore performed a validation experiment in which PCR reactions were carried out in quadruplets for both reference (β-actin) and target (CBX7) genes in a series of seven dilutions. For both primers to be compatible, the absolute value of the slope of log input amount versus CT should be <0.1 (see the Q-PCR section in Materials and Methods). In all 13 cases, CBX7 expression in the tumors was at least 3 times lower than in the normal corpus callosum control (Table 2).
LOH Analysis by Q-PCR and Mutation Analysis
Subsequently, we investigated the copy number status of the CBX7 gene in 11 ependymoma samples, all of which were previously used either in the microarray or in the Q-PCR sections (see Table 1). The β-actin gene was used as a reference, and as a control we used DNA obtained from blood samples from 20 individuals. Results from normal DNA samples were subsequently analyzed on the ABI sequence analyzer 7000 and averaged before they were used to calculate copy number changes in the tumor samples. (For methodology, see Senchenko et al. [2003]; http://www.appliedbiosystems.com.)
From our data, the mean value from the pooled blood samples was 1 (95% reference range, 1 ± 0.16). Taking account that normal tissue contamination could reach up to 40%, one could expect values less than 0.5 and 0.9 to account for homozygous and hemizygous deletions, respectively (Senchenko et al., 2003). The Mann-Whitney nonparametric t test was applied to both groups (blood and tumor), producing highly significant results (P = 0.003). Samples with values ⩽0.84 (mean value of normal samples minus 95% reference range) were considered hemizygously deleted. Alleles were considered as homozygously deleted if their highest SDM value was <0.56 (0.4 plus 2 × SDM of controls). Our Q-PCR results suggest that 1/11 (9%) samples was homozygously deleted and 5/11 (46%) were hemizygously deleted (Table 4). These data show allelic loss at 22q13.1 in 55% of the cases, which is significantly higher than previously reported in pediatric intracranial ependymoma (Bhattacharjee et al., 1997; Carter et al., 2002; Ward et al., 2001). Mutation analysis of CBX7 in seven of the above samples which had retained one or two copies of the gene did not reveal any sequence alterations in the coding region.
Table 4.
Deletion analysis results for the CBX7 ge ene by Q-PCR on 11 ependymoma samples
| Sample ID | ΔCT | SDM ΔCT | ΔΔCT | SDM ΔΔCT | Copy no.2−Δ ΔCT | SEM 2−ΔΔCT | No. of Alleles |
|---|---|---|---|---|---|---|---|
| Controlsa | 1.23 | 0.03 | 0 | 0.13 | 1.01 | 0.02 | 2 |
| IN1134 | 1.32 | 0.06 | 0.08 | 0.06 | 0.94 | 0.01 | 2 |
| IN1258 | 1.55 | 0.09 | 0.32 | 0.09 | 0.81 | 0.01 | 1 |
| IN1759 | 1.99 | 0.01 | 0.76 | 0.01 | 0.59 | 0.01 | 1 |
| IN2931 | 1.28 | 0.04 | 0.05 | 0.15 | 0.97 | 0.02 | 2 |
| IN2935 | 1.99 | 0.06 | 0.76 | 0.06 | 0.59 | 0.01 | 1 |
| IN2939 | 2.24 | 0.08 | 1.01 | 0.08 | 0.5 | 0.01 | 0 |
| IN3087 | 1.29 | 0.1 | 0.06 | 0.08 | 0.96 | 0.01 | 2 |
| IN3108 | 1.43 | 0.12 | 0.19 | 0.1 | 0.88 | 0.01 | 2 |
| IN2376 | 1.61 | 0.06 | 0.37 | 0.12 | 0.77 | 0.01 | 1 |
| IN3121 | 1.85 | 0.00 | 0.61 | 0.02 | 0.62 | 0.00 | 1 |
| IN3125 | 1.01 | 0.00 | −0.22 | 0.09 | 1.17 | 0.00 | 2 |
Abbreviations: SDM, standard deviation from the mean; SEM, standard error of the mean. The terms CT, ΔCT, ΔΔCT, and 2−ΔΔCT are defined elsewhere (Applied Biosystems, 1997).
Control values were derived from normal DNA obtained from blood from 20 individuals.
Number of alleles was estimated as heterozygously deleted ⩽2−ΔΔCT of controls minus 95% reference range of controls (or 2 × SDM of controls), homozygously deleted ⩽0.4 + 2 × 0.08 (SDM of controls). See “LOH Analysis by Q-PCR and Mutation Analysis” in the Results section.
Discussion
Microarray technology allows the generation of multiple gene expression profiles in a single experiment, with the potential to accelerate the identification of prognostic markers and improve diagnosis and choice of therapy. Several studies have used microarray technology to identify patterns of gene expression in time course experiments (Arbeitman et al., 2002; Mandel et al., 2002), classify tumor samples (Perou et al., 2000; van ’t Veer et al., 2003), and reliably map novel genes to molecular pathways (Voehringer et al., 2000; Zhao et al., 2000), giving an unprecedented insight into the function of unknown genes and their interactions with other genes in different cellular pathways.
At the molecular level very little is known regarding the etiology of ependymoma, and to date, most studies have employed cytogenetic and CGH analysis to elucidate the genetic makeup of these tumors. The aim of the present study was to identify candidate genes responsible for pediatric ependymoma development by using a microarray-based approach, validated by Q-PCR followed by LOH analysis of a candidate gene mapping to 22q13.1. We report a group of 112 abnormally expressed genes, four of which mapping to 22q12-13, which constitutes the most recurrent region of loss in ependymoma. We have also shown that deletions in 22q in pediatric ependymoma are more common than previously reported.
Abnormally Expressed Genes in Pediatric Ependymoma
Some of the genes identified in this study have been associated with other types of cancer, particularly brain tumors, and are likely to play an important role in ependymoma development. These include COL4A1 (>29.5-fold change), IBP2 (>13.5-fold change), HOX7 (>11.5-fold change), Wee1 (>11-fold change), GAC1 (>7-fold change), WNT5A (>5.5-fold change), and FN1 (>5.5-fold change) (Almeida et al., 1998; Howng et al., 2002; Masaki et al., 2003; Wang et al., 2003; Wasenius et al., 2003). We also showed underexpression of EB1 and SCHIP-1 by both expression profiling and Q-PCR. These genes interact with the tumor suppressors APC and NF2, respectively (Renner et al., 1997; Rouleau et al., 1993). NF2 is a tumor suppressor gene responsible for an autosomal dominant disease that predisposes to the development of central nervous system tumors. Mutations in this gene have been found in intraspinal ependymoma (Alonso et al., 2002; Rubio et al., 1994) but are rarely observed in intracranial ependymomas. Although the biological function of SCHIP-1 remains unknown, our results suggest that it may play an important role in ependymoma development and should be further investigated.
One previous study by Korshunov et al. (2003) investigated gene expression in ependymoma by comparing a heterogeneous mix of spinal and intracranial biopsy samples to a pooled control comprising 10 different cell lines. The authors were able to differentiate between spinal/intracranial, adult/pediatric, and benign/anaplastic samples and concluded that subgroups of ependymoma patients may be suffering from molecularly distinct diseases (Carter et al., 2002; Korshunov et al., 2002, 2003). In the present study we have produced a molecular signature of pediatric intracranial ependymoma compared to three biopsy controls to identify genes involved in tumor development. We did not observe abnormal expression of other genes found to be abnormally expressed in other types of brain tumors such as EGFR, PDGF, PDGFR, CDK2, Rb, MDM2, and PTEN, and we therefore postulate that development of ependymoma in pediatric patients may depend on alternative molecular pathways.
Expression of Genes Located in Common Regions of Loss or Gain
By cytogenetic analysis, we and others have identified gains and amplifications at 1q and losses at 6q and 22q as the most common genomic imbalances in ependymoma (Mazewski et al., 1999; Reardon et al., 1999; Ward et al., 2001). Chromosome 22q has also been involved in translocations and interstitial deletions in ependymoma, both in sporadic and familial cases, supporting the presence of a tumor suppressor gene at this location (Nijssen et al., 1994; von Haken et al., 1996). By cytogenetic analysis, monosomy 22 has been reported in up to 31% of pediatric samples (Mazewski et al., 1999), compared to an incidence of 56% in adult samples (Kramer et al., 1998).
Four candidate genes have previously been proposed as potential tumor suppressors mapping to 22q. One such gene, NF2, is mutated in sporadic cases of NF2 associated schwannomas and meningiomas but has been rarely reported in astrocytic tumors and ependymomas (Rubio et al., 1994). Another candidate gene is the tumor suppressor hSNF5/INI1, found to be mutated in pediatric rhabdoid tumors, atypical teratoid and rhabdoid tumors of the brain, renal and extrarenal rhabdoid tumors, choroid plexus tumors, and primitive neuroectodermal tumors (Biegel et al., 1999; Sevenet et al., 1999; Versteege et al., 1998). However, screening of the hSNF5/INI gene in 52 ependymomas failed to identify any mutations (Sevenet et al., 1999). Chk2 and EP300, also mapping to 22q, have been associated with various types of cancers, such as gastric carcinoma and colorectal and breast cancer, but so far no association between Chk2 and EP300 and brain tumors has been found (Bryan et al., 2002; Hartmann et al., 2004; Muraoka et al., 1996; Vahteristo et al., 2002). Recently, by using high-resolution mapping analysis of 22q, two groups have shown 22q12.3-22q13.2 to be a critical region associated with astrocytoma grade and progression (Ino et al., 1999; Oskam et al., 2000).
In this study we have identified a group of four under-expressed genes mapping to 22q12-q13.1, suggesting the presence of a microdeletion at this site of 22q. By Q-PCR we have shown that CBX7 is also underexpressed in 13 further ependymoma samples as compared to a commercially available control from normal human brain.
The use of Q-PCR in the detection of genomic deletions has been applied in a number of cancer studies (Braga et al., 2002; M’soka et al., 2000; Senchenko et al., 2003). Recently, Senchenko et al. (2003) showed that this technique is sensitive enough to distinguish between one and two alleles by examining differences in gene dosage between the X and Y chromosomes. We used Q-PCR to show that abnormal expression of CBX7 was due to allelic loss in 55% of cases (6/11), where under-expression was due to loss of one allele in 46% of cases and both alleles in 9% of cases. Subsequently, we performed mutation analysis of the coding region of the CBX7 gene in seven samples, showing the presence of one or two copies by Q-PCR. We were unable to identify any sequence alteration of the CBX7 coding region, which suggests that other mechanisms, such as promoter methylation or histone deacetylation, may be responsible for the silencing of this gene in our ependymoma samples. Extensive methylation of promoters and regulatory sequences that control gene activity have previously been reported near tumor suppressor genes (Esteller et al., 2000a, b; Herman and Baylin, 2000). Histone acetylation and deacetylation are major regulatory mechanisms of transcription that function by modulating the accessibility of transcription factors to their binding site on DNA. An example of such a mechanism is performed by the Rb gene, which transmits active repression to E2F-responsive genes by modifying chromatin architecture (Brehm et al., 1998; Magnaghi-Jaulin et al., 1998).
CBX7 belongs to a family of protein coding genes containing the chromodomain motif, which are involved in gene silencing by mediating changes in higher order chromatin structure (Paro and Hogness, 1991). CBX7 is highly expressed in a number of different normal tissue types, including brain, kidney, heart, and skeletal muscle, although it has not previously been investigated in tumor cells (Gil et al., 2004). A recent study has demonstrated that CBX7 expression is associated with extension of cellular life span in mouse embryonic fibroblasts and human prostate primary epithelial cells by downregulating expression of the Ink4a/Arf locus (Gil et al., 2004). The role of the p16Ink4a/Rb and Arf/p53 pathways in ependymoma is unclear. Although gene mutations are rare, hypermethylation of p16Ink4a, Rb, and p14ARF has been reported in a subgroup of tumors (4%–32%) (Alonso et al., 2003, 2004; Sato et al., 1996; Tong et al., 1999). However, in view of our findings that CBX7 was consistently underexpressed in 19 ependymoma samples and that at least one copy is deleted in 55% of cases examined, further work is required to discern an alternative mechanism for CBX7 function in these tumors.
Loss of 6q was the most common genomic imbalance in one study, present in 23% of ependymoma (Reardon et al., 1999). In our CGH analysis, loss of 6q was the sole abnormality in one case (Ward et al., 2001). We have found underexpression of three genes located on 6q by microarray analysis; two such genes, ADM1 and CDK11, map at 6q21 ~200 kb away from each other (http://www.ensembl.org). Statistical analysis of microarray data did not detect any further underexpressed genes mapping to 6q21. A third gene, namely SASH1 (6q24), also underexpressed in our ependymoma cohort, has recently been reported to be downregulated in breast cancer (Zeller et al., 2003).
Gains of 1q constitute the most common region of gain in ependymoma (Carter et al., 2002; Ward et al., 2001) and have also been involved with drug resistance (Muleris et al., 1994; Schrock et al., 1994) and poor prognosis in other types of solid tumors (Gronwald et al., 1997; Nishida et al., 2003; Petersen et al., 2000). We identified two overexpressed genes mapping to 1q, namely laminin and GAC1, which represent potential oncogenes mapping to the 1q32 amplicon.
Our knowledge regarding the molecular pathways leading to ependymoma development is scarce. In this study, we present a group of 112 genes that are abnormally expressed in ependymoma and that may contribute to the development of this pediatric brain tumor. We have also validated the expression of five genes, namely WNT5A, FN1, p63, SCHIP-1, and EB1, in a further eight samples. These genes have potential roles in cancer development and have not been previously involved in ependymoma. The expression of a sixth gene, CBX7 which maps to the 22q13.1 CGH hot spot, was further analyzed in 13 samples by Q-PCR. We have identified a cluster of abnormally expressed genes mapping to 1q21-q31, 6q21-24, and 22q12-q13 that constitute the most common regions of genomic imbalance identified by CGH. We also provide evidence that underexpression of one gene, CBX7, was due to homozygous allele loss in 9% of cases and heterozygous loss in 45%, an overall incidence of 55%, higher than previously reported following other methodologies (Table 4). Q-PCR is emerging as an alternative method to microsatellite deletion mapping and CGH to detect copy number changes (Braga et al., 2002; Senchenko et al., 2003). CGH is limited by poor resolution of 20 Mb for losses, and microsatellite mapping requires the identification of polymorphic markers and paired normal somatic tissue. Homozygous deletions are excellent indicators of the location of tumor suppressor genes, and therefore, the genes identified in this study mapping to hot spot CGH regions should be further investigated, as they constitute potential tumor suppressors responsible for the neoplastic phenotype in pediatric ependymoma patients.
Acknowledgment
The authors acknowledge Anthony Lynch and Catherine Smith at GlaxoSmithKline UK for helpful assistance and discussions on Q-PCR technology.
Footnotes
This work was supported by the Brain Research Trust and Charlie’s Challenge.
Supplemental data are available at http://neuro-oncology.dukejournals.org/cgi/content/full/7/1/20/DC1/1 on request to authors.
Abbreviations used are as follows: ΔΔCT method, comparative CT method; APC, adenomatous polyposis coli CGH, comparative genomic hybridization; LOH, loss of heterozygosity; Q-PCR, quantitative real-time polymerase chain reaction analysis; SDM, standard deviation from the mean.
References
- Affymetrix (2004) GeneChip Expression Analysis Technical Manual. Affymetrix, Santa Clara, Calif.
- Almeida A, Zhu XX, Vogt N, Tyagi R, Muleris M, Dutrillaux AM, Dutrillaux B, Ross D, Malfoy B, Hanash S. GAC1, a new member of the leucine-rich repeat superfamily on chromosome band 1q32.1, is amplified and overexpressed in malignant gliomas. Oncogene. 1998;16:2997–3002. doi: 10.1038/sj.onc.1201828. [DOI] [PubMed] [Google Scholar]
- Alonso ME, Bello MJ, Arjona D, Gonzalez-Gomez P, Lomas J, de Campos JM, Kusak ME, Isla A, Rey JA. Analysis of the NF2 gene in oligodendrogliomas and ependymomas. Cancer Genet Cytogenet. 2002;134:1–5. doi: 10.1016/s0165-4608(01)00591-x. [DOI] [PubMed] [Google Scholar]
- Alonso ME, Bello MJ, Gonzalez-Gomez P, Arjona D, Lomas J, de Campos JM, Isla A, Sarasa JL, Rey JA. Aberrant promoter methylation of multiple genes in oligodendrogliomas and ependymomas. Cancer Genet Cytogenet. 2003;144:134–142. doi: 10.1016/s0165-4608(02)00928-7. [DOI] [PubMed] [Google Scholar]
- Alonso ME, Bello MJ, Gonzalez-Gomez P, Arjona D, de Campos JM, Gutierrez M, Rey JA. Aberrant CpG island methylation of multiple genes in ependymal tumors. J Neurooncol. 2004;67:159–165. doi: 10.1023/b:neon.0000021862.41799.f7. [DOI] [PubMed] [Google Scholar]
- Applied Biosystems (1997) User Bulletin #2, Relative Quantitation of Gene Expression. Foster City, Calif.: Applied Biosystems.
- Arbeitman MN, Furlong EE, Imam F, Johnson E, Null BH, Baker BS, Krasnow MA, Scott MP, Davis RW, White KP. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–2275. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57:289–300. [Google Scholar]
- Bhattacharjee MB, Armstrong DD, Vogel H, Cooley LD. Cytogenetic analysis of 120 primary pediatric brain tumors and literature review. Cancer Genet Cytogenet. 1997;97:39–53. doi: 10.1016/s0165-4608(96)00330-5. [DOI] [PubMed] [Google Scholar]
- Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- Bigner SH, McLendon RE, Fuchs H, McKeever PE, Friedman HS. Chromosomal characteristics of childhood brain tumors. Cancer Genet Cytogenet. 1997;97:125–134. doi: 10.1016/s0165-4608(96)00404-9. [DOI] [PubMed] [Google Scholar]
- Braga E, Senchenko V, Bazov I, Loginov W, Liu J, Ermilova V, Kazubskaya T, Garkavtseva R, Mazurenko N, Kisseljov F, Lerman MI, Klein G, Kisselev L, Zabarovsky ER. Critical tumor-suppressor gene regions on chromosome 3p in major human epithelial malignancies: Allelotyping and quantitative real-time PCR. Int J Cancer. 2002;100:534–541. doi: 10.1002/ijc.10511. [DOI] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FCP, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genetics. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- Brehm A, Miska EA, McCance DJ, Reid JL, Bannister AJ, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- Bryan EJ, Jokubaitis VJ, Chamberlain NL, Baxter SW, Dawson E, Choong DY, Campbell IG. Mutation analysis of EP300 in colon, breast and ovarian carcinomas. Int J Cancer. 2002;102:137–141. doi: 10.1002/ijc.10682. [DOI] [PubMed] [Google Scholar]
- Carter M, Nicholson J, Ross F, Crolla J, Allibone R, Balaji V, Perry R, Walker D, Gilbertson R, Ellison DW. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br J Cancer. 2002;86:929–939. doi: 10.1038/sj.bjc.6600180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer S, Prebble E, Davison V, Davies P, Ramani P, Ellison D, Grundy R. Genomic imbalances in pediatric intracranial ependymomas define clinically relevant groups. Am J Pathol. 2002;161:2133–2141. doi: 10.1016/S0002-9440(10)64491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrskjot L, Thykjaer T, Kruhoffer M, Jensen JL, Marcussen N, Hamilton-Dutoit S, Wolf H, Orntoft TF. Identifying distinct classes of bladder carcinoma using microarrays. Nat Genet. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, Bussaglia E, Prat J, Harkes IC, Repasky EA, Gabrielson E, Schutte M, Baylin SB, Herman JG. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000a;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ, Baylin SB, Herman JG. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000b;60:4366–4371. [PubMed] [Google Scholar]
- Gil J, Bernard D, Martinez D, Beach D. Polycomb CBX7 has a unifying role in cellular lifespan. Nat Cell Biol. 2004;6:67–72. doi: 10.1038/ncb1077. [DOI] [PubMed] [Google Scholar]
- Gilbertson RJ, Bentley L, Hernan R, Junttila TT, Frank AJ, Haapasalo H, Connelly M, Wetmore C, Curran T, Elenius K, Ellison DW. ERBB receptor signaling promotes ependymoma cell proliferation and represents a potential novel therapeutic target for this disease. Clin Cancer Res. 2002;8:3054–3064. [PubMed] [Google Scholar]
- Gronwald J, Storkel S, Holtgreve-Grez H, Hadaczek P, Brinkschmidt C, Jauch A, Lubinski J, Cremer T. Comparison of DNA gains and losses in primary renal clear cell carcinomas and metastatic sites: Importance of 1q and 3p copy number changes in metastatic events. Cancer Res. 1997;57:481–487. [PubMed] [Google Scholar]
- Hartmann C, Numann A, Mueller W, Holtkamp N, Simon M, von Deimling A. 2004. Fine mapping of chromosome 22q tumor suppressor gene candidate regions in astrocytoma. 844;Int J Cancer:108–839. doi: 10.1002/ijc.11638. [DOI] [PubMed] [Google Scholar]
- Heideman RL. Tumors of the central nervous system in children. Rocky Mt Med J. 1979;77:27A–32A. [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Promoter-region hypermethylation and gene silencing in human cancer. Curr Top Microbiol Immunol. 2000;249:35–54. doi: 10.1007/978-3-642-59696-4_3. [DOI] [PubMed] [Google Scholar]
- Howng SL, Wu CH, Cheng TS, Sy WD, Lin PC, Wang C, Hong YR. Differential expression of Wnt genes, beta-catenin and E-cadherin in human brain tumors. Cancer Lett. 2002;183:95–101. doi: 10.1016/s0304-3835(02)00085-x. [DOI] [PubMed] [Google Scholar]
- Ino Y, Silver JS, Blazejewski L, Nishikawa R, Matsutani M, von Deimling A, Louis DN. 1999. Common regions of deletion on chromosome 22q12.3-q13.1 and 22q13.2 in human astrocytomas appear related to malignancy grade. 885;J Neuropathol Exp Neurol:58–881. doi: 10.1097/00005072-199908000-00010. [DOI] [PubMed] [Google Scholar]
- Kamiya M, Nakazato Y. The expression of p73, p21 and MDM2 proteins in gliomas. J Neurooncol. 2002;59:143–149. doi: 10.1023/a:1019633910603. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58:255–270. doi: 10.1023/a:1016222202230. [DOI] [PubMed] [Google Scholar]
- Korshunov A, Neben K, Wrobel G, Tews B, Benner A, Hahn M, Golanov A, Lichter P. Gene expression patterns in ependymomas correlate with tumor location, grade, and patient age. Am J Pathol. 2003;163:1721–1727. doi: 10.1016/S0002-9440(10)63530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer DL, Parmiter AH, Rorke LB, Sutton LN, Biegel JA. Molecular cytogenetic studies of pediatric ependymomas. J Neurooncol. 1998;37:25–33. doi: 10.1023/a:1005925613992. [DOI] [PubMed] [Google Scholar]
- Lewandowicz GM, Harding B, Harkness W, Hayward R, Thomas DG, Darling JL. Chemosensitivity in childhood brain tumours in vitro: Evidence of differential sensitivity to lomustine (CCNU) and vincristine. Eur J Cancer. 2000;36:1955–1964. doi: 10.1016/s0959-8049(00)00245-8. [DOI] [PubMed] [Google Scholar]
- Ljubimova JY, Khazenzon NM, Chen Z, Neyman YI, Turner L, Riedinger MS, Black KL. 2001. Gene expression abnormalities in human glial tumors identified by gene array. 295;Int J Oncol:18–287. doi: 10.3892/ijo.18.2.287. [DOI] [PubMed] [Google Scholar]
- Luo JH. Gene expression alterations in human prostate cancer. Drugs Today (Barc) 2002;38:713–719. doi: 10.1358/dot.2002.38.10.704653. [DOI] [PubMed] [Google Scholar]
- Mackay A, Jones C, Dexter T, Silva RL, Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, Reis LF, Lakhani SR, O’Hare MJ. cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene. 2003;22:2680–2688. doi: 10.1038/sj.onc.1206349. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L, Groisman R, Naguibneva I, Robin P, Lorain S, Le Villain JP, Troalen F, Trouche D, Harel-Bellan A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature. 1998;391:601–605. doi: 10.1038/35410. [DOI] [PubMed] [Google Scholar]
- Mandel S, Grunblatt E, Maor G, Youdim MB. Early and late gene changes in MPTP mice model of Parkinson’s disease employing cDNA microarray. Neurochem Res. 2002;27:1231–1243. doi: 10.1023/a:1020989812576. [DOI] [PubMed] [Google Scholar]
- Masaki T, Shiratori Y, Rengifo W, Igarashi K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y, Yoshiji H, Watanabe S, Omata M, Kuriyama S. 2003. Cyclins and cyclin-dependent kinases: Comparative study of hepatocellular carcinoma versus cirrhosis. 543;Hepatology:37–534. doi: 10.1053/jhep.2003.50112. [DOI] [PubMed] [Google Scholar]
- Mazewski C, Soukup S, Ballard E, Gotwals B, Lampkin B. Karyotype studies in 18 ependymomas with literature review of 107 cases. Cancer Genet Cytogenet. 1999;113:1–8. doi: 10.1016/s0165-4608(99)00046-1. [DOI] [PubMed] [Google Scholar]
- M’soka TJ, Nishioka J, Taga A, Kato K, Kawasaki H, Yamada Y, Yu A, Komada Y, Nobori T. Detection of methylthio-adenosine phosphorylase (MTAP) and p16 gene deletion in T cell acute lymphoblastic leukemia by real-time quantitative PCR assay. Leukemia. 2000;14 :935–940. doi: 10.1038/sj.leu.2401771. [DOI] [PubMed] [Google Scholar]
- Muleris M, Almeida A, Dutrillaux AM, Pruchon E, Vega F, Delattre JY, Poisson M, Malfoy B, Dutrillaux B. Oncogene amplification in human gliomas: A molecular cytogenetic analysis. Oncogene. 1994;9:2717–2722. [PubMed] [Google Scholar]
- Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–1569. [PubMed] [Google Scholar]
- Nijssen PC, Deprez RH, Tijssen CC, Hagemeijer A, Arnoldus EP, Teepen JL, Holl R, Niermeyer MF. Familial anaplastic ependymoma: Evidence of loss of chromosome 22 in tumour cells. J Neurol Neurosurg Psychiatry. 1994;57:1245–1248. doi: 10.1136/jnnp.57.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Nishimura T, Ito T, Komeda T, Fukuda Y, Nakao K. Chromosomal instability and human hepatocarcinogenesis. Histol Histopathol. 2003;18:897–909. doi: 10.14670/HH-18.897. [DOI] [PubMed] [Google Scholar]
- Oskam NT, Bijleveld EH, Hulsebos TJ. A region of common deletion in 22q13.3 in human glioma associated with astrocytoma progression. Int J Cancer. 2000;85:336–339. [PubMed] [Google Scholar]
- Paro R, Hogness DS. The Polycomb protein shares a homologous domain with a heterochromatin-associated protein of Drosophila. Proc Natl Acad Sci USA. 1991;88:263–267. doi: 10.1073/pnas.88.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Petersen I, Hidalgo A, Petersen S, Schluns K, Schewe C, Pacyna-Gengelbach M, Goeze A, Krebber B, Knosel T, Kaufmann O, Szymas J, von Deimling A. Chromosomal imbalances in brain metastases of solid tumors. Brain Pathol. 2000;10:395–401. doi: 10.1111/j.1750-3639.2000.tb00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, Gerszten PC, Martinez AJ, Lo KH, Shultz B, Albright AL, Janosky J, Deutsch M. Intracranial ependymomas of childhood: Long-term outcome and prognostic factors. Neurosurgery. 1995;37:655–666. doi: 10.1227/00006123-199510000-00008. [DOI] [PubMed] [Google Scholar]
- Reardon DA, Entrekin RE, Sublett J, Ragsdale S, Li H, Boyett J, Kepner JL, Look AT. Chromosome arm 6q loss is the most common recurrent autosomal alteration detected in primary pediatric ependymoma. Genes Chromosomes Cancer. 1999;24:230–237. doi: 10.1002/(sici)1098-2264(199903)24:3<230::aid-gcc8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Renner C, Pfitzenmeier JP, Gerlach K, Held G, Ohnesorge S, Sahin U, Bauer S, Pfreundschuh M. RP1, a new member of the adenomatous polyposis coli-binding EB1-like gene family, is differentially expressed in activated T cells. J Immunol. 1997;159:1276–1283. [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, Pulst SM, Lenoir G, Bijlsma E, Fashold R, Dumanski J, de Jong P, Parry D, Eldrige R, Aurias A, Delattre O, Thomas G. Alteration in a new gene encoding a putative membrane-organizing protein causes neurofibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Rubio MP, Correa KM, Ramesh V, MacCollin MM, Jacoby LB, von Deimling A, Gusella JF, Louis DN. Analysis of the neurofibromatosis 2 gene in human ependymomas and astrocytomas. Cancer Res. 1994;54:45–47. [PubMed] [Google Scholar]
- Sato K, Schauble B, Kleihues P, Ohgaki H. Infrequent alterations of the p15, p16, CDK4 and cyclin D1 genes in non-astrocytic human brain tumors. Int J Cancer. 1996;66:305–308. doi: 10.1002/(SICI)1097-0215(19960503)66:3<305::AID-IJC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schrock E, Thiel G, Lozanova T, du Manoir S, Meffert MC, Jauch A, Speicher MR, Nurnberg P, Vogel S, Janisch W. Comparative genomic hybridization of human malignant gliomas reveals multiple amplification sites and nonrandom chromosomal gains and losses. Am J Pathol. 1994;144:1203–1218. [PMC free article] [PubMed] [Google Scholar]
- Senchenko V, Liu J, Braga E, Mazurenko N, Loginov W, Seryogin Y, Bazov I, Protopopov A, Kisseljov FL, Kashuba V, Lerman MI, Klein G, Zabarovsky ER. Deletion mapping using quantitative real-time PCR identifies two distinct 3p21.3 regions affected in most cervical carcinomas. Oncogene. 2003;22:2984–2992. doi: 10.1038/sj.onc.1206429. [DOI] [PubMed] [Google Scholar]
- Sevenet N, Lellouch-Tubiana A, Schofield D, Hoang-Xuan K, Gessler M, Birnbaum D, Jeanpierre C, Jouvet A, Delattre O. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum Mol Genet. 1999;8:2359–2368. doi: 10.1093/hmg/8.13.2359. [DOI] [PubMed] [Google Scholar]
- Slavc I, MacCollin MM, Dunn M, Jones S, Sutton L, Gusella JF, Biegel JA. Exon scanning for mutations of the NF2 gene in pediatric ependymomas, rhabdoid tumors and meningiomas. Int J Cancer. 1995;64:243–247. doi: 10.1002/ijc.2910640406. [DOI] [PubMed] [Google Scholar]
- Suzuki SO, Iwaki T. Amplification and overexpression of mdm2 gene in ependymomas. Mod Pathol. 2000;13:548–553. doi: 10.1038/modpathol.3880095. [DOI] [PubMed] [Google Scholar]
- Tong CY, Ng HK, Pang JC, Hui AB, Ko HC, Lee JC. Molecular genetic analysis of non-astrocytic gliomas. Histopathology. 1999;34:331–341. doi: 10.1046/j.1365-2559.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- Vahteristo P, Bartkova J, Eerola H, Syrjakoski K, Ojala S, Kilpivaara O, Tamminen A, Kononen J, Aittomaki K, Heikkila P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H. A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet. 2002;71:432–438. doi: 10.1086/341943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Bernards R, Friend SH. Expression profiling predicts outcome in breast cancer. Breast Cancer Res. 2003;5:57–58. doi: 10.1186/bcr562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O. 1998. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. 206;Nature:394–203. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, Herzenberg LA, Steinman L, Herzenberg LA. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci USA. 2000;97:2680–2685. doi: 10.1073/pnas.97.6.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haken MS, White EC, Daneshvar-Shyesther L, Sih S, Choi E, Kalra R, Cogen PH. Molecular genetic analysis of chromosome arm 17p and chromosome arm 22q DNA sequences in sporadic pediatric ependymomas. Genes Chromosomes Cancer. 1996;17:37–44. doi: 10.1002/(SICI)1098-2264(199609)17:1<37::AID-GCC6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H, Shen W, Huang H, Hu L, Ramdas L, Zhou YH, Liao WS, Fuller GN, Zhang W. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- Ward S, Harding B, Wilkins P, Harkness W, Hayward R, Darling JL, Thomas DG, Warr T. Gain of 1q and loss of 22 are the most common changes detected by comparative genomic hybridisation in paediatric ependymoma. Genes Chromosomes Cancer. 2001;32:59–66. doi: 10.1002/gcc.1167. [DOI] [PubMed] [Google Scholar]
- Wasenius VM, Hemmer S, Kettunen E, Knuutila S, Franssila K, Joensuu H. Hepatocyte growth factor receptor, matrix metal-loproteinase-11, tissue inhibitor of metalloproteinase-1, and fibronectin are up-regulated in papillary thyroid carcinoma: A cDNA and tissue microarray study. Clin Cancer Res. 2003;9:68–75. [PubMed] [Google Scholar]
- Watson MA, Gutmann DH, Peterson K, Chicoine MR, Kleinschmidt-DeMasters BK, Brown HG, Perry A. Molecular characterization of human meningiomas by gene expression profiling using high-density oligonucleotide microarrays. Am J Pathol. 2002;161:665–672. doi: 10.1016/S0002-9440(10)64222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller C, Hinzmann B, Seitz S, Prokoph H, Burkhard-Goettges E, Fischer J, Jandrig B, Schwarz LE, Rosenthal A, Scherneck S. SASH1: A candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene. 2003;22:2972–2983. doi: 10.1038/sj.onc.1206474. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, Tom E, Mack DH, Levine AJ. 2000. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. 993;Genes Dev:14–981. [PMC free article] [PubMed] [Google Scholar]