Abstract
Most neurocytomas are well differentiated, being associated with better long-term survival than the more aggressive atypical lesions. Atypical neurocytomas are characterized by an MIB-1 labeling index >3% or atypical histologic features. This analysis focuses on well-differentiated neurocytomas in order to define the optimal treatment. A case with a follow-up of 132 months is presented. The patient developed two recurrences two and four years after first surgery, each showing an increasing proliferation activity. Furthermore, all published well-differentiated neurocytoma cases were reviewed for surgery, radiotherapy, and prognosis. Additional relevant data were obtained from the authors. Complete resection (CTR), complete resection plus radiotherapy (CTR + RT), incomplete resection (ITR), and incomplete resection plus radiotherapy (ITR + RT) were compared for outcome by using the Kaplan-Meier method and the log-rank test. Data were complete in 301 patients (CTR, 108; CTR + RT, 27; ITR, 81; ITR + RT, 85). Local control and survival were better after CTR than after ITR (P < 0.0001 and P = 0.0085, respectively). Radiotherapy improved local control after ITR (P < 0.0001) and after CTR (P = 0.0474), but not survival (P = 0.17 and P = 1.0, respectively). In the ITR + RT group, doses ⩽ 54 Gy (n = 33) and >54 Gy (n = 32) were not significantly different for local control (P = 0.88) and survival (P = 0.95). The data demonstrated CTR to be superior to ITR for local control and survival. After CTR and ITR, radiotherapy improved local control, but not survival. A radiation dose of 54 Gy appeared sufficient. Application of postoperative radiotherapy should be decided individually, taking into account the risk of local failure, the need for another craniotomy, and potential radiation toxicity.
In 1998, a total number of 34,345 individuals were newly diagnosed in the United States with a benign or malignant tumor of the central nervous system (Preston-Martin, 2003). Only 0.25% to 0.5% of these tumors are central neurocytomas (Hassoun et al., 1993). These rare lesions can be divided in two major subgroups, well-differentiated neurocytomas and atypical neurocytomas. About 75% of the neurocytomas are well differentiated, representing a variant considered benign. Well-differentiated neurocytomas are characterized by an MIB-1 labeling index ⩽3% and absence of atypical histologic features such as focal necrosis, increased mitotic activity, and vascular proliferation (Rades et al., 2004a). Well-differentiated lesions show a less aggressive behavior than lesions representing the other entity, atypical neurocytomas characterized by an MIB-1 labeling index > 3% and presence of atypical histologic features.
The most serious complications, which may occur in both types of neurocytoma, are intracerebral hemorrhage and transformation to more aggressive malignant lesions (Elek et al., 1999; Eng et al., 1997; Hanel et al., 2001; Jamshidi et al., 2001; Metellus et al., 2001; Namiki et al., 1998; Smoker et al., 1991; Taylor et al., 1998; Tomura et al., 1997; Vates et al., 2001; Yamamoto et al., 1996). In 1982, Hassoun et al. first coined the term “neurocytoma.” These lesions occurred mainly in adults, compared to neuroblastomas, which occurred mainly in children (Hassoun et al., 1982). Since that time, about 500 neurocytoma cases have been reported. The ratio of male to female was approximately 1.25:1. The age at diagnosis was between 20 and 35 years in the majority of patients. Most of the tumors were located in the ventricular system, mainly the lateral ventricles (Hassoun et al., 1993; Majos et al., 1997; Salvati et al., 1997; Tacconi et al., 1997; von Deimling et al., 1990). Extra-ventricular occurrence was rare (Brat et al., 2001). Fewer than 10 spinal lesions have been described (Ashkan et al., 2000; Coca et al., 1994; Louis et al., 1990; Stapleton et al., 1997; Stephan et al., 1999; Tatter et al., 1994).
Macroscopically, neurocytomas appear as gray, partly calcified masses. On light microscopy, the tumor is composed of small, round cells with intercellular fibrillar zones and ill-defined rosette-like structures. The nucleus is round or oval with a finely specked chromatin and an occasionally prominent nucleolus. Clear cells are common, resulting in a honeycomb appearance similar to that of oligodendrogliomas. Mitoses and necrosis are very rare. High mitotic activity and necrosis have to be considered as indicators for malignancy. Calcifications and well-developed vascularization are common. On electron microscopy, tumor cells have regular, round nuclei with finely dispersed chromatin and sometimes a neat small nucleolus. The cytoplasm contains parallel ergastoplasmic channels, a prominent Golgi apparatus, and lysosome-like inclusions. Numerous thin and intermingled cell processes have been observed containing mitochondria and microtubules (Favereaux et al., 2000; Kim et al., 1992; Kubota et al., 1991; Mackenzie 1999; Tsuchida et al., 1996; Uematsu et al., 2001)
Neurocytomas may show immunoreactivity for a variety of neuronal marker proteins such as neuron-specific enolase, synaptophysin, synapsin, neurofilament protein, neuron-associated class III beta-tubulin, microtubule-associated proteins MAP2 and tau, neuron-specific antigene L1, S-100 protein, or Leu-7. An occasional staining for glial fibrillary acidic protein as seen by immunohistology (von Deimling et al., 1990) shows the potential for astrocytic differentiation as observed in cell culture (Westphal et al., 1998). Most of the markers are nonspecific. Synaptophysin is considered to be the most reliable one (Brown et al., 2001; Enam et al., 1997; Hessler et al., 1992; Ishiuchi and Tamura, 1997).
On CT scans, neurocytomas appear as well-circumscribed, round or poly-lobed, hypodense masses showing homogeneous enhancement with contrast medium. The lesion may contain cystic areas or calcifications. Magnetic resonance imaging shows an isointense or slightly hyperintense signal compared to the cortex on T1- and on T2-weighted images. Administration of gadolinium-DTPA (diethylenetriamine pentaacetic acid) leads to a slight homogeneous enhancement. The enhancement may be inhomogeneous in cases of cysts or calcifications (Bolen et al., 1989; Chang et al., 1993; Sgouros et al., 1998; Wichmann et al., 1991).
Surgical resection is the most frequently used treatment for neurocytomas. There is still debate regarding optimal therapy for both well-differentiated and atypical lesions, especially regarding the potential benefit of postoperative radiotherapy.
This analysis focuses on the treatment of well-differentiated neurocytomas. It is the first large cohort of patients analyzed with the goal of defining the best available therapy for this entity. With respect to local control and survival, four treatment options are compared: complete tumor resection alone (CTR),2 complete resection followed by conventional radiotherapy (CTR + RT), incomplete resection alone (ITR), and incomplete resection followed by conventional radiotherapy (ITR + RT). Furthermore, we include a case with a long-term follow-up after incomplete resection of a well-differentiated neurocytoma showing an increase of MIB-1 labeling index at the time of recurrence.
Materials and Methods
Case Study
In February 1993, a 23-year-old woman presented with a two-month history of progressive headache, nausea, ataxia, and weakness of the left arm. Cranial CT and MRI showed a unifocal enhancing tumor (5 cm) located mainly in the right lateral ventricle. The tumor was incompletely resected in March 1993. Histology revealed a well-differentiated neurocytoma without increased mitotic activity. The MIB-1 labeling index was 0.7%. After surgery, the neurologic symptoms disappeared completely.
In February 1995, follow-up MRI study showed a progression of the remaining tumor. Although the patient did not complain about symptoms, she wanted the lesion to be resected. The tumor was almost completely removed by laser surgery in March 1995. The MIB-1 labeling index was now 2.8%.
In January 1997, the patient presented with a two-month history of progressive headache. The MRI study demonstrated a second recurrence (Fig. 1), which turned out to be multifocal, with three separate lesions along the septum pellucidum. An almost total resection of the three lesions was performed in February 1997. For the first time, histology showed mitotic figures. Immunohistochemistry demonstrated a further increase of the MIB-1 labeling index, which was now 5.4%. Postoperative radiotherapy was performed with a total dose of 50.4 Gy (dose per fraction, 1.8 Gy) from April to June 1997, resulting in complete response. Since then, the patient has remained symptom free. MRI has been performed twice a year and has not shown any evidence of recurrence.
Fig. 1.
T2-weighted MRI at the time of the second recurrence showing a multifocal tumor.
Treatment Options
All reports of neurocytomas published since 1982 were reviewed for age and gender of the patient, extent of resection (complete, incomplete), confirmation of diagnosis (surgeon’s impression alone, CT scan, or MRI), MIB-1 labeling index, atypical histologic features, radiotherapy, local control, and survival. Patients with tumors classified as atypical (MIB-1 labeling index > 3%, presence of atypical histologic features such as focal necrosis, increased mitotic activity, and vascular proliferation) were excluded from the analysis.
About 75% of the authors (one per previously published report) were directly contacted for additional data by phone, mail, fax, or e-mail, because the data stated in the literature were not complete. Of the contacted authors, about 80% provided us with additional data. Inclusion criteria were as follows: histologic confirmation of well-differentiated neurocytoma; treatment with either CTR, CTR + RT, ITR + RT, or ITR; complete data for the parameters described above; and minimal follow-up of 12 months. The interval between surgery and radiotherapy was usually a few weeks. Conventional irradiation was usually given with five fractions per week.
Local control, defined as absence of a recurrence of the original tumor, and survival, calculated from the date of the initial surgery, at 5 years and at 10 years were calculated with the Kaplan-Meier method and correlated with the four different therapies. The four therapies were compared with the log-rank test.
Results
Of about 500 reviewed cases, 301 patients could be included. About 90 patients had atypical lesions, and about 100 patients had incomplete data, in most cases because of a follow-up of <12 months. The data of the 301 patients included for evaluation were obtained from 94 studies. Seventy-six of these studies included one to three cases (n = 107), 18 studies included four to seven cases (n = 97), and eight studies included more than seven cases (n = 97). No significant difference was found among these three groups for five-year survival (93%, 95%, and 97%, respectively, P = 0.98) or five-year local control (75%, 82%, and 74%, respectively, P = 0.87). Thus, a potential study effect did not have a significant impact on our results.
The 301 patients were categorized according to the treatment schedule: CTR, 108; CTR + RT, 27; ITR, 81; or ITR + RT, 85. Table 1 summarizes the patient characteristics. There was no striking imbalance for age, gender, or method of confirming the extent of resection among the four groups. Follow-up in survivors ranged from 12 to 456 months (median, 48 months; mean, 66 months).
Table 1.
Comparison of the four treatment groups for age, gender, and confirmation of the extent of resection
| Characteristic | CTR | CTR + RT | ITR | ITR + RT |
|---|---|---|---|---|
| Median age (range) | 26 years (1–67) | 24 years (8–64) | 28 years (3–60) | 26 years (7–68) |
| Females | 47% (51/108) | 37% (10/270 | 36% (29/81) | 48% (41/85) |
| Males | 53% (57/108) | 63% (17/27) | 64% (52/81) | 52% (44/85) |
| Method for confirming extent of resection | ||||
| CT scan or MRI | 91% (98/108) | 93% (25/27) | 89% (72/81) | 93% (79/85) |
| Surgeon alone | 9% (10/108) | 7% (2/27) | 11% (9/81) | 7% (6/85) |
Abbreviations: CTR, complete tumor resection; CTR + RT, complete resection followed by radiotherapy; ITR, incomplete resection; ITR + RT, incomplete resection followed by radiotherapy.
In the series of 301 patients, local failure occurred in 60 patients (20%) during the period of follow-up. The data of our analysis span two decades. However, no significant difference was observed for local control between the 103 patients presented from 1982 to 1995 and the 198 patients presented after 1995. Survival rates at five years were 91% and 96%, respectively (P = 0.89). Local control rates at five years were 75% and 71%, respectively (P = 0.89).
MIB-1 labeling index, age, and Eastern Cooperative Oncology Group (ECOG) performance status (Oken et al., 1982) might be considered as potential prognostic factors for outcome in patients with well-differentiated neurocytoma. The MIB-1 index was available in 89 patients, ⩽0.5% in 37 patients and >0.5% in 52 patients. The comparison of MIB-1 index ⩽0.5% vs. >0.5% did not show a significant difference for five-year survival (95% vs. 90%, P = 1.0) or for five-year local control (90% vs. 72%, P = 0.61). In 11 of the patients with local failure, the MIB-1 index was also available for the recurrent tumor. In three patients (including the case presented here at the time of the second recurrence), an increase of the MIB-1 index from ⩽3% (well differentiated) to >3% (atypical) was observed. Our patient was treated with surgery plus irradiation instead of surgery alone as performed for the primary tumor and the first recurrence. The two cases from the literature were treated for both the primary tumor and the recurrent tumor with surgery alone.
The 49 children (age ⩽18 years) were compared to the 252 adult patients (age >18 years) for outcome. There was no significant difference observed for survival (95% vs. 95%, P = 1.0) or for local control (81% vs. 73%, P = 0.79).
The ECOG performance status was available in 185 patients. The comparison of ECOG grade 1 versus grade 2 to 3 did not reveal a significant difference, either for five-year survival (98% vs. 89%, P = 0.75) or for five-year local control (76% vs. 73%, P = 0.89).
Death occurred in seven patients, in two patients after ITR + RT (after 26 and 36 months), and in five patients after ITR (after 6, 10, 14, 15, and 18 months). Cause of death was local recurrence in six patients (two patients after ITR + RT and four patients after ITR) and Salmonella infection in one patient (after ITR, at 14 months after surgery). Median follow-up in the surviving patients was 48 months (range, 12–456 months). Follow-up was more than 40 months in 164 of the surviving patients.
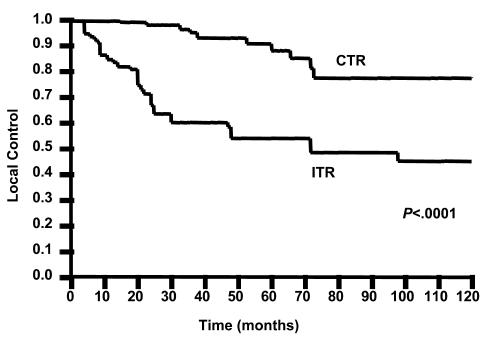
CTR was associated with a significantly better local control (Fig. 2, P < 0.0001) and survival (Fig. 3, P = 0.0085) than ITR. Local control rates were 91% versus 54% at five years and 77% versus 45% at 10 years, respectively. Survival rates were 100% versus 93% at five years and 100% versus 93% at 10 years, respectively.
Fig. 2.
Comparison of complete tumor resection alone (CTR) versus incomplete resection alone (ITR) for local control using the Kaplan-Meier method and the log-rank test.
Fig. 3.
Comparison of complete tumor resection alone (CTR) versus incomplete resection alone (ITR) for survival using the Kaplan-Meier method and the log-rank test.
As shown in Fig. 4, CTR + RT achieved better local control than CTR at five years and at 10 years (100% vs. 91% and 100% vs. 77%, respectively; P = 0.0474). Survival was 100% in both groups at five years and at 10 years (P = 1.0).
Fig. 4.
Comparison of complete tumor resection alone (CTR) versus complete resection followed by radiotherapy (CTR + RT) for local control using the Kaplan-Meier method and the log-rank test.
After ITR, RT significantly improved local control (Fig. 5, P < 0.0001), but not survival (P = 0.17). At five years, local control was 92% after ITR + RT versus 54% after ITR, and at 10 years, it was 86% after ITR + RT versus 45% after ITR. Survival rates were 97% versus 93%, respectively, both at five years and at 10 years. In the ITR group, 11 patients were identified with an MIB-1 labeling index of ⩽1.0%, and 10 patients were identified with an MIB-1 labeling index of 1.1% to 3.0%. Crude overall local failure rates were 9% (1/11) and 60% (6/10), respectively (P = 0.0954, chi-squared test).
Fig. 5.
Comparison of incomplete tumor resection alone (ITR) versus incomplete resection followed by radiotherapy (ITR + RT) for local control using the Kaplan-Meier method and the log-rank test.
Median time to local failure was 53 months after CTR (range, 15–128 months), 55 months after ITR + RT (range, 20–276 months), and 21 months after ITR (range, 4–218 months). After CTR + RT, local failure was not observed during the period of follow-up.
To investigate a possible dose-effect relationship, the ITR + RT group was divided into two subgroups of almost equal size according to the total radiation dose, ⩽54 Gy (n = 33) and >54 Gy (n = 32). The radiation dose was unknown in 20 patients. Local control rates at five years and at 10 years were 89% and 83% after ⩽54 Gy versus 93% and 86% after >54 G, respectively (P = 0.88). Overall survival rates for the ⩽54-Gy group and for the >54-Gy group were 97% and 96%, respectively, both at 5 years and at 10 years (P = 0.95).
Treatment for recurrence was known in 55/60 patients (92%). The majority of these patients received surgery, either surgery alone (n = 21), surgery plus RT (n = 7), or surgery plus chemotherapy with MCNU (ranimustine) or CCNU (lomustine) (n = 4). Radiosurgery alone was given in nine patients, conventional irradiation alone in seven patients, chemotherapy alone in two patients, and no treatment in five patients. Median follow-up after treatment for recurrence was 43 (range, 9–132) months after surgery alone, 45 (range, 18–132) months after surgery plus RT, 43 (range, 8–120) months after surgery plus chemotherapy, and 34 (range, 12–63) months after radiosurgery alone. Follow-up in the two patients receiving chemotherapy alone was four and eight months. The five patients without treatment for recurrence died within three months. Of the other 55 patients, two died during the period of follow-up, one patient at 11 months (after surgery alone) and one patient at 14 months (after conventional irradiation alone).
Discussion
The present study was performed to determine the best available treatment for well-differentiated neurocytomas. Four different therapeutic options were compared for local control and survival. Data were obtained from the literature and supplemented by further follow-up data obtained from direct contact with the majority of the authors. This approach, compared with the analysis of published data alone, provided more detailed information with a longer follow-up period.
According to our results, CTR was expectedly associated with both better local control and survival than ITR. There was a better local control observed after CTR + RT than after CTR, whereas radiotherapy did not lead to an improved survival after complete resection.
If only incomplete resection was achieved, postoperative radiotherapy also significantly improved local control, but not survival. This is different from the treatment of atypical neurocytomas, where radiotherapy significantly improves both local control and survival (Rades et al., 2004b).
The fact that no survival benefit was observed for radiotherapy after complete resection or incomplete resection of well-differentiated neurocytomas could be explained by the benign character of the well-differentiated lesions associated with a high rate of long-term survival, even in presence of residual tumor. This may lead to the question of whether radiotherapy should be administered during the initial treatment or be saved for the treatment of a possible local recurrence.
We think that both survival and local control are important end points because, at the time of recurrence, neurocytomas may manifest malignant behavior such as craniospinal dissemination or intraventricular hemorrhage (Elek et al., 1999; Eng et al., 1997; Hanel et al., 2001; Jamshidi et al., 2001; Metellus et al., 2001; Namiki et al., 1998; Smoker et al., 1991; Taylor et al., 1998; Tomura et al., 1997; Vates et al., 2001; Yamamoto et al., 1996). Furthermore, a second cranial operation appears to entail significant risk. With respect to our own case, the tumor showed an increase of the proliferation activity at each recurrence. The increase of the MIB-1 labeling index from 0.7% to 5.4% may reflect transformation from a well-differentiated neurocytoma (MIB-1 index ⩽ 3%) to a more aggressive atypical neurocytoma (MIB-1 index > 3%) (Rades et al., 2004a). This suggestion is supported by the fact that the tumor was unifocal at the time of the first surgery (MIB-1 index 0.7%) and of the first recurrence (MIB-1 index 2.8%), but was multifocal at the time of the second recurrence (MIB-1 index 5.4%).
This analysis was based on a retrospective analysis of heterogeneous data from patients treated in different institutions all over the world during various periods of time (between 1982 and 2001). No significant difference with respect to survival and local control was observed between patients reported from 1982 to 1995 and patients reported after 1995. There was also no significant difference between patients from studies with one to three cases, patients from studies with four to seven cases, and patients from studies with more than seven cases. However, the potential effect of a publication bias has to be taken into account in interpretation of our results. There was no striking imbalance for age, gender, and confirmation of the extent of resection among the four compared groups. Therefore, these parameters did not have a relevant impact on the results. No difference in surgery-related toxicity has been reported for complete versus incomplete tumor resection. Potential prognostic factors for outcome such as MIB-1 labeling index, age, and performance status have been investigated. However, no significant impact was observed for any of these factors. Regarding the MIB-1 labeling index, the finding is not unexpected, as the “cut-off point” for the MIB-1 labeling index associated with a worse prognosis is 3% (Rades et al., 2004a). Also, age and performance status appeared not to be of prognostic significance in the treatment of well-differentiated neurocytoma, at least in the series presented here, which is the largest series ever published on well-differentiated neurocytoma.
In the ITR + RT group, a radiation dose of 54 Gy appeared sufficient to improve local control. Because of the small number of patients who had a local failure within five years after surgery and subsequent radiotherapy, the recommendation of a particular radiation schedule was not possible. Radiosurgery may be an alternative option for the treatment of well-differentiated neurocytomas, but there is still a lack of experience (Cobery et al., 2001; Kim et al., 2003; Tyler-Kabara et al., 2001).
In contrast with the more aggressive atypical neurocytomas, well-differentiated neurocytomas are associated with an excellent long-term survival. The data of this analysis suggest complete tumor resection to be more effective than incomplete resection for the treatment of well-differentiated neurocytomas, as it was associated with significantly better local control and survival. After both complete resection and incomplete resection, radiotherapy significantly improved local control, but not survival. The decision to proceed with irradiation needs to be made on a case-by-case basis, taking into account the patient’s risk tolerance for needing another craniotomy and considerations of potential radiation toxicity, which may be influenced by tumor location. If postoperative radiation is performed, a total dose of 54 Gy appears sufficient to achieve a high probability for freedom from recurrence.
Acknowledgment
We thank the contacted authors, who provided us with additional data, for their kind cooperation and support.
Footnotes
Abbreviations used are as follows: CTR, complete tumor resection; CTR + RT, complete tumor resection followed by radiotherapy; ECOG, Eastern Cooperative Oncology Group; ITR, incomplete tumor resection; ITR + RT, incomplete tumor resection followed by radiotherapy; RT, radiotherapy.
References
- Ashkan K, Casey AT, D’Arrigo C, Harkness WF, Thomas DG. Benign central neurocytoma. Cancer. 2000;89:1111–1120. [PubMed] [Google Scholar]
- Bolen JW, Jr, Lipper MH, Caccamo D. Intraventricular central neurocytoma: CT and MR findings. J Comput Assist Tomogr. 1989;13:495–497. doi: 10.1097/00004728-198905000-00024. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Scheithauer BW, Eberhart CG, Burger PC. Extra-ventricular neurocytomas: Pathologic features and clinical outcome. Am J Surg Pathol. 2001;25:1252–1260. doi: 10.1097/00000478-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Brown DM, Karlovits S, Lee LH, Kim K, Rothfus WE, Brown HG. Management of neurocytomas: Case report and review of the literature. Am J Clin Oncol. 2001;24:272–278. doi: 10.1097/00000421-200106000-00013. [DOI] [PubMed] [Google Scholar]
- Chang KH, Han MH, Kim DG, Chi JG, Suh DC, Kim SJ, Cha SH, Han MC. MR appearance of central neurocytoma. Acta Radiol. 1993;34:520–526. [PubMed] [Google Scholar]
- Cobery ST, Noren G, Friehs GM, Chougule P, Zheng Z, Epstein MH, Taylor W. Gamma knife surgery for treatment of central neurocytomas. J Neurosurg. 2001;94:327–330. doi: 10.3171/jns.2001.94.2.0327. [DOI] [PubMed] [Google Scholar]
- Coca S, Moreno M, Martos JA, Rodriguez J, Barcena A, Vaquero J. Neurocytoma of spinal cord. Acta Neuropathol. 1994;87:537–540. doi: 10.1007/BF00294182. [DOI] [PubMed] [Google Scholar]
- Elek G, Slowik F, Eross L, Toth S, Szabo Z, Balint K. Central neurocytoma with malignant course. Neuronal and glial differentiation and craniospinal dissemination. Pathol Oncol Res. 1999;5:155–159. doi: 10.1053/paor.1999.0164. [DOI] [PubMed] [Google Scholar]
- Enam SA, Rosenblum ML, Ho KL. Neurocytoma in the cerebellum. Case report. J Neurosurg. 1997;87:100–102. doi: 10.3171/jns.1997.87.1.0100. [DOI] [PubMed] [Google Scholar]
- Eng DY, DeMonte F, Ginsberg L, Fuller GN, Jaeckle K. Craniospinal dissemination of central neurocytoma. Report of two cases. J Neurosurg. 1997;86:547–552. doi: 10.3171/jns.1997.86.3.0547. [DOI] [PubMed] [Google Scholar]
- Favereaux A, Vital A, Loiseau H, Dousset V, Caille J, Petry K. Histopathological variants of central neurocytoma: Report of 10 cases. Ann Pathol. 2000;20:558–563. [PubMed] [Google Scholar]
- Hanel R.A, Montano JC, Gasparetto E, Ditzel LF, Torres LF, Araujo JC. Uncommon presentation of central neurocytoma causing intraventricular hemorrhage: Case report. Arq Neuropsiquiatr. 2001;59:628–632. [PubMed] [Google Scholar]
- Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, Toga M. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151–156. doi: 10.1007/BF00690587. [DOI] [PubMed] [Google Scholar]
- Hassoun J, Soylemezoglu F, Gamberelli D, Figarella-Branger D, von Ammon K, Kleihues P. Central neurocytoma: A synopsis of clinical and histological features. Brain Pathol. 1993;3:297–306. doi: 10.1111/j.1750-3639.1993.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Hessler RB, Lopes MB, Frankfurter A, Reidy J, VandenBerg SR. Cytoskeletal immunohistochemistry of central neurocytomas. Am J Surg Pathol. 1992;16:1031–1038. doi: 10.1097/00000478-199211000-00001. [DOI] [PubMed] [Google Scholar]
- Ishiuchi S, Tamura M. Central neurocytoma: An immunohistochemical, ultrastructural and cell culture study. Acta Neuropathol. 1997;94:425–435. doi: 10.1007/s004010050729. [DOI] [PubMed] [Google Scholar]
- Jamshidi J, Izumoto S, Yoshimine T, Maruno M. Central neurocytoma presenting with intratumoral hemorrhage. Neurosurg Rev. 2001;24:48–52. doi: 10.1007/pl00011968. [DOI] [PubMed] [Google Scholar]
- Kim CY, Paek SH, Kim DG. Linear accelerator radiosurgery for central neurocytoma: A case report. J Neurooncol. 2003;61:249–254. doi: 10.1023/a:1022540929253. [DOI] [PubMed] [Google Scholar]
- Kim DG, Chi JG, Park SH, Chang KH, Lee SH, Jung HW, Kim HJ, Cho BK, Choi KS, Han DH. Intraventricular neurocytoma: Clinicopathological analysis of seven cases. J Neurosurg. 1992;76:759–765. doi: 10.3171/jns.1992.76.5.0759. [DOI] [PubMed] [Google Scholar]
- Kubota T, Hayashi M, Kawano H, Kabuto M, Sato K, Ishise J, Kawamoto K, Shirataki K, Iizuka H, Tsunoda S, Katsuyama J. Central neurocytoma: Immunohistochemical and ultrastructural study. Acta Neuropathol. 1991;81:418–427. doi: 10.1007/BF00293463. [DOI] [PubMed] [Google Scholar]
- Louis DN, Swearingen B, Linggood RM, Dickersin GR, Kretschmar C, Bhan AK, Hedley-Whyte ET. Central nervous system neurocytoma and neuroblastoma in adults—report of eight cases. J Neurooncol. 1990;9:231–238. doi: 10.1007/BF02341154. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR. Central neurocytoma: Histologic atypia, proliferation potential, and clinical outcome. Cancer. 1999;85:1606–1610. doi: 10.1002/(sici)1097-0142(19990401)85:7<1606::aid-cncr24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Majos C, Coll S, Pons LC. Central neurocytoma arising in the third ventricle. Neuroradiology. 1997;39:270–272. doi: 10.1007/s002340050406. [DOI] [PubMed] [Google Scholar]
- Metellus P, Dufour H, Fuentes S, Do L, Figarella-Branger G, Grisoli F. [Central neurocytoma revealed by intraventricular hemorrhage. A case report and review of the literature] Neurochirurgie. 2001;47:445–447. [PubMed] [Google Scholar]
- Namiki J, Nakatsukasa M, Murase I, Yamazaki K. Central neurocytoma presenting with intratumoral hemorrhage 15 years after initial treatment by partial removal and irradiation Neurol. Med Chir. 1998;38 :278–282. doi: 10.2176/nmc.38.278. [DOI] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- Preston-Martin, S. (2003) Epidemiology of primary brain tumors. In: Petrovich, Z., Brady, L.W., Apuzzo, M.L., and Bamberg, M. (Eds.), Combined Modality Therapy of Central Nervous System Tumors. Berlin-Heidelberg-New York: Springer, pp. 1–17.
- Rades D, Schild SE, Fehlauer F. The prognostic value of the MIB-1 labeling index for central neurocytomas. Neurology. 2004a;62:987–989. doi: 10.1212/01.wnl.0000115392.21898.e3. [DOI] [PubMed] [Google Scholar]
- Rades D, Fehlauer F, Schild SE. Treatment of atypical neurocytomas. Cancer. 2004b;100:814–817. doi: 10.1002/cncr.20032. [DOI] [PubMed] [Google Scholar]
- Salvati M, Cervoni L, Caruso R, Gagliardi FM. Central neurocytoma: Clinical features of 8 cases. Neurosurg Rev. 1997;20:39–43. doi: 10.1007/BF01390524. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Carey M, Aluwihare N, Barber P, Jackowski A. Central neurocytoma: A correlative clinicopathologic and radiologic analysis. Surg Neurol. 1998;49:197–204. doi: 10.1016/s0090-3019(97)00017-7. [DOI] [PubMed] [Google Scholar]
- Smoker W, Townsend JJ, Reichman MV. Neurocytoma accompanied by intraventricular hemorrhage: Case report and literature review. AJNR Am J Neuroradiol. 1991;12:765–770. [PMC free article] [PubMed] [Google Scholar]
- Stapleton SR, David KM, Harkness WF, Harding BN. Central neurocytoma of the cervical spinal cord. J Neurol Neurosurg Psychiatry. 1997;63:119. doi: 10.1136/jnnp.63.1.119. (letter) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan CL, Kepes JJ, Arnold P, Green KD, Chamberlin F. Neurocytoma of the cauda equina. Case report. J Neurosurg Spine. 1999;90:247–251. doi: 10.3171/spi.1999.90.2.0247. [DOI] [PubMed] [Google Scholar]
- Tacconi L, Thom M, Symon L. Central neurocytoma: A clinico-pathological study of five cases. Br J Neurosurg. 1997;11:286–291. doi: 10.1080/02688699746050. [DOI] [PubMed] [Google Scholar]
- Tatter SB, Borges LF, Louis DN. Central neurocytomas of the cervical spinal cord. Report of two cases. J Neurosurg. 1994;81:288–293. doi: 10.3171/jns.1994.81.2.0288. [DOI] [PubMed] [Google Scholar]
- Taylor CL, Cohen ML, Cohen AR. Neurocytoma presenting with intraparenchymal cerebral hemorrhage. Pediatr Neurosurg. 1998;29 :92–95. doi: 10.1159/000028696. [DOI] [PubMed] [Google Scholar]
- Tomura N, Hirano H, Watanabe O, Watarai J, Itoh Y, Mineura K, Kowada M. Central neurocytoma with clinically malignant behavior. Am J Neuroradiol. 1997;18:1175–1178. [PMC free article] [PubMed] [Google Scholar]
- Tsuchida T, Matsumoto M, Shirayama Y, Imahori T, Kasai H, Kawamoto K. Neuronal and glial characteristics of central neurocytoma: Electron microscopical analysis of two cases. Acta Neuropathol. 1996;91:573–577. doi: 10.1007/s004010050469. [DOI] [PubMed] [Google Scholar]
- Tyler-Kabara E, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for residual neurocytoma. Report of four cases. J Neurosurg. 2001;95:879–882. doi: 10.3171/jns.2001.95.5.0879. [DOI] [PubMed] [Google Scholar]
- Uematsu Y, Tanakla Y, Shimizu M, Oobayashi S, Fujita K, Nakai K, Itakura T, Moriwaki H, Kamei I. Histogenesis and proliferative activity of central neurocytomas. Brain Tumor Pathol. 2001;18:29–36. doi: 10.1007/BF02478922. [DOI] [PubMed] [Google Scholar]
- Vates GE, Arthur KA, Ojemann SG, Williams F, Lawton MT. A neurocytoma and an associated lenticulostriate artery aneurysm presenting with intraventricular hemorrhage: Case report. Neurosurgery. 2001;49:721–725. doi: 10.1097/00006123-200109000-00036. [DOI] [PubMed] [Google Scholar]
- von Deimling A, Janzer R, Kleihues P, Wiestler OD. Patterns of differentiation in central neurocytoma. An immunohistochemical study of eleven biopsies. Acta Neuropathol. 1990;79:473–479. doi: 10.1007/BF00296105. [DOI] [PubMed] [Google Scholar]
- Westphal M, Meissner H, Matschke J, Herrmann HD. Tissue culture of human neurocytomas induces the expression of glial fibrillary acidic protein. J Neurocytol. 1998;27:805–816. doi: 10.1023/a:1006903430869. [DOI] [PubMed] [Google Scholar]
- Wichmann W, Schubiger O, von Deimling A, Schenker C, Valavanis A. Neuroradiology of central neurocytoma. Neuroradiology. 1991;33:143–148. doi: 10.1007/BF00588253. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Komori T, Shibata N, Toyoda C, Kobayashi M. Multifocal neurocytoma/gangliocytoma with extensive leptomeningeal dissemination in the brain and spinal cord. Am J Surg Pathol. 1996;20:363–370. doi: 10.1097/00000478-199603000-00014. [DOI] [PubMed] [Google Scholar]