Abstract
Childhood malignant gliomas are rare, but their clinical behavior is almost as aggressive as in adults, with resistance to therapy, rapid progression, and not uncommonly, dissemination. Our study protocol incorporated sequential chemotherapy and high-dose thiotepa in the preradiant phase, followed by focal radiotherapy and maintenance with vincristine and lomustine for a total duration of one year. The induction treatment consisted of two courses of cisplatin (30 mg/m2) plus etoposide (150 mg/m2) × 3 days and of vincristine (1.4 mg/m2) plus cyclophosphamide (1.5 g/m2) plus high-dose methotrexate (8 g/m2), followed by high-dose thiotepa (300 mg/m2 × 3 doses), with harvesting of peripheral blood progenitor cells after the first cisplatin/etoposide course. From August 1996 to March 2003, 21 children, 14 females and 7 males, with a median age of 10 years were enrolled, 18 presenting with residual disease after surgery. Histologies were glioblastoma multiforme in 10, anaplastic astrocytoma in nine, and anaplastic oligodendroglioma in two; sites of origin were supratentorial areas in 17, spine in two, and posterior fossa in two. Of the 21 patients, 12 have died (10 after relapse, with a median time to progression for the whole series of 14 months; one with intratumoral bleeding at 40 months after diagnosis; and one affected by Turcot syndrome for duodenal cancer relapse). Four of 12 relapsed children had tumor dissemination. At a median follow-up of 57 months, overall survival and progression-free survival at four years were 43% and 46%, respectively. Sequential and high-dose chemotherapy can be afforded in front-line therapy of childhood malignant glioma without excessive morbidity and rather encouraging results.
Because of the rare incidence of high-grade glioma in children, that is, approximately 5% to 10% of all childhood brain tumors (Marchese and Chang, 1990), and because of the dismal prognosis for this disease, no treatment strategy can be defined as a gold standard in care for this patient population.
In the only randomized study published so far, Sposto et al. in 1986 reported that adopting adjuvant chemotherapy with vincristine (VCR), lomustine, and prednisone after radiotherapy resulted in a patient outcome that was superior to results with radiotherapy only (Sposto et al., 1989). This difference remained for smaller numbers of patients when histological samples were reviewed, and new, correct nosological entities were therefore assigned (Packer, 1996). Since the 1989 publication, chemotherapy has been added to radiotherapy in different schedules: as a preradiant “sandwich” phase (Kedar et al., 1994; Kuhl et al., 1998; Pendergrass et al., 1987; Wolff, et al. 2002a), concomitantly (Doz et al., 2002; Massimino et al., 2000a; Wolff et al., 2002b), and as maintenance (Finlay et al., 1995). High-dose chemotherapy has already been explored for relapsing or newly diagnosed malignant glioma (Finlay et al., 1996), either in preradiant or postradiant schedules, with the aim of tumor reduction before delivering radiotherapy (Heideman et al., 1993), or as radiation treatment consolidation (Bouffet et al., 1997; Finlay, 1996). In our institutional experience that is here reported, we have included a sequence of intensive and high-dose chemotherapy before radiation treatment with the aim of obtaining maximal tumor reduction before radiotherapy, thus improving probability of tumor control and overall survival (OS). We discuss the efficacy, feasibility, and survival rate of this postoperative regimen.
Patients and Methods
Patients
Children were referred to our institution after surgical excision, and the original histological diagnoses were made at the referral pathology service of the neurosurgical unit. Histological diagnoses were reviewed after admission for adjuvant treatment in all cases by two of the authors (P.C., F.G.). Criteria of inclusion were age between 3 and 21 years and no other treatment for brain tumor after surgery but steroids. All patients were fitted with a central line catheter before beginning the chemotherapy program. All images used for tumor diagnosis as well as for re-evaluation during and after treatment were centrally reviewed, and if postoperative scans had been performed more than two weeks before the start of chemotherapy, a gadolinium-enhanced MRI was requested. Staging was completed with spinal MRI and cytological spinal fluid examination in all cases.
In three cases a previous tumor not originating in the central nervous system had been diagnosed. In one 16-year-old girl, an adrenal adenocarcinoma had been surgically treated 14 years before the diagnosis of a glioblastoma multiforme. In a 10-year-old girl, a subcutaneous neurothecoma had been excised from the right forearm two years before. In a 17-year-old boy, who had undergone surgery and been treated with chemotherapy two years before the diagnosis of malignant brain tumor for a duodenal adenocarcinoma, the staging consisted also of an abdominal CT scan and clysis of the bowel. In three other children with a family history indicating a “cancer family syndrome,” genetic counseling and tailored blood analyses were proposed to those who were probands.
The scientific and ethics committees of each institution approved the protocol.
Treatment
Radiotherapy was administered between weeks 17 and 23 after the start of chemotherapy. The preradiation and postradiation scheduled medical treatment is shown in Table 1.
Table 1.
Treatment schedule
| Week | Treatment |
|---|---|
| 1 | CDDP (40 mg/m2) plus VP-16 (150 mg/m2) daily for 3 days) |
| plus G-CSF plus PBPC leukapheresis | |
| 4 | CDDP plus VP-16 |
| MRI | |
| 7 | VCR (1.4 mg/m2) plus CTX (1.5 g/m2) plus hd-MTX (8 g/m2) |
| 10 | VCR plus CTX plus hd-MTX |
| MRI | |
| 13 | hd-Thiotepa (300 mg/m2 × 3 in one day) plus PBPC plus G-CSF |
| (two cycles in four patients with residual tumor response after the first course) | |
| MRI | |
| 17–23 | Radiotherapy |
| MRI | |
| 23–52 | VCR (every 3 weeks), CCNU (80 mg/m2 p.o. every 9 weeks) |
Abbreviations used: CCNU, lomustine; CDDP, cisplatin; CTX, cyclophosphamide; G-CSF, μl granulocyte colony–stimulating factor; hd, high-dose; MTX, methotrexate; PBPC, peripheral blood progenitor cells; VCR, vincristine; VP-16, etoposide.
After the first cisplatin/etoposide (VP-16) course, on day 7 after the beginning of chemotherapy, granulocyte colony–stimulating factor (10 γ/kg/day s.c.) was prescribed. Between day 8 and day 12, CD34+ peripheral blood progenitor cell (PBPC)2 monitoring and collection were conducted.
Leukapheresis procedure
PBPCs were collected by using the COBE Spectra AutoPBSC (COBE BCT Inc., Lakewood, Col., USA) blood cell separator. The criterion for starting the procedure was the increase of CD34+ cells to >20/μl. The optimum target number of CD34+ cells to collect was 5 × 106 kg, although the minimum required for reinfusion was 3 × 106 kg. For patients weighing less than 15 kg (about 33 lb), the extracorporeal line of the circuit (165 ml) was primed with reconstituted whole blood that had been irradiated and leukodepleted, and the procedure was performed under general anesthesia in the operating room. A temporary femoral intravenous cannula was used if no central line had previously been inserted and/or if it was impossible to ensure adequate blood flow with peripheral veins. For anticoagulation, an i.v. bolus of 50 IU/kg of heparin was given before starting the leukapheresis, whereas during the procedure, acid citrate dextrose formula A was administered in a 1:12 to 1:18 ratio with whole blood. To prevent hypocalcemia-related symptoms, calcium gluconate was given in bolus doses of 0.2 g every 30 min after the beginning of the collection procedure. The number of CD34+ cells collected was determined by flow cytometric analysis. The processed blood volume was 2.5-fold the individual total blood volume. The apheresis product was divided into aliquots and cryopreserved.
Radiotherapy
Radiotherapy was scheduled to be administered five weeks after thiotepa administration. Radiotherapy was generally planned to be delivered to a volume defined by T1-weighted, contrast-enhanced images of the preoperative MRI with 2-cm margins. The prescribed total dose of radiation also depended, however, on extent of tumor at diagnosis and thus varied from 50 to 60 Gy. For most children, the planned total dose was 60 Gy, with a standard fractionation of 1.8 Gy per day, five treatment days per week. The children were treated with high-energy photon beams, and immobilization devices were adopted for all patients to guarantee treatment reproducibility. Two- or three-dimensional computerized treatment plans to optimize dose distribution around the target volume were adopted.
Postradiation therapy
In concomitance with the radiological re-evaluation after radiotherapy, the maintenance phase was started, to cover a total time span of one year from the beginning of chemotherapy. Since the treatment was studied to explore the activity of different drug combination courses, progression after any course of preradiant chemotherapy (cisplatin + VP-16 or VCR + cyclophosphamide [CTX] + methotrexate [MTX], first or second course) did not prevent proceeding to the next phase of treatment. Only in the case of progression after radiotherapy was treatment stopped and eventually modified with a second-line treatment.
After radiotherapy, all children were maintained in active follow-up, with MRI performed every three months for the first year, every four months for the second and third years, and every six months thereafter. During weeks 23 to 52 of the treatment schedule, children were placed on a regimen of VCR and lomustine. One year after radiotherapy, all patients were subjected to an endocrinological profile with stimulation tests if required. Surviving patients were also included in a program of neuropsychological tests to explore disease and iatrogenic sequelae and to organize a personal program aimed at rehabilitation.
Statistical Methods
Progression-free survival (PFS) and OS were computed in years according to the Kaplan and Meier method, considering failure and toxicity events from the date of chemotherapy start and censoring data for progression and survival at the last follow-up visit (Kaplan and Meier, 1958). Differences in survival among patient groups were evaluated by using the log-rank test (Peto and Peto, 1972).
Evaluation of Response
Radiological evaluation was performed before every other chemotherapy course that was scheduled at three-week intervals and thereafter five weeks after the end of radiotherapy. Radiological response was assessed according to the International Society of Pediatric Oncology criteria (Gnekov, 1995): Complete response is defined as no evidence of disease, partial response requires a reduction in size of more than 50%, and stable disease is the absence of tumor progression. In the particular evaluation of response used for high-grade glioma, where it is difficult to achieve tumor shrinkage, we also noted any minor responses, which we defined as more than 25% but less than 50% reduction of residual tumor size as obtained by the product of the diameters in the plane with the largest extent of tumor, with the perpendicular diameter measured on the same scan. However, for the purposes of response shown in Table 2, we used only the sum of complete responses and partial responses, which we termed objective responses. In the case of stable neoplastic volume with clinical improvement and weaning from steroids, disease was considered as stable for the purposes of statistical analysis in this study.
Table 2.
Response to each treatment phase*
| Treatment# | Response |
|---|---|
| CDDP plus VP-16 (18) | 2 OR (11%), 10 SD, 6 PD |
| hd-MTX plus VCR plus CTX (17) | 8 OR (47%), 7 SD, 2 PD |
| hd-TT (14) | 3 OR (21%), 9 SD, 2 PD |
| RT (14) | 2 OR (14%), 8 SD, 4 PD |
Abbreviations: CDDP, cisplatin; CTX, cyclophosphamide; hd-MTX, high-dose methotrexate; hd-TT, high-dose thiotepa; OR, objective response; PD, progressive disease; RT, radiotherapy; SD, stable disease; VCR, vincristine; VP-16, etoposide.
Eighteen of 21 patients were assessable because three patients had no residual tumor after surgery.
Number in parentheses is the number of assessable patients.
Results
The patients included in this study represent all children affected by high-grade glioma that did not originate in the brain stem who were admitted between August 1996 and March 2003 at Istituto Nazionale Tumori of Milan; two other children were treated at Policlinico in Pavia. There were thus 21 patients, 14 females and 7 males, with a median age of 10 years (range, 3.5–19 years).
Treatment Results
All had received a first surgery that consisted of macroscopically complete excision of tumor in three cases, partial excision in 10, and biopsy only in the other eight children. Table 3 illustrates sites of tumor origin and histological diagnoses. In four cases, the presence of multiple foci in supratentorial areas, together with the histological diagnosis of malignant glioma, could have led to the clinical-iconographic diagnosis of gliomatosis. In two other patients, the tumor infiltrated all of the spinal cord in one case and was extended from C3 to C7 in the other one. In the remaining 12 cases, with residual tumor after surgery, the maximal tumor diameter ranged from 1.5 to 7 cm. Genetic examinations performed at diagnosis or during treatment and follow-up indicated a familial multiple cancer syndrome such as Li-Fraumeni (Hisada et al., 1989) in five cases and Turcot syndrome (Hamilton et al., 1995) and astrocytoma-melanoma syndrome (Bahuau et al., 1997) in one case each.
Table 3.
Tumor sites and histotypes
| Histological diagnosis | |
| Glioblastoma multiforme | 10 |
| Anaplastic astrocytoma | 9 |
| Anaplastic oligodendroglioma | 2 |
| Site of tumor origin | |
| Spine | 2 |
| Posterior fossa | 2 |
| Supratentorial | 17 |
| Thalamomesencephalon | 6 |
| Multicentric | 4 |
All children received the first scheduled course of chemotherapy, and an adequate amount of PBPC (mean, 14 × 106 cells/kg; range, 3.5–43 × 106 cells) was collected. Leukaphereses were performed at days 8 to 12 (median, day 9) from the beginning of chemotherapy: Fifteen patients required only one apheresis procedure, and six required two procedures. One of the two children less than six years of age required general anesthesia for leukapheresis.
In the 18 patients assessable for tumor response to chemotherapy (since three patients had no residual tumor after surgery), we evaluated the responses to the single-treatment phases according to MRI performed after each phase. These data are illustrated by Table 2. Other patients had minor responses to the different phases of treatment, namely, five after cisplatin/VP-16, one after VCR/CTX/MTX, three after high-dose thiotepa, and four after radiotherapy. The three children without macroscopic tumor residual did not progress during treatment. Patients who progressed during chemotherapy did not have tumor reduction after radiotherapy, while two children who had had a tumor reduction after thiotepa showed disease progression at the first radiological evaluation after the end of radiotherapy. Four children with measurable disease after surgery who experienced a reduction in tumor volume after high-dose thiotepa received a second course of this drug with the same modalities of administration at an interval of four weeks from the first one, and two continued to show tumor reduction after the second course. Radiotherapy dose and treatment techniques were selected according to site of origin and extent of disease. Three children received a whole–supratentorial brain irradiation at a total dose of 50 Gy because of multiple tumor foci that did not allow us to exclude safely any part of the supratentorial parenchyma from radiation treatment, three children were treated with fractionated-stereotactic radiotherapy at a dose of 60 Gy, one child with a spinal tumor received 45 Gy on the tumor site, and the remaining 13 children received 60 Gy with a 2D or 3D conformal technique. One child with a holocord glioblastoma had a rapid progression of the disease after the first two courses of chemotherapy and died, while all 20 of the other children were able to complete the program at least until the radiotherapy phase. The total time span from surgery to radiotherapy reached a median of 22 weeks.
Patient Outcome
A total of 12 patients have progressed so far, with a median time to progression for the whole series of 14 months from diagnosis. Eleven of 12 relapsing children have died. One girl with glioblastoma obtained a second remission with chemotherapy and is alive two years after failure and four years after diagnosis; the patient with Turcot syndrome received surgery for local brain relapse nine months after diagnosis, and at 14 months, the patient developed lymph-nodal and hepatic metastases from a previous duodenal adenocarcinoma, dying from bowel tumor 23 months after the diagnosis of glioblastoma. The latest-relapsing girl, already proven to be affected by Li-Fraumeni syndrome, developed a second glioblastoma multiforme 53 months after the diagnosis of the first one. One patient with no documented progressive disease suddenly died elsewhere from bleeding arising from residual tumor 40 months after diagnosis; three months before, a brain MRI and a PET scan had shown no overt tumoral metabolic activity. The girl with melanoma-glioma syndrome, 24 months after diagnosis, developed multiple scalp melanomas that were surgically treated: She remained in complete remission from both glioma and melanoma.
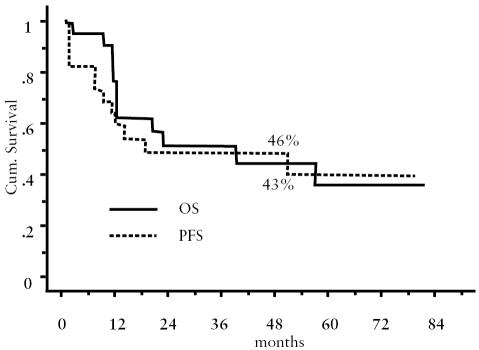
Median follow-up of the series was 57 months, with a range from 13 to 84 months. PFS and OS at four years were 46% and 43%, respectively (Fig. 1).
Fig. 1.
Overall survival and progression-free survival of the series.
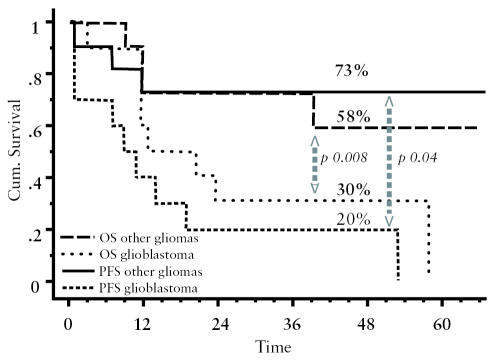
We evaluated the four-year PFS for the 10 patients with glioblastoma multiforme as compared to the PFS of the 11 with other histotypes, determining a PFS of 20% and 73%, respectively (P = 0.008); OS rates were 30% and 58%, respectively (P = 0.004). These curves are shown in Fig. 2. If we compare PFS and OS according to surgical results, PFS and OS are both 67% for patients without tumor residual after surgery, and 38% and 40%, respectively, for those with macroscopic tumor residual. Such a comparison is not feasible in grouping patients according to histological subtype and surgical results because 9 of 10 patients with glioblastoma had residual tumor after surgery. The subgroup of four patients who received two myeloablative courses with high-dose thiotepa had a PFS comparable to that of the other patients in the series: Two of them relapsed and two remained in continuous remission.
Fig. 2.
Overall survival and progression-free survival according to histology (glioblastoma multiforme vs. other malignant gliomas).
We also checked for any difference in PFS according to the presence of a “cancer syndrome.” We found no statistical difference, with a PFS of 38% for the seven patients carrying a genetic syndrome and a PFS of 57% for the other 14 children.
Since the p53 status was not systemically determined in the histological samples at the time of this report, we could not derive any prognostic consideration according to this feature.
Pattern of Failure
Site of relapse
The MRIs of the 12 relapsing children were examined to evaluate sites of relapse. Sites were only local in seven, local together with distant deposits in two, disseminated without local relapse in two, and marginal to radiotherapy field in one.
Myeloablative treatment toxicity
All 20 of the children who were administered high-dose thiotepa experienced grade 3–4 (NCI 2, National Cancer Institute, Bethesda, Md., USA) neutropenia, followed by fever, requiring antibiotics for a median of 14 days. Transfusional support was required in 83% of patients. Neutrophils and platelets taken (neutrophils >500/mm3 and platelets >50,000/mm3) were obtained in a median time of 10 (range, 3–12) and 14 (range 3–27) days, respectively. Grade 2 stomatitis was present in 77% of patients and required artificial nutrition support, which was carried out in the majority of cases by enteral tube. Pain deriving from stomatitis was treated with opioids. In 33% of cases, 24 to 48 h after the end of thiotepa infusion, a mild mood depression was evident, and low oral doses of amitriptyline were administered with success.
Discussion
Different strategies have been tried to ameliorate the prognosis for children with malignant glioma, whose median survival with conventional-dose chemotherapy and radiotherapy is around 11 to 24 months and whose OS is around 5% to 20% at five years (Al-Mefty et al., 1987; Sposto et al., 1989). Prognostic factors derive mainly from adult series (Al-Mefty et al., 1987; Kreth et al., 1999) or from biological studies (Pollack et al., 2002) that have not yet been supported by prospective studies. The amount of surgical resection is felt to be of prognostic value (Finlay and Wisoff 1999; Wisoff et al., 1998). Incomplete resection is, however, the rule in pediatric malignant gliomas, and generally no more than 20% of patients have had near-total or gross total resections (Sposto et al., 1989). Our results are in line with these numbers, with 86% of patients having gross residual disease after surgery.
The aim of our treatment strategy was therefore to pursue the maximal possible tumor reduction before delivering radiotherapy. The choice of drugs depended on data already published on phase 2 or window studies (Bertolone et al., 1989; Longee et al., 1990; McCowage et al., 1998). The association of cisplatin and VP-16 alternating with VCR and CTX reached a response rate of 60% in a subset of children less than three years of age with high-grade glioma (Duffner et al., 1993). The addition of high-dose methotrexate (hd-MTX) was based on the good clinical experience in other histological brain tumor subtypes and on the activity already shown in glioma lines exposed to antifolate drugs (Massimino et al., 2000b; Terzis et al., 1993).
The objective responses that we obtained with cisplatin/VP-16 and VCR/CTX/MTX therapies were comparable to what has been reported. For the combination containing MTX, we found a surprising efficacy, within the limits of a small number of patients to evaluate, in inducing tumor reduction. The combination of cisplatin and VP-16 was active in inducing PBPC mobilization, with very good harvest of stem cells and a moderate myelotoxicity. We have no data from other studies using this combination therapy for mobilizing PBPC. Recently, Wolff reported the results of a sandwich chemotherapy including hd-MTX used by a German cooperative pediatric brain tumor study group. Their response rate, 7 of 19, is comparable to the 8 of 17 patients obtained in our study (Wolff et al., 2002a).
The drugs used against malignant glioma are, however, few, and conventional-dose chemotherapy has marginally improved the prognosis of this disease. The aim of high-dose chemotherapy is to increase the tumor’s exposure to cytotoxic agents by overcoming the limited permeability of the blood-brain barrier (Jakacki et al., 1998). Because its lipid solubility gives a high degree of CNS penetration when administered intravenously, thiotepa has been chosen in the treatment of many brain tumor histotypes and has shown activity especially in medulloblastoma/PNET (Kalifa et al., 1992; Mason et al., 1998). In vitro and in vivo studies have shown a steep dose-response curve for thiotepa (Ahmed et al., 1990; Heideman et al., 1989). In relapsed and newly diagnosed malignant glioma of childhood, thiotepa has been used mostly in combination with other drugs at myeloablative dosages, such as CTX (Heideman et al., 1993; Kedar et al., 1994), VP-16 (Balmaceda et al., 1997; Bouffet et al., 1997; Busca et al., 1997; Finlay et al., 1996; Papadopoulos et al., 1998), carboplatin and VP-16 (Gururangan et al., 1998; Mason et al., 1998), and carmustine and VP-16 (Grovas et al., 1999; Papadakis et al., 2000). Heideman et al. (1993) have reported a 31% OR rate after high-dose thiotepa and CTX in a heterogeneous pediatric population with newly diagnosed and relapsing high-grade glioma, and a comparable response rate of 4/14 was reported by Finlay et al. (1997). Finlay et al. (1996) also used the same dosage of thiotepa (300 mg/m2 for three days), and obtained, with the addition of VP-16, more than 20% long-term survivors in a subset of adult and young patients with relapsing high-grade glioma (Finlay et al., 1996).
The response rate that we observed to high-dose thiotepa used as a single myeloablative drug was encouraging, and taking into account its manageable toxicity, we were able to administer a subsequent course to four patients and obtain further shrinkage of tumor in two of the four. Multiple cycles of high-dose chemotherapy followed by PBPC support can be efficacious in reducing tumor burden. The use of single drugs, while maintaining efficacy, could reduce transplant-related complications (Finlay et al., 1997; Jakacki et al., 1997). No transplant-related death occurred, even under our intense conditioning regimen. Our regimen achieved a control of disease progression in 9 of 21 patients at a median follow-up longer than four years, which compares well with results obtained by more aggressive regimens (Grovas et al., 1999).
Our vigorous chemotherapy regimen demanded a time span for delivering radiotherapy that was around 22 weeks after surgery. This interval does not seem to have compromised feasibility of radiotherapy and patients’ prognosis, especially when considering the number of patients with residual disease in our series, which precluded forming any statistical assumption about the value of surgical excision. It is possible that chemotherapy induces resistance to irradiation (Poppenborg et al., 1997). On the other hand, it may be that drugs delivered to the highly vascular tumor bed soon after surgery can better penetrate brain parenchyma and, if given in sequence, can maintain an altered state of permeability of the blood-brain barrier and can possibly create a synergistic activity. The delay of radiotherapy can therefore be a non-negative event, provided that the chemotherapy regimen is sufficiently intensive and effective. However, despite the use of an intense multidisciplinary strategy, patients with glioblastoma maintained a dramatically poorer outcome than patients with grade 3 tumors, which thus confirms the aggressiveness of this disease in children.
Seven of 21 children presented with genetic predisposition to develop a brain tumor and other malignancies. This high percentage implied a particular need for attention at diagnosis and during follow-up to check not only for brain tumor evolution but also for any new neoplastic events. As a matter of fact, two of our children developed other tumors during follow-up, and another girl with a proven Li-Fraumeni syndrome developed a new glioblastoma 53 months after the diagnosis of the first one, which had become radiologically and clinically silent. This impact of genetic constitutional alteration might be a feature of high-grade glioma when it occurs in children, although there are no reports of such a prevalence in a pediatric population with malignant glioma. In any case, the challenges of cure and quality of outcome are compounded when dealing with a constitutional genetic disease.
Conclusions
The outcome of this series has allowed us to form some conclusions. Sequential and high-dose chemotherapy can be afforded in pediatric high-grade glioma without excessive morbidity, and the addition of hd-MTX to standard regimens can improve tumor response. High-dose thiotepa does not have a “consolidation” role but can be a further tool for obtaining remission and possibly cure of this ominous disease. Radiotherapy is still an important step for obtaining tumor control, and its activity could be enhanced by reducing the bulk of tumor before its application, thus diminishing the disease to the greatest extent possible, and by using more intensive chemotherapy regimens, which could include multiple cycles of high-dose chemotherapy.
Footnotes
Abbreviations used are as follows: CTX, cyclophosphamide; hd-MTX, high-dose methotrexate; OS, overall survival; PBPC, peripheral blood progenitor cells; PFS, progression-free survival; VP-16, etoposide; VCR, vincristine.
References
- Ahmed T, Feldman E, Helson L, Biguzzi S, Szalyga A. Phase 1–2 trial of high dose thiotepa (HDT) with autologous bone marrow transplantation (ABMT) and localized radiotherapy (RT) for patients (pts) with astrocytoma III–IV. Proc Am Assoc Cancer Res. 1990;31:172. (abstract) [Google Scholar]
- Al-Mefty O, Al-Rodhan NR, Phillips RL, el-Senossi M, Fox JL. Factors affecting survival of children with malignant gliomas. Neurosurgery. 1987;20:416–420. doi: 10.1227/00006123-198703000-00010. [DOI] [PubMed] [Google Scholar]
- Bahuau M, Vidaud D, Kujas M, Palangie A, Assouline B, Chaignaud-Lebreton M, Prieur M, Vidaud M, Harpey JP, Lafourcade J, Caille B. Familial aggregation of malignant melanoma/dysplastic naevi and tumours of the nervous system: An original syndrome of tumour proneness. Ann Genet. 1997;40:78–91. [PubMed] [Google Scholar]
- Balmaceda C, Fetell MR, Hesdorffer C. Thiotepa and etoposide treatment of recurrent malignant gliomas: Phase I study. Cancer Chemother Pharmacol. 1997;40:72–74. doi: 10.1007/s002800050628. [DOI] [PubMed] [Google Scholar]
- Bertolone SJ, Baum ES, Krivit W, Hammond GD. A phase II study of cisplatin therapy in recurrent childhood brain tumors. A report from the Children’s Cancer Study Group. J Neurooncol. 1989;7:5–11. doi: 10.1007/BF00149372. [DOI] [PubMed] [Google Scholar]
- Bouffet E, Mottolese C, Jouvet A, Philip I, Frappaz D, Carrie C, Brunat-Mentigny M. Etoposide and thiotepa followed by ABMT (autologous bone marrow transplantation) in children and young adults with high-grade gliomas. Eur J Cancer. 1997;33:91–95. doi: 10.1016/s0959-8049(96)00369-3. [DOI] [PubMed] [Google Scholar]
- Busca A, Miniero R, Besenzon L, Cordero di Montezemolo L, Cenni M, Fagioli F, Sandri A, Vassallo E, Ricardi U, Madon E. Etoposide-containing regimens with autologous bone marrow transplantation in children with malignant brain tumors. Childs Nerv Syst. 1997;13:572–577. doi: 10.1007/s003810050142. [DOI] [PubMed] [Google Scholar]
- Doz F, Neuenschwander S, Bouffet E, Gentet JC, Schneider P, Kalifa C, Mechinaud F, Chastagner P, De Lumley L, Sariban E, Plantaz D, Mosseri V, Bours D, Alapetite C, Zucker JM. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: A study by the Société Française d’Oncologie Pédiatrique. Eur J Cancer. 2002;38:815–819. doi: 10.1016/s0959-8049(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, Sanford RA, Mulhern RK, James HE, Freeman CR, Seidel FG, Kun LE. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- Finlay JL. The role of high-dose chemotherapy and stem cell rescue in the treatment of malignant brain tumors. Bone Marrow Transplant. 1996;18 (suppl 3):S1–S5. [PubMed] [Google Scholar]
- Finlay JL, Wisoff JH. The impact of extent of resection in the management of malignant gliomas of childhood. Childs Nerv Syst. 1999;15:786–788. doi: 10.1007/s003810050471. [DOI] [PubMed] [Google Scholar]
- Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M, Turski PA, Wara WM, Edwards M, Sutton LN, Berger MS, Epstein F, Ayers G, Allen JC, Packer RJ. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Children’s Cancer Group. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- Finlay JL, Goldman S, Wong MC, Cairo M, Garvin J, August C, Cohen BH, Stanley P, Zimmerman RA, Bostrom B, Geyer JR, Harris RE, Sanders J, Yates AJ, Boyett JM, Packer RJ. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. Children’s Cancer Group. J Clin Oncol. 1996;14:2495–2503. doi: 10.1200/JCO.1996.14.9.2495. [DOI] [PubMed] [Google Scholar]
- Finlay JL, Gardner S, Moreno S, Bayer L, Dunkel I, Johnson J, Petriccione M, Halpern S. Sequential high-dose thiotepa with autologous stem cell rescue (ASCR) in patients with primary CNS tumors. J Neurooncol. 1997;35(suppl. 1):S16. (abstract 46) [Google Scholar]
- Gnekow AK. Recommendations of the Brain Tumor Subcommittee for the reporting of trials. SIOP Brain Tumor Subcommittee International Society of Pediatric Oncology. Med Pediatr Oncol. 1995;24:104–108. doi: 10.1002/mpo.2950240209. [DOI] [PubMed] [Google Scholar]
- Grovas AC, Boyett JM, Lindsley K, Rosenblum M, Yates AJ, Finlay JL. Regimen-related toxicity of myeloablative chemotherapy with BCNU, thiotepa, and etoposide followed by autologous stem cell rescue for children with newly diagnosed glioblastoma multiforme: Report from the Children’s Cancer Group. Med Pediatr Oncol. 1999;33:83–87. doi: 10.1002/(sici)1096-911x(199908)33:2<83::aid-mpo4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gururangan S, Dunkel IJ, Goldman S, Garvin JH, Rosenblum M, Boyett JM, Gardner S, Merchant TE, Gollamudi S, Finlay JL. Myeloablative chemotherapy with autologous bone marrow rescue in young children with recurrent malignant brain tumors. J Clin Oncol. 1998;16:2486–2493. doi: 10.1200/JCO.1998.16.7.2486. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, Krush AJ, Berk T, Cohen Z, Tetu B, Burger PC, Wood PA, Taqi F, Booker SV, Petersen GM, Offerhaus GJA, Tersmette AC, Giardiello FM, Vogelstein B, Kinzler KW. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ, Singher LJ, Ettinger LJ, Gillespie A, Sam J, Poplack DG. Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: Evidence for dose-dependent plasma clearance of thiotepa. Cancer Res. 1989;49:736–741. [PubMed] [Google Scholar]
- Heideman RL, Douglass EC, Krance RA, Fontanesi J, Langston JA, Sanford RA, Kovnar EH, Ochs J, Kuttesch J, Jenkins JJ, Fairclough DL, Kun LE. High-dose chemotherapy and autologous bone marrow rescue followed by interstitial and external-beam radiotherapy in newly diagnosed pediatric malignant gliomas. J Clin Oncol. 1993;11:1458–1465. doi: 10.1200/JCO.1993.11.8.1458. [DOI] [PubMed] [Google Scholar]
- Hisada M, Garber JE, Fung CY, Fraumeni JF, Jr, Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst. 1998;90:606–611. doi: 10.1093/jnci/90.8.606. [DOI] [PubMed] [Google Scholar]
- Jakacki RI, Jamison C, Heifetz SA, Caldemeyer K, Hanna M, Sender L. Feasibility of sequential high-dose chemotherapy and peripheral blood stem cell support for pediatric central nervous system malignancies. Med Pediatr Oncol. 1997;29:553–559. doi: 10.1002/(sici)1096-911x(199712)29:6<553::aid-mpo6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Jakacki RI, Jamison C, Mathews VP, Heilman DK, Dropcho E, Cornetta K, Macdonald DR, Williams DA. Dose-intensification of procarbazine, CCNU (lomustine), vincristine (PCV) with peripheral blood stem cell support in young patients with gliomas. Med Pediatr Oncol. 1998;31:483–490. doi: 10.1002/(sici)1096-911x(199812)31:6<483::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kalifa C, Hartmann O, Demeocq F, Vassal G, Couanet D, Terrier-Lacombe MJ, Valteau D, Brugieres L, Lemerle J. High-dose busulfan and thiotepa with autologous bone marrow transplantation in childhood malignant brain tumors: A phase II study. Bone Marrow Transplant. 1992;9:227–233. [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Non parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kedar A, Maria BL, Graham-Pole J, Ringdahl DM, Quisling RG, Mickle JP, Mendenhall NP, Marcus RB, Jr, Gross S. High-dose chemotherapy with marrow reinfusion and hyperfractionated irradiation for children with high-risk brain tumors. Med Pediatr Oncol. 1994;23:428–436. doi: 10.1002/mpo.2950230507. [DOI] [PubMed] [Google Scholar]
- Kreth FW, Berlis A, Spiropoulou V, Faist M, Scheremet R, Rossner R, Volk B, Ostertag CB. The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer. 1999;86:2117–2123. [PubMed] [Google Scholar]
- Kuhl J, Muller HL, Berthold F, Kortmann RD, Deinlein F, Maass E, Graf N, Gnekow A, Scheurlen W, Gobel U, Wolff JE, Bamberg M, Kaatsch P, Kleihues P, Rating D, Sorensen N, Wiestler OD. Preradiation chemotherapy of children and young adults with malignant brain tumors: Results of the German pilot trial HIT’88/’89. Klin Padiatr. 1998;210:227–233. doi: 10.1055/s-2008-1043883. [DOI] [PubMed] [Google Scholar]
- Longee DC, Friedman HS, Albright RE, Jr, Burger PC, Oakes WJ, Moore JO, Schold SC., Jr Treatment of patients with recurrent gliomas with cyclophosphamide and vincristine. J Neurosurg. 1990;72:583–588. doi: 10.3171/jns.1990.72.4.0583. [DOI] [PubMed] [Google Scholar]
- Marchese MJ, Chang CH. Malignant astrocytic gliomas in children. Cancer. 1990;65:2771–2778. doi: 10.1002/1097-0142(19900615)65:12<2771::aid-cncr2820651227>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mason WP, Grovas A, Halpern S, Dunkel IJ, Garvin J, Heller G, Rosenblum M, Gardner S, Lyden D, Sands S, Puccetti D, Lindsley K, Merchant TE, O’Malley B, Bayer L, Petriccione MM, Allen J, Finlay JL. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998;16:210–221. doi: 10.1200/JCO.1998.16.1.210. [DOI] [PubMed] [Google Scholar]
- Massimino M, Gandola L, Casanova M, Cefalo G, Ferrari A, Luksch R, Riganti G, Lombardi F. Concomitant chemoradiotherapy for childhood poor-prognosis gliomas. Med Pediatr Oncol. 2000a;34:147–150. doi: 10.1002/(sici)1096-911x(200002)34:2<147::aid-mpo16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Massimino M, Gandola L, Cefalo G, Lasio G, Riva D, Fossati-Bellani F, Gianni MC, Luksch R, Tesoro-Tess JD, Lombardi F. Management of medulloblastoma and ependymoma in infants: A single-institution long-term retrospective report. Childs Nerv Syst. 2000b;16:15–20. doi: 10.1007/PL00007279. [DOI] [PubMed] [Google Scholar]
- McCowage GB, Friedman HS, Moghrabi A, Kerby T, Ferrell L, Stewart E, Duncan-Brown M, Fuchs HE, Tien R, McLendon RE, Meier L, Kurtzberg J, Ashley D, Colvin OM, Longee DC. Activity of high-dose cyclophosphamide in the treatment of childhood malignant gliomas. Med Pediatr Oncol. 1998;30:75–80. doi: 10.1002/(sici)1096-911x(199802)30:2<75::aid-mpo1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Packer, R.J., Yates, A., and Sposto, R. Re-evaluation of outcome in children with high-grade gliomas treated on a Children’s Cancer Group Study CCG-943: A randomized study of radiation alone versus radiation plus adjuvant CCNU, prednisone and vincristine chemotherapy. 7th International Symposium: Pediatric Neuro-Oncology, May 15–18, 1996, Washington, D.C. (abstract).
- Papadakis V, Dunkel IJ, Cramer LD, Kramer E, Papadopoulos E, Goldman S, Packer RJ, Willoughby M, Baker D, Garvin J, Strandjord S, Coccia P, Kaplan AM, Klemperer M, Finlay JL. High-dose carmustine, thiotepa and etoposide followed by autologous bone marrow rescue for the treatment of high risk central nervous system tumors. Bone Marrow Transplant. 2000;26:153–160. doi: 10.1038/sj.bmt.1702475. [DOI] [PubMed] [Google Scholar]
- Papadopoulos KP, Garvin JH, Fetell M, Vahdat LT, Garrett TJ, Savage DG, Balmaceda C, Bruce J, Sisti M, Isaacson S, DeLa Paz R, Hawks R, Bagiella E, Antman KH, Hesdorffer CS. High-dose thiotepa and etoposide-based regimens with autologous hematopoietic support for high-risk or recurrent CNS tumors in children and adults. Bone Marrow Transplant. 1998;22:661–667. doi: 10.1038/sj.bmt.1701408. [DOI] [PubMed] [Google Scholar]
- Pendergrass TW, Milstein JM, Geyer JR, Mulne AF, Kosnik EJ, Morris JD, Heideman RL, Ruymann FB, Stuntz JT, Bleyer WA. Eight-drugs-in-one-day chemotherapy for brain tumors: Experience in 107 children and rationale for pre-radiation chemotherapy. J Clin Oncol. 1987;5:1221–1231. doi: 10.1200/JCO.1987.5.8.1221. [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J. Asymptomatically efficient rank invariant test procedures. J R Stat Soc Ser A. 1972;135:185–207. [Google Scholar]
- Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R. Children’s Cancer Group. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346:420–427. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- Poppenborg H, Munstermann G, Knupfer MM, Hotfilder M, Wolff JE. C6 cells cross-resistant to cisplatin and radiation. Anticancer Res. 1997;17:2073–2077. [PubMed] [Google Scholar]
- Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: Results of a randomized trial. A report from the Children’s Cancer Study Group. J Neurooncol. 1989;7:165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- Terzis AJ, Fiskerstrand T, Refsum H, Ueland PM, Arnold H, Bjerkvig R. Proliferation, migration and invasion of human glioma cells exposed to antifolate drugs. Int J Cancer. 1993;54:112–118. doi: 10.1002/ijc.2910540118. [DOI] [PubMed] [Google Scholar]
- Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, Packer RJ, Finlay JL. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: A report of the Children’s Cancer Group trial no. CCG-945. J Neurosurg. 1998;89:52–59. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- Wolff JE, Gnekow AK, Kortmann RD, Pietsch T, Urban C, Graf N, Kuhl J. Pre-radiation chemotherapy for pediatric patients with high-grade glioma. Cancer. 2002a;94:264–271. doi: 10.1002/cncr.10114. [DOI] [PubMed] [Google Scholar]
- Wolff JE, Wagner S, Sindichakis M, Pietsch T, Gnekow A, Kortmann RD, Strater R, Kuehl J. Simultaneous radiochemotherapy in pediatric patients with high-grade glioma: A phase I study. Anticancer Res. 2002b;22:3569–3572. [PubMed] [Google Scholar]