Abstract
Most children with medulloblastoma (MB), the second most common pediatric brain tumor, have a 70% probability of survival. However, survivors who receive aggressive therapy are at significant risk of cognitive deficits that have been associated with lower volumes of normal-appearing white matter (NAWM). We hypothesized that cranial irradiation inhibited normal brain volume development in these survivors. We retrospectively analyzed 324 MRI studies of 52 patients with histologically proven MB treated with surgery and 35 to 40 Gy cranio-spinal irradiation, with or without chemotherapy. The volume of NAWM and that of cerebrospinal fluid were quantified from a single index section and compared with those of healthy, age-similar control subjects. A quadratic random coefficient model was used to identify trends in brain volume with increasing age. Patients treated for MB at younger ages demonstrated substantially less development of NAWM volume than did their healthy peers. Younger age at the time of irradiation and the need for a ventricular shunt were significantly associated with reduced NAWM volume. NAWM and craniospinal fluid volume differences between patients who had shunts and those without resolved over a period of four to five years. NAWM volume is known to be associated with neurocognitive test performance, which shows deficiencies after cranial irradiation early in life. Therefore, volumetric monitoring of brain development can be used to guide the care of survivors, assess the toxicity of previous and current clinical trials, and aid in the design of therapies that minimize toxicity.
Brain tumors constitute approximately 20% of pediatric malignancies. Because of the inherent risk of these tumors, patients receive aggressive CNS therapy that often comprises maximal surgical resection, local and craniospinal irradiation (CSI), and adjuvant chemotherapy. Consequently, long-term survivors are at risk of cognitive delays or deficits that impair their academic performance, employment opportunities, and quality of life (Dennis et al., 1996; Mulhern et al., 1998; Ris et al., 2001).
In survivors of childhood medulloblastoma (MB), deficits in IQ and academic achievement appear to reflect a diminished ability to acquire new information (Palmer et al., 2001). One or more cognitive processing mechanisms, including attention, short-term memory, speed of processing, visual-motor coordination, and sequencing ability, may be impaired (Schatz et al., 2000). These processes depend on the integrity of widely distributed neural networks supported by interhemispheric and intrahemispheric white matter tracts. Recent findings have shown that in pediatric patients treated for brain tumors, a reduced volume of normal-appearing white matter (NAWM)3 is associated with reduced attentional ability and a decline in IQ and academic achievement (Reddick et al., 2003).
The proportion of intracranial volume that is NAWM is normally expected to increase into early adulthood. This increase is usually modeled as a quadratic function in which growth is most rapid in the first five years, continues to rise at a moderate rate over the next 10 years, and then slows to asymptotically approach the adult volume (Giedd et al., 1999; Sowell et al., 2002). Previous studies of the association between NAWM and cognitive function have yielded mixed results (Andreasen et al., 1993; Reiss et al., 1996). NAWM volume is not strongly related to IQ in healthy children but is significantly associated in other populations with pathological conditions such as attention deficit-hyperactivity disorder (Castellanos et al., 2002). At least one author has suggested a threshold effect in which cognitive impairment becomes apparent only below a certain volume of NAWM (Inzitari, 2000).
Studies that have quantified toxic effects on white matter and investigated the association between neurotoxicity and cognitive deficits in children have focused primarily on survivors of MB of the posterior fossa (approximately 20% of pediatric brain tumors). One such study compared patients treated for MB with age-similar controls who had received surgery alone for low-grade tumors of the posterior fossa; the survivors of MB had a significantly smaller volume of NAWM, a substantially greater volume of cerebrospinal fluid (CSF), and an equal volume of gray matter (Reddick et al., 1998). This study also demonstrated that chemotherapy did not have a significant detectable impact on tissue volumes. The MB patients also had significantly lower IQs (Mulhern et al., 1999). However, because of their cross-sectional design, these studies could not discern whether the smaller NAWM volume reflected loss of tissue, decreased myelination, or both. A subsequent longitudinal study revealed a significant loss of NAWM volume in patients undergoing treatment for MB; this loss was more rapid among patients who received a CSI dose of 36 Gy versus CSI of 23.4 Gy (Reddick et al., 2000). However, this study was limited by a relatively short median follow-up period of one year.
NAWM volume can explain approximately 70% of the association between IQ impairment and age at the time of irradiation (Mulhern et al., 2001). In a recent cross-sectional study, patients treated for MB showed significantly impaired performance on all neurocognitive measures of intellect, attention, memory, and academic achievement (Reddick et al., 2003). The study produced a developmental model in which academic achievement was predicted by NAWM volume, attentional ability, and IQ; these factors explained approximately 60% of the variance observed in reading and spelling and almost 80% of the variance observed in mathematics. The primary consequence of reduced NAWM volume was decreased attentional ability, which reduced patients’ IQ and academic achievement (Reddick et al., 2003).
We designed a retrospective longitudinal study to compare brain volume development of patients treated for MB with that of healthy, age-similar peers. To control for the effect of irradiation dose, we included only patients who received a CSI dose of 35 to 40 Gy (once used to treat all cases of MB and now used for patients at high risk). This retrospective design has three limitations that could conceivably cause results to differ from more comprehensive prospective trials: (1) Imaging was limited to a single representative section, (2) diffusion tensor imaging was not acquired as a routine part of clinical imaging during this period, and (3) extent and incidence of regions of T2 hyperintensity in other locations could not be assessed by the single index section. However, this retrospective study was designed to comprise as homogeneous a group of subjects as possible: Patients received similar doses of CSI to treat the same type of tumor, which arose in the same location. This study builds on previous work by including serial magnetic resonance (MR) studies to determine the effect of age at irradiation, time since irradiation, gender, use of chemotherapy, and use of ventricular shunt on the development of brain parenchyma (Reddick et al., 1998).
Methods
Patients
A review of patient records identified 133 patients with histologically proven MB who were treated at St. Jude Children’s Research Hospital between 1985 and 2000. Criteria for inclusion in our analysis were treatment with surgery; a CSI dose of 35 to 40 Gy with or without chemotherapy; initiation of CSI one to eight years prior to MR examination; two or more MRI studies; and no disease progression within the first year after beginning therapy. To ensure that comparable, high-quality MRI data were analyzed, we included only MR examinations performed after July 1996. Fifty-four patients had received CSI outside the targeted dose range, 21 patients had fewer than two MR studies, two patients’ imaging was limited by metal artifacts, two patients had started CSI less than one year previously, and two had progressive disease within the first year. Therefore, 52 patients were included in the study.
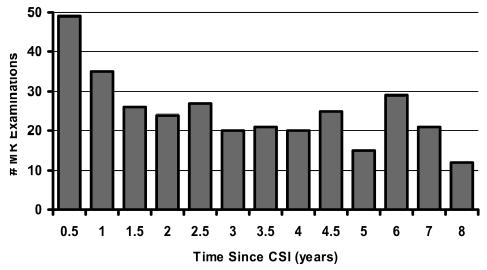
All 52 patients had undergone maximal surgical tumor resection and postoperative CSI (35–40 Gy fractionated, 1.8 Gy per day) with a boost to the posterior fossa (1.8 Gy per day to a total dose of 50.8–59.4 Gy). The group included 35 male and 17 female patients, who had had 324 MRI studies. Nineteen patients had shunts placed before CSI. Thirty-eight patients received adjuvant chemotherapy (Table 1). The median age at the time of CSI was 8.3 years (range, 3.4–20.0 years). As demonstrated in Fig. 1, MRI was performed within a range of 0.2 to 7.9 years (median, 2.5 years) after CSI.
Table 1.
Chemotherapy regimens used to treat 38 of the 52 patients in this study
| Protocol | N | Description* |
|---|---|---|
| CNS8 | 8 | Carbo/VP-16 + Cyclo/VCR (post-RT for patients with PD) |
| CNS14 | 1 | Carbo/Cyclo/VP-16 (pre-RT) |
| P9031 | 10 | CDDP/VP-16 (± pre-RT) + Cyclo/VCR (post-RT) |
| PACKER | 1 | CCNU/CDDP/VCR (post-RT) |
| CCSG921 | 1 | “8-in-1” (Zeltzer et al., 1999) |
| SJMB96 | 17 | Topotecan (pre-RT), Cyclo/CDDP/VCR (post-RT) |
Abbreviations: Carbo, carboplatin; CCNU, lomustine; CDDP, cisplatin; Cyclo, cyclophosphamide; PD, progressive disease; RT, radiation treatment; VCR, vincristine; VP-16, etoposide.
Fig. 1.
Number of MR examinations performed at each time interval postirradiation.
Three of the 52 patients had T2 signal abnormalities in the single section evaluated in this study. The regions of hyperintensity were segmented separately and not included in the volumes of NAWM. The sizes of these regions were generally small, 1% to 5% of the NAWM volume, and stable across the longitudinal evaluations. Exclusion of these regions means the NAWM volume would not correspond to total white matter volume in these three cases. However, since the regions were small and stable over time, they would have no effect on the slope and very little impact on the intercepts resulting from the quadratic random coefficient model described below. Five other patients had lacunar infarctions due to treatment, which represent local loss of white matter, and these areas were not included in the NAWM volumes.
Healthy Control Group
A group of 26 healthy control (HC) subjects was recruited from among the siblings of survivors of leukemia and brain tumors who were participating in an unrelated screening protocol. No control subjects were currently being treated for neurological or psychiatric conditions, including attention deficit-hyperactivity disorder. They ranged in age from 7.0 to 16.5 years (median, 12.4 years). The group comprised 13 male and 13 female subjects. Subjects underwent a single MRI examination without sedation.
MR Imaging
The MR instrument was a 1.5 T Magnetom (Siemens Medical Systems, Iselin, N.J.) whole-body imager. All subjects underwent a clinical imaging protocol approved by the hospital’s Institutional Review Board, after informed consent had been obtained from the subject, parent, or guardian, as appropriate. T1-, T2-, and proton density-weighted images of all subjects were acquired as 5-mm transverse slices with a 1-mm gap interleaved between slices to avoid cross-excitation. The imaging protocol was designed as part of the routine MR evaluation of patients with MB at our institution. Examinations consisted of 19 images centered on the posterior fossa so that the most superior section in the imaging set approximately covered the top of the ventricles. The whole brain was not imaged and therefore could not be evaluated in this retrospective analysis. Diffusion tensor imaging was also not a part of routine clinical imaging at our institution before 2000.
Volumetric MR Studies
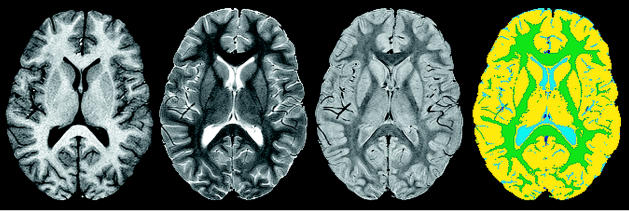
Image registration was performed within and between all examinations. The index section chosen for volumetric study was a single transverse section at the level of the basal ganglia that included both genu and splenium of the corpus callosum and generally showed the puta-men and the lateral ventricle. This representative index section allowed quantification of both interhemispheric and intrahemispheric NAWM tracts. It allowed us to sample cortical gray matter, NAWM, central gray matter structures, and ventricular CSF, and it has been shown to be highly predictive of the full cerebral NAWM and CSF volumes in other patient populations (Glass et al., 2003). The volume of regional brain parenchyma was quantified from MR images by using a fully automated hybrid neural network segmentation and classification method (Reddick et al., 1997). The resulting classified regions were mapped to a color scheme as shown in Fig. 2. A histogram was then completed for each color to determine the number of pixels; this value was multiplied by pixel volume to determine the sampled volume of each tissue type. Robust reliability and validity have been established for these methods, resulting in a predicted variance of approximately 2% in the repeated measure of NAWM (Reddick et al., 1997, 2002).
Fig. 2.
Examples of types of MR images acquired. From left to right: T1-weighted, T2-weighted, and proton density images at the level of the index section. Far right: the resulting segmented map used to quantify the volume of normal-appearing white matter (green) and CSF (blue).
Statistical Analysis
A regression model was used to examine NAWM volume and CSF volume as a function of age in the control group. For the MB group, a random coefficient model was used to examine NAWM and CSF volume as a function of time since CSI, in which the influence of age at the time of CSI, gender, use of chemotherapy, and the presence of a shunt were included as covariate variables. CSF volume was used as an index of brain atrophy/hydrocephalus. The final fitted models included only variables that were significant or whose interactions were significant. The random coefficient model, a subclass of mixed models, was suitable for analyzing longitudinal data in which each patient has been observed several times longitudinally (Littell et al., 1996). This model could be understood intuitively as estimating the longitudinal trend of a population by averaging the trends of individual patients. The 324 MRI observations from 52 patients provided a sufficient sample size for applying the random coefficient model appropriately to this analysis. The goodness of fit for all fitted models was examined by the patterns of residuals against fitted predictions and by checking the normality of residuals. All models reported in this paper have been fitted appropriately, and tests in all fitted models were tested at a 0.05 alpha level (two-tailed) using SAS (Cary, NC) software.
Because the control group was imaged only once and the MB group was imaged longitudinally, the resulting models could not be statistically compared. However, another analysis was performed to directly compare between the HC subjects and the most recent examination of patients without shunts under the age of 19 (to match age of control subjects). Student’s t tests were conducted assuming unequal variance between the two groups for age at examination and NAWM volume. Two-tailed probability tests of significance (alpha = 0.05) were used.
Results
Using the quadratic random coefficient model, we analyzed the volume of NAWM and CSF in the index MR section of MB patients as a function of time since CSI, and we assessed the impact of age at the time of CSI, gender, chemotherapy status, and shunt status on changes in volume (Table 2). Female gender was associated with a smaller volume of NAWM and CSF at presentation, but gender was not associated with change in volume over time after CSI. Chemotherapy status was also not significantly associated with change in volume. However, shunt status and age at the time of CSI were statistically significant factors.
Table 2.
Estimated volume of normal-appearing white matter and cerebrospinal fluid as a function of time since irradiation (Time) with the covariates age at time of irradiation (Age) and shunt status (Shunt)a
| Model | Parameter Estimate | Standard Error | Pvalue |
|---|---|---|---|
| White Matter Model | <0.0001 | ||
| Intercept | 24.773 | 0.871 | <0.0001 |
| Shunt (Yes) | −4.177 | 1.348 | 0.0032 |
| Time | −2.310 | 0.606 | 0.0002 |
| Time × Shunt (Yes) | 1.652 | 0.536 | 0.0028 |
| Time × Age | 0.235 | 0.052 | <0.0001 |
| Time2 | 0.308 | 0.092 | 0.0011 |
| Time2 × Age | −0.030 | 0.008 | 0.0001 |
| Time2 × Shunt (Yes) | −0.202 | 0.070 | 0.0045 |
| CSF Model | <0.0001 | ||
| Intercept | 10.998 | 1.234 | <0.0001 |
| Shunt (Yes) | 4.006 | 1.895 | 0.0386 |
| Time | −1.528 | 0.688 | 0.0274 |
| Time × Shunt (Yes) | −1.520 | 0.651 | 0.0213 |
| Time × Age | 0.133 | 0.057 | 0.0215 |
| Time2 | 0.187 | 0.108 | 0.0866 |
| Time2 × Age | −0.014 | 0.009 | 0.1251 |
| Time2 × Shunt (Yes) | 0.166 | 0.082 | 0.0436 |
Estimates were derived by using the random coefficient model. Since the longitudinal volume versus time data was assessed with a quadratic random coefficient model, the second-order time component is shown as Time2
Impact of Shunt Status in Medulloblastoma Group
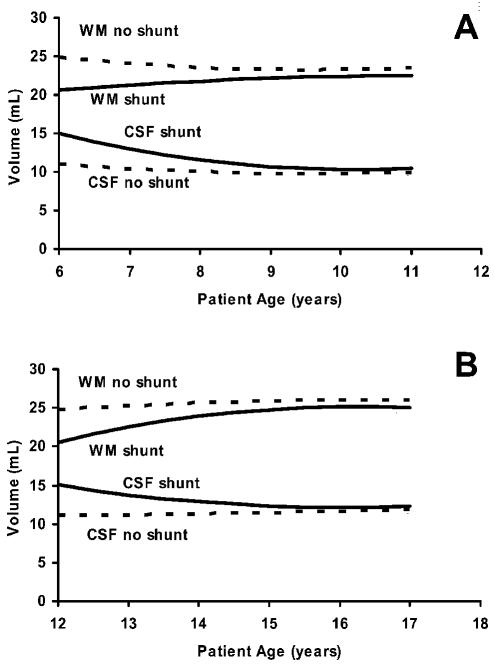
Figure 3 shows the NAWM and CSF volumes predicted by the random coefficient model for patients receiving CSI at 6 years of age (panel A) or at 12 years of age (panel B), with and without a shunt. In both cases, use of a shunt initially predicted a larger CSF volume and a smaller NAWM volume. Surprisingly, however, over the next five years, these volumes asymptotically approached those of patients who did not require shunts. To remove the confounding changes associated with shunt placement, we eliminated the patients with shunts from further comparison with the HC group. However, because the modeled data predicted very similar NAWM and CSF volume four to five years after therapy regardless of shunt status, conclusions derived from the comparison would also be relevant for long-term follow-up of patients with shunts.
Fig. 3.
Estimated volume of normal-appearing white matter and CSF projected by the study data over a five-year period after treatment of medulloblastoma at the age of 6 years (A) or 12 years (B). Patients did (solid lines) or did not (dashed lines) have ventricular shunts. Values represent absolute volume calculated for the index section. The curves were generated by using the quadratic random coefficient model.
Comparison of Medulloblastoma Group with Healthy Control Group for Change over Time in NAWM Volume
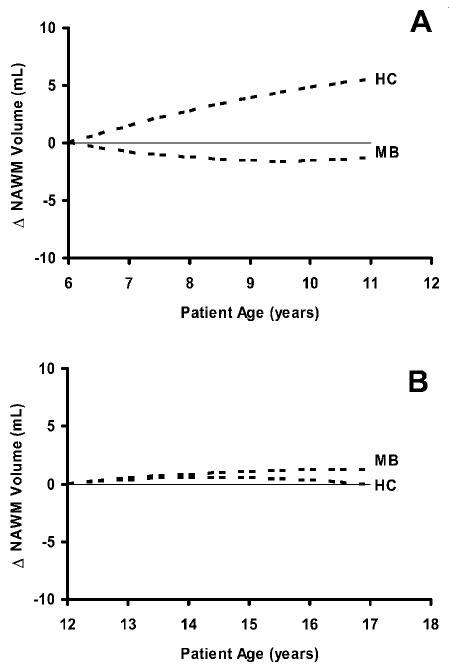
In control subjects, the volume of NAWM was positively associated with increasing age (P = 0.07), but the volume of CSF was not (P = 0.72). Figure 4 illustrates the volumetric changes observed at the level of the index section during normal maturation of controls. The study data were used to model the projected change in NAWM volume in HC subjects imaged at ages 6 to 12 years (HC, Fig. 3A) and at ages 12 to 18 years (HC, Fig. 3B). By using the generalized models reported in the previous sections for the MB group, we projected changes over a five-year period in NAWM volume for patients without shunts who received CSI at age 6 (MB, Fig. 3A) or at age 12 (MB, Fig. 3B). Values were standardized by the intercepts at 6 and 12 years of age to allow comparison with projected values for the HC subjects.
Fig. 4.
Change in the volume of normal-appearing white matter (NAWM) projected by the model in healthy control subjects (HC) and patients treated for medulloblastoma (MB) without ventricular shunts starting at the age of 6 years (A) or 12 years (B). A solid line at zero change is shown for reference. Absolute volume is standardized to the intercept at ages 6 and 12 to facilitate comparison. The curves were generated by using quadratic regression models.
As would be expected, HC children between ages 6 and 12 years exhibited more rapid maturational changes in NAWM volume, with the mean NAWM volume increasing approximately 1.1 ml (5.4%) per year. In contrast, changes over these five years in NAWM volume in the MB group irradiated at age 6 differed markedly from those in age-similar controls. NAWM volume in the MB group decreased at a rate of −0.3 ml (−1.1%) per year over the next five years. In contrast to the younger children, HC adolescents between ages 12 and 18 exhibited mean NAWM volumes that remained almost constant, as expected, increasing by only 0.1 ml (0.5%) per year, which is well within the expected measurement error of approximately 2%. Projected changes in NAWM volume in patients irradiated at age 12 appeared to be relatively stable and comparable to control subjects.
Direct Comparison of Medulloblastoma Group with Healthy Control Group
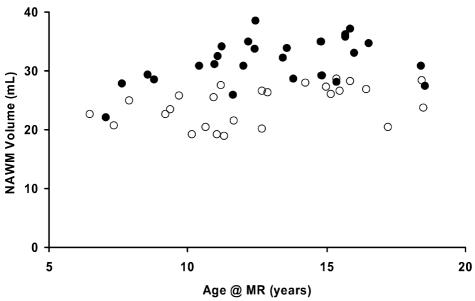
In the most recent examination of MB patients without shunts under the age of 19, 26 MB patients were directly compared to the HC group (Fig. 5). The median age at the time of CSI for the MB group was 8.3 years (range, 4.3–14.2 years). MRI was performed a median of 4.7 years after CSI (range, 0.9–7.2 years). Student t tests revealed that the age at examination for the MB group (13.0 ± 3.1 years) was not significantly different than the age at examination for the HC group (12.6 ± 3.4 years). However, the MB group did have a highly significant (P < 0.0001) deficit in NAWM volume (24.2 ± 3.3 cm3) compared to the HC group (31.6 ± 3.8 cm3).
Fig. 5.
Volume of normal-appearing white matter (NAWM) observed in healthy control subjects (solid circles) and NAWM volume at the most recent examination observed in patients treated for medulloblastoma (empty circles) without ventricular shunts plotted against age of the subject at the time of examination.
Discussion
This study is the first to use quantitative MRI to compare changes in the volumetric brain development of children surviving MB with those of HC subjects. This study is also the first to analyze longitudinal MR changes to quantify the impact of clinical variables such as age at the time of CSI and shunt status on brain development in children surviving MB.
When compared to healthy peers, young patients treated for MB exhibited an observable deficit in NAWM volume development. This finding implies that the neurocognitive deficits observed in this patient group may be caused by the absence of normal maturation of white matter after CSI. It is reasonable to speculate that these survivors do not experience normal myelination of axons. Lack of normal myelination could reduce the efficiency of signal transfer, yielding longer response time and possibly difficulty in learning new information at an age-appropriate rate. These findings are consistent with the lower rate of intellectual development observed among children treated for MB (Palmer et al., 2003).
Age at the time of CSI was significantly related to development of NAWM. The reduced development of NAWM volume in patients who receive CSI at a younger age implies that myelination is halted at an earlier stage in these patients and is consistent with the more severe problems with learning and intellectual development observed in this set of patients (Mulhern et al., 1998; Palmer et al., 2001).
Although patients requiring shunts initially demonstrated a higher volume of CSF and a lower volume of NAWM than the CSF and NAWM volumes observed in other MB patients, these differences resolved over a four-to five-year period of follow-up. The gradual normalization of CSF volume in patients who require shunts may explain why the effect of shunts on volumetric measures varies with the length of follow-up in cross-sectional studies.
Our study was designed to comprise as homogeneous a group of subjects as possible: Patients received similar doses of CSI to treat the same type of tumor, which arose in the same location. However, this study had several limitations. Imaging was limited to a single representative section and therefore could not detect changes in NAWM volume that may have occurred in other locations. CSI-induced changes are known to occur most frequently in the centrum semiovale and periventricular regions. The index section used in this study did sample the periventricular region, and it is highly predictive of the full cerebral volume in other patient populations (Glass et al., 2003). Another limitation is the fact that although all patients received approximately the same dose of CSI (35–40 Gy) and had approximately the same dose of irradiation to the posterior fossa or tumor bed, the NAWM examined in the index section did not receive a uniform dose of irradiation. Differences in the methods used to irradiate the posterior fossa and in the sizes of the original tumors are likely to have caused different doses of irradiation to be delivered to different regions of NAWM. Despite the marked differences we observed between the young MB and HC groups, the nonlinear nature of these models and the cross-sectional (HC) versus longitudinal (MB) observations did not allow a direct statistical comparison between models. However, a direct comparison between the HC subjects and the most recent examination of patients without shunts under the age of 19 revealed a highly significant deficit in NAWM volumes in the MB group, which is consistent with the differences predicted by the nonlinear models.
This study was the first to use quantitative MRI to conclude that patients treated for MB at earlier ages demonstrated substantially less development of NAWM volume than did their healthy peers. The next logical steps in this line of investigation would be to integrate the digital radiation dosimetry maps with the segmented MR data to determine the NAWM volume–dose response and establish a critical dose threshold; to expand the volume of interest by sampling a larger proportion of the cerebrum; and to combine regional analyses with more sensitive and specific neurocognitive testing. Such studies would be necessary to establish the precise relationship between therapy, the location of deficient development of NAWM volume, and impact on neurocognitive performance. A critical dose threshold for NAWM could be defined and then incorporated into optimized radiation therapy planning. Combining regional NAWM analyses with more sensitive and specific neurocognitive testing could predict specific neurocognitive deficits, which would then be targeted with remedial or rehabilitative interventions (Butler and Copeland, 2002; Thompson et al., 2001).
Acknowledgments
The authors wish to acknowledge and thank Rhonda Simmons for her assistance in the collection and processing of the MRI data and Sharon Naron for editorial consultation.
Footnotes
This work was supported in part by Cancer Center Support (CORE) grant P30CA21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations used are as follows: CSF, cerebrospinal fluid; CSI, craniospinal irradiation; HC, healthy control; MB, medulloblastoma; MR, magnetic resonance; NAWM; normal-appearing white matter
References
- Andreasen NC, Flaum M, Swayze V, II, . O’Leary, D.S. Alliger, R. Cohen, G. Ehrhardt, J. and, Yuh WTC. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Butler RW, Copeland DR. 2002. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. 124;J Int Neuropsychol Soc:8–115. [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol. 1996;29:91–101. doi: 10.1007/BF00165522. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glass JO, Ji Q, Glas LS, Reddick WE. Prediction of total cerebral tissue volumes in normal appearing brain from sub-sampled segmentation volumes. Magn Reson Imaging. 2003;21:977–982. doi: 10.1016/j.mri.2003.05.010. [DOI] [PubMed] [Google Scholar]
- Inzitari D. Age-related white matter changes and cognitive impairment. Ann Neurol. 2000;47:141–143. [PubMed] [Google Scholar]
- Littell, R.C., Milliken, G.A., Stroup, W.S., and Wolfinger, R.D. (1996) SAS System for Mixed Models, Cary, N.C.: SAS Institute Inc.
- Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: A Pediatric Oncology Group study. J Clin Oncol. 1998;16:1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Reddick WE, Palmer SL, Glass JO, Elkin TD, Kun LE, Taylor JS, Langston JW, Gajjar A. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol. 1999;46:834–841. doi: 10.1002/1531-8249(199912)46:6<834::aid-ana5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor JS, Langston JA, Gajjar A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun LE, Merchant TE, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, Xiong X, Mulhern RK. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Cook EN, Elkin TD, Deaton RJ. Automated segmentation and classification of multispectral magnetic resonance images of brain using artificial neural networks. IEEE Trans Med Imaging. 1997;16:911–918. doi: 10.1109/42.650887. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Mulhern RK, Elkin TD, Glass JO, Merchant TE, Langston JW. A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magn Reson Imaging. 1998;16:413–421. doi: 10.1016/s0730-725x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Russell JM, Glass JO, Xiong X, Mulhern RK, Langston JW, Merchant TE, Kun LE, Gajjar A. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. [DOI] [PubMed] [Google Scholar]
- Reddick WE, Glass JO, Langston JW, Helton KJ. Quantitative MRI assessment of leukoencephalopathy. Magn Reson Med. 2002;47:912–920. doi: 10.1002/mrm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Leigh L, Mulhern RK. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: A developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14:189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Leigh L, Christensen R, Xiong X, Kun LE, Heideman RL, Reddick WE, Gajjar A, Merchant T, Pui CH, Hudson MM, Mulhern RK. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19:1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer R.J. s. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: Conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]