Abstract
While the introduction of carmustine wafers (Gliadel wafers) into the tumor resection cavity has been shown to be a beneficial therapy for malignant glioma, it is recognized that clinically significant cerebral edema is a potential adverse effect. Following are two clinical case reports demonstrating profound cerebral edema associated with implantation of Gliadel wafers. As a result, one of these individuals had premature death. A brief literature review is provided to assist in explaining the mechanisms by which clinically significant cerebral edema may develop.
The introduction of Gliadel (carmustine; Guilford Pharmaceuticals, Baltimore, Md.) wafers in the 1990s was a significant breakthrough in the management of glioblastoma multiforme (GBM).2 Well-performed clinical trials have consistently demonstrated Gliadel’s efficacy, extending life on average up to six months in some studies (Brem et al., 1991, 1995; Olivi et al., 2003; Valtonen et al., 1997; Westphal et al., 2003). The localized chemotherapy administered with Gliadel obviously lowers systemic adverse effects, but importantly for Gliadel wafers, the local adverse effects are only mildly significantly increased in comparison to resection alone.
Among the adverse effects that are increased are postoperative wound infection and the concern in the two patients presented—cerebral edema (Brem et al., 1991, 1995; Olivi et al., 2003; Valtonen et al., 1997; Westphal et al., 2003). The severity of this edema and the associated clinical morbidity are the subjects of this paper.
Following are two clinical case reports demonstrating profound cerebral edema apparently caused by implantation of Gliadel wafers. This cerebral edema led to repeated and extended hospitalizations, significant morbidity and neurological deficit, and delays in subsequent therapeutic modalities, such as radiation therapy and systemic chemotherapy. In one case, premature death eventually ensued.
Case 1
This 53-year-old white female was admitted to the hospital on July 24, 2000, with a two-week history of severe headache, altered gait, distortion of vision, and impaired memory for the names of close friends and relatives. Her neurological examination was significant for a dense left homonymous hemianopsia and slight left-sided facial weakness. A CT scan demonstrated a large right temporal–parietal mass with greatest diameter of 7 cm. (The images in Figs. 1 and 2 demonstrate actual tumor configuration, and the T2-weighted image in Fig. 2 depicts the associated edema and ventricular shift). The patient was subsequently placed on dexamethasone 6 mg every 6 h.
Fig. 1.
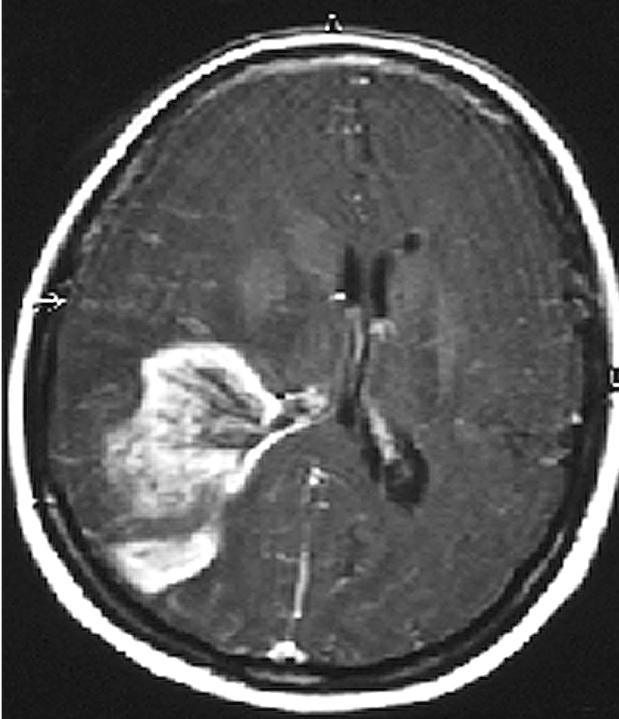
Case 1 preoperative CT scan showing tumor configuration.
Fig. 2.
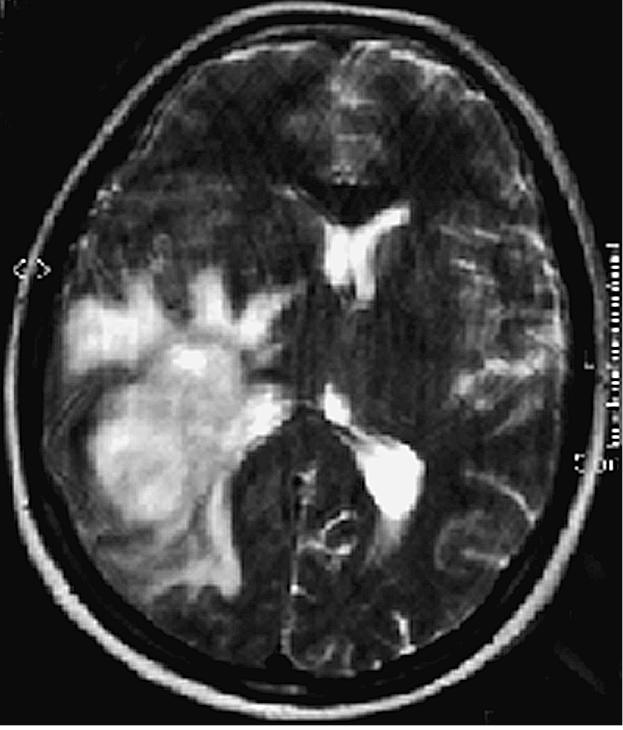
Case 1 preoperative MRI T2-weighted image depicts the associated edema and ventricular shift.
On July 26, a right posterior temporal–parietal craniotomy was performed with gross total resection of a histologically confirmed GBM. The cavity was subsequently lined with eight Gliadel wafers. In the immediate postoperative period, there was no neurological change, and she was continued on the prior dexamethasone regimen. The stitches were removed, and she was discharged home on phenytoin and 1 mg every 8 h of dexamethasone. In follow-up on August 9, 2000, she again demonstrated a dense left homonymous hemianopsia and left facial weakness, but had also developed a degree of cortical sensory loss.
On August 14, the patient began vomiting and had increased headache and affect change. A CT scan (Fig. 3) demonstrated a marked midline shift with edema throughout the right hemisphere, which was more pronounced than on preoperative images. There was concern over possible uncal herniation, and she was readmitted to the hospital. A drug regimen of 10 mg dexamethasone every 6 h and 20 g mannitol intravenous every 4 h was initiated. With this treatment she improved and was discharged two days later on oral dexamethasone 8 mg every 8 h.
Fig. 3.
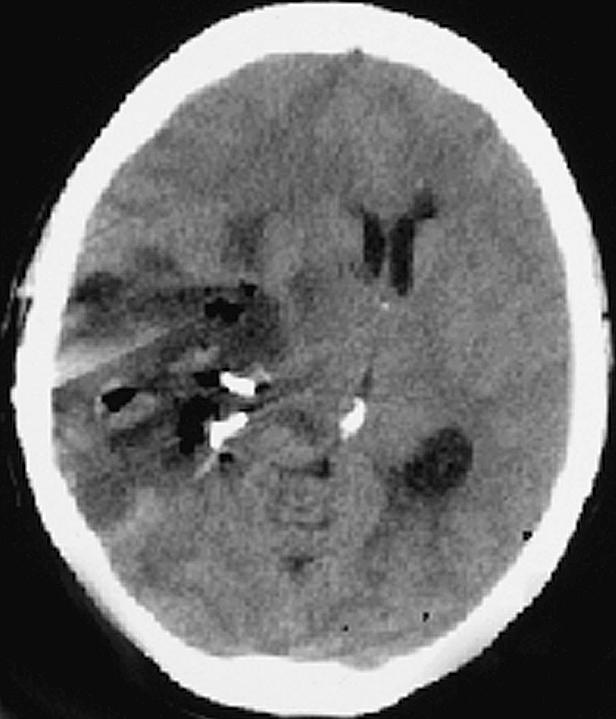
Case 1 postoperative day 19 CT scan demonstrating a marked midline shift with edema throughout the right hemisphere that is more pronounced than on preoperative images.
On exam one week later, the patient had developed further proprioceptive loss on the left side, but was otherwise stable neurologically. Then on August 28, she returned to the emergency room with somnolence and new weakness in her left lower extremity. A CT scan (Fig. 4) was repeated and demonstrated slight improvement over her condition two weeks prior. Radiation therapy, which normally would have been initiated at this point, was not started because of the severity of the edema and the midline shift. The patient was again treated with dexamethasone and mannitol over the course of the next day and subsequently discharged. Two days later, she again returned to the emergency room and was admitted with a severe headache, a dense left hemiparesis, and increased somnolence; however, she was alert, oriented, and responding appropriately. A CT scan (Fig. 5) demonstrated increased edema throughout the right hemisphere and further shift of the midline structure. Dexamethasone 8 mg intravenous every 4 h, mannitol 20 g every 4 h, and furosemide were given intravenously. The patient became progressively more obtunded, pupils became dilated, and she ceased to respond to painful stimuli. On September 3, just 39 days following her craniotomy and Gliadel wafer implantation, the patient died.
Fig. 4.
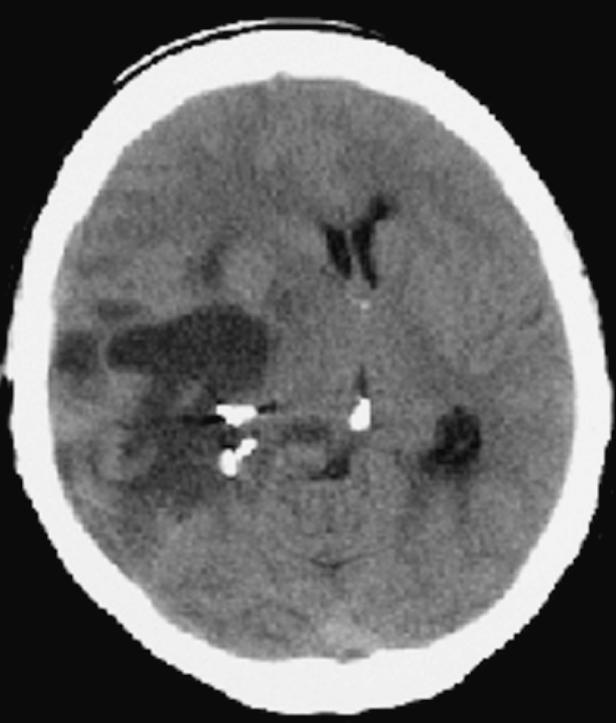
Case 1 postoperative day 33 CT scan demonstrating slight improvement over day 19 condition.
Fig. 5.
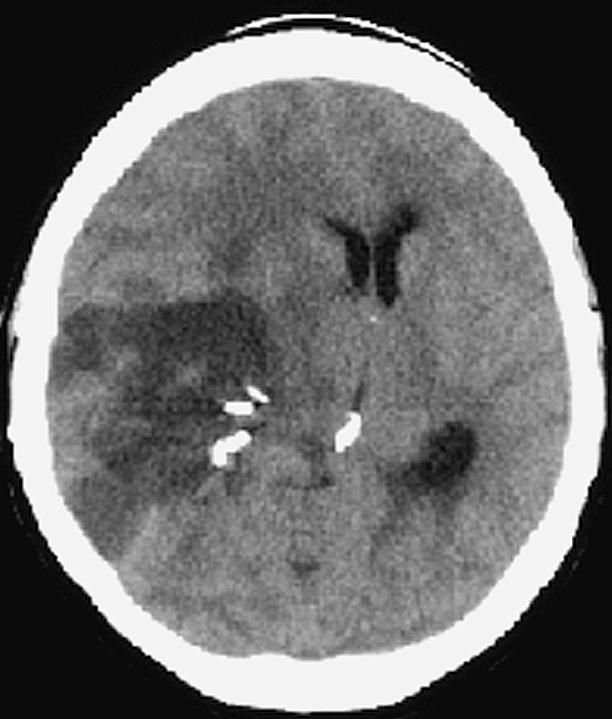
Case 1 postoperative day 35 CT demonstrating increased edema throughout the right hemisphere and further shift of the midline structure.
Case 2
In December 2002, this 60-year-old white female began having focal motor and sensory seizures on the right side. These increased in frequency and severity, and an MRI scan (Figs. 6 and 7) revealed a mass lesion in the high left frontal–parietal area with minimal edema. Her exam demonstrated a right-sided hemisensory deficit with good gross muscle strength. On February 10, 2003, dexamethasone was initiated at 4 mg every 8 h.
Fig. 6.
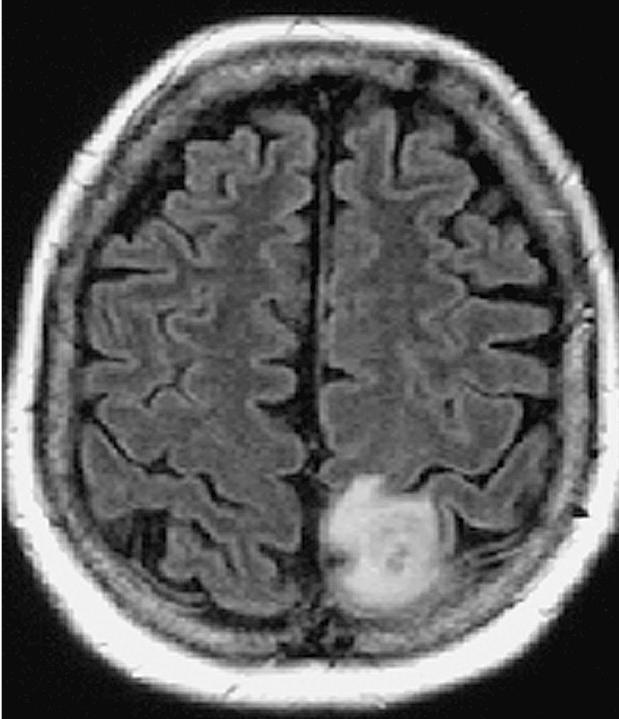
Case 2 preoperative MRI T1-weighted image revealing a mass lesion in the high left frontal–parietal area with minimal edema.
Fig. 7.
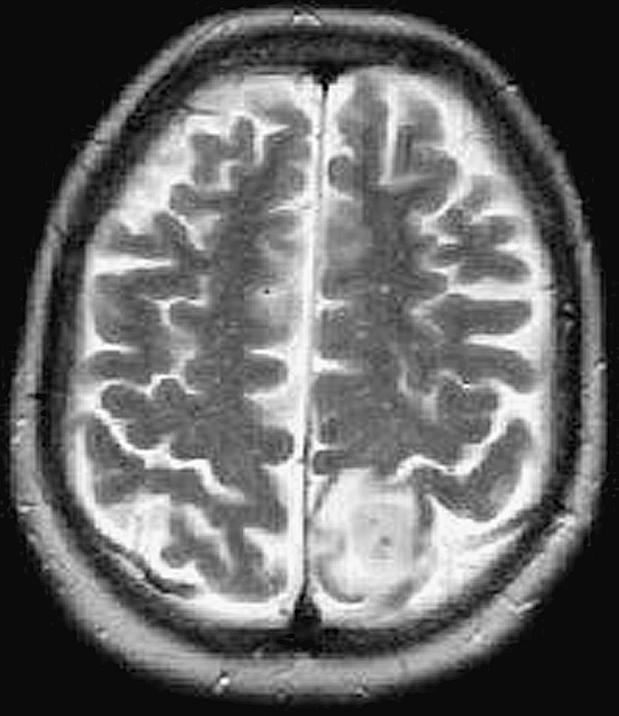
Case 2 preoperative MRI T2-weighted image revealing a mass lesion in the high left frontal–parietal area with minimal edema.
On February 12, a left frontal-parietal craniotomy was performed. A discrete mass lesion was grossly resected and classified as a grade III fibrillary astrocytoma. As the size of the resection cavity was limited, only four Gliadel wafers were implanted on the brain surface. Immediately postoperation, the patient had no sign of increased paresis, cortical sensory deterioration, or speech loss. She was continued on the dexamethasone 4 mg every 8 h. The following day, the patient was without evidence of seizure activity or aphasia, but the right-sided weakness and sensory loss had increased. On February 14, a CT scan (Fig. 8) demonstrated increased edema in the left hemisphere; furthermore, the hemiparesis continued to worsen. Two days later, on February 16, the hemiparesis had increased further, and on February 17, the patient had a focal seizure. Anticonvulsants were adjusted, and a repeat CT scan (Fig. 9) demonstrated increasing edema of the left hemisphere with mass effect. The dexamethasone dosage was increased to 6 mg every 6 h, and the patient was transferred back to the intensive care unit, where mannitol was initiated. Over this period she developed aphasia with worsening of the hemiparesis.
Fig. 8.
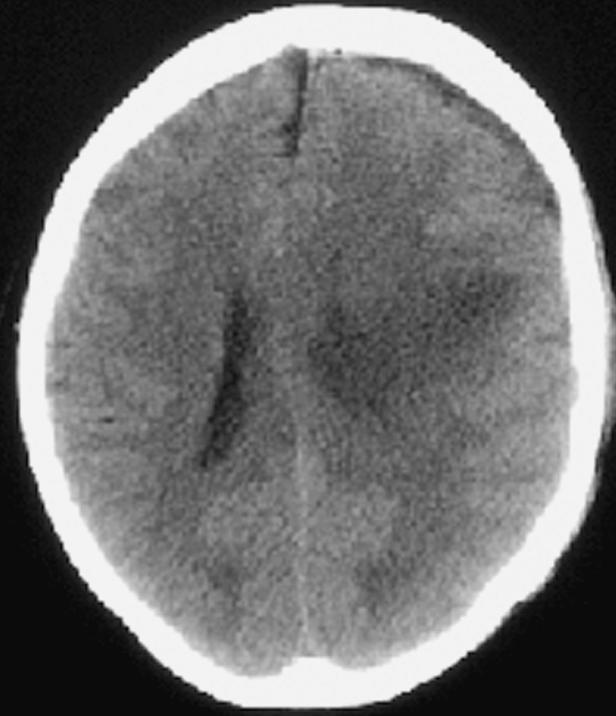
Case 2 postoperative day 2 CT scan demonstrating increased edema in the left hemisphere.
Fig. 9.
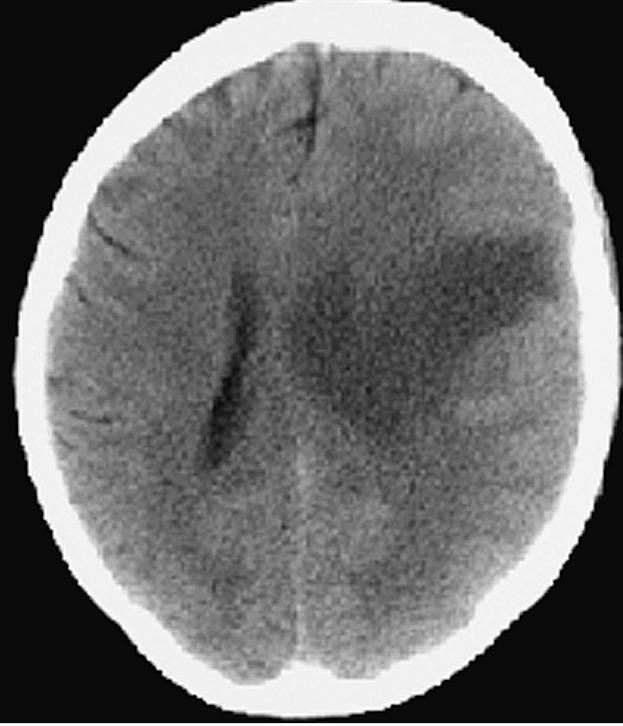
Case 2 postoperative day 5 CT scan demonstrating increasing edema of the left hemisphere with mass effect.
On February 19, a CT scan (Fig. 10) demonstrated a decrease in the amount of cerebral edema, and her speech began to improve. Three days later, the patient was still somewhat somnolent and aphasic with a persistent right hemiparesis. On February 24, a right homonymous heminopsia presented on examination for the first time, and subsequently, her dexamethasone was increased to 10 mg every 6 h. On February 27, an MRI and magnetic resonance angiography were performed; the tests showed no signs of vascular occlusion, but did show extensive cerebral edema throughout the left hemisphere with a left to right midline shift, and dexamethasone was increased further to 15 mg every 6 h.
Fig. 10.
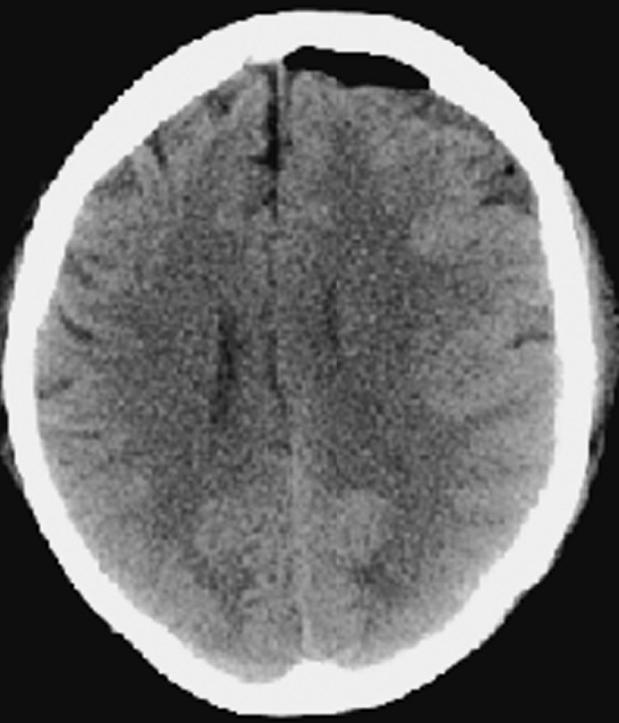
Case 2 postoperative day 7 CT scan demonstrating a decrease in the amount of cerebral edema.
By March 3, the patient began moving her right side slightly, and her speech had improved moderately. She was transferred, 21 days after her admission, to a rehabilitation facility, still on dexamethasone 12 mg every 6 h, as well as anticonvulsants. In the subsequent four months she exhibited some resolution of the aphasia, hemiparesis, and visual field loss, but at the time of this writing remains unable to walk independently. Subsequently, radiation therapy and temozolomide were initiated, followed by further chemotherapy under the guidance of Neuro-Oncology physicians at Duke University. As of the patient’s last office visit in August 2004, the patient was obviously alive and doing well.
Discussion
Malignant glioma resection alone inherently produces some degree of cerebral edema, but this is typically localized to the areas adjacent to the resection site and well managed with corticosteroids (Brem et al., 1991, 1995; Olivi et al., 2003; Valtonen et al., 1997; Westphal et al., 2003). The two individuals presented in this case review, however, demonstrated extensive postoperative cerebral edema, particularly involving the ipsilateral hemisphere. In both individuals, the cerebral edema was sufficient to produce significant mass effects and subsequent morbidity, as well as mortality in one patient.
One might contend that rapid tumor recurrence played some role in the edema formation in these two patients, but this seems unreasonable for even malignant gliomas (Giese and Westphal, 1996). This seems to be supported radiographically, as the mass effect in both patients is edema related and not due to tumor. The patient in Case 1 died after only 39 days, which is not typical after GBM resection. The Case 2 patient is still alive, and subsequent to the resolution of the edema, additional therapy was instituted; her clinical deterioration began one day postoperative, and this event would not fit with tumor recurrence.
One might also contend that the seizure in Case 2 contributed to the clinical symptoms and signs, but these would be expected to reverse with time, as well as with use of medications for seizure control. This was not the case and the patient currently suffers greater deficits than were present preoperatively or even immediately postoperatively. In both individuals, there were no comorbid conditions that might have exacerbated or facilitated the occurrence of edema. It seems most likely that the Gliadel wafer caused the profound postoperative edema seen in these two patients.
Carmustine was initially introduced intravascularly for treatment of malignant gliomas, as the drug elicited beneficial clinical responses experimentally. As expected, the intravascular route produced dose-limiting systemic cytotoxicity. The 15-min half-life of carmustine also made it a suboptimal drug (Brem et al., 1991). Subsequently, the development and implantation of carmustine-impregnated wafers (Gliadel wafers) into the tumor resection cavity began in the 1990s. This mechanism allowed for significantly higher local concentrations of carmustine over a greater period of time; subsequent improvements in survival after either primary or recurrent resection of malignant gliomas were found (Brem et al., 1991, 1995; Olivi et al., 2003, Valtonen et al., 1997, Westphal et al., 2003).
Overall, morbidity has been minimal, and only one other case of mortality associated with Gliadel (standard dose of 3.85% carmustine [BCNU] by weight) use has been reported (Subach et al., 1999). In a study performed on 21 patients in 1991, Brem et al. did note having to increase steroid doses and reoperate on several patients secondary to neurological decline with mass effect. These patients were found to have extensive necrotic debris in the resection cavity. The patients subsequently improved with reoperation and debridement of necrotic tissue. In these individuals, the cerebral edema occurred several weeks postoperation. This clinical course is similar to the developments in our Case 1 patient.
A more recent study published by Olivi et al. (2003) assessed escalating doses (ranging from 6.5% to 27% BCNU) of BCNU wafers on recurrent malignant gliomas in 44 human beings. Only at the level of 27% BCNU by weight did severe morbidity result as an individual developed anoxic brain injury. As dosing levels were increased, complications of seizures and cerebral edema increased, yet no mortality occurred.
Brem et al. (1994) further studied Gliadel wafers on nonhuman primates. This study demonstrated edema production to be more pronounced, yet subtle, with wafer (without carmustine) implantation versus resection alone. Furthermore, wafers with carmustine yielded greater edema production with mild midline shifts in most cases. The thickness of necrotic debris was also 2- to 6-fold greater in the carmustine-wafer group versus wafer group only, which may correlate with the problems seen in some of Brem’s 1991 study patients and our Case 1 patient.
In order to understand the mechanism by which carmustine might elicit cerebral edema, understanding the pharmacokinetics of carmustine is important. First, the distribution of carmustine within the brain is actually dependent upon the state of the brain parenchyma, that is, a nontraumatized versus traumatized state. Obviously, the traumatized state is the one of interest in investigating carmustine distribution postresection of brain parenchyma. Theoretically, drug distribution within the brain has four basic patterns: (1) diffusion, (2) bulk or convective flow, (3) intravascular invasion and extravasation, and (4) cerebrospinal fluid (CSF) invasion and extravasation. In nontraumatized brain parenchyma, diffusion is the predominant mechanism by which carmustine distributes. This may well be an important mechanism for distribution to the tissue adjacent the resection cavity, as most recurrences occur within centimeters of the cavity (Fleming and Saltzman, 2002).
However, in the case of traumatized tissue, results of studies performed indicate a predominance of other mechanisms of distribution. The mechanism of bulk flow was demonstrated in cats by Reulen et al. (1977). Vasogenic edema, which is felt to be the determinant of bulk flow, was induced in cats. The distribution of several molecules and concomitant measurement of interstitial pressures within the parenchyma were assessed, and the findings were consistent with bulk flow as the predominant mechanism of molecule distribution rather than diffusion. The pressure gradient resulting from the vasogenic edema secondarily produces convective flow and spread of drug in the white matter (a low-resistance pathway) (Reulen et al., 1977), as well as potential uptake into the CSF. The increased uptake into the CSF was a notion supported in studies on rat and monkeys (Brem et al., 1994; Fung et al., 1996). The idea of CSF permeation by carmustine, particularly in monkeys, may prove to be a better explanation not only for the widespread distribution, but also the distance traveled in the monkey brain. It was felt that the length of carmustine distribution could not be explained by diffusion and/or convective flow alone, supporting CSF or vascular permeation as another mechanism of carmustine distribution. It was also notable that in monkey brains the contralateral CSF contained carmustine one day postimplantation, lending further support to this mechanism (Fung et al., 1996; Reulen et al., 1977).
In assessing carmustine levels post–polymer implantation in rats, Fung et al. (1996) also demonstrated distribution fivefold greater one to three days postimplantation in comparison to that determined on days 3 to 14. It was concluded that carmustine distribution was increased in the first three days secondary to vasogenic edema and bulk flow. Subsequently, as the vasogenic edema resolved, the area of carmustine distribution decreased markedly. A study done by Grossman et al. (1992) with rabbits further supports the widespread distribution of carmustine in the first three days postimplantation, as radiolabeled carmustine was extensively distributed throughout the ipsilateral hemisphere. When measurements were taken 7 to 21 days postimplantation, carmustine was detected only directly adjacent to the implantation site.
Briefly stated, these two individuals appeared to have two separate mechanisms by which carmustine initiated their downhill courses. The patient in Case 1 had a delayed onset of approximately two weeks, responded poorly to standard treatment for brain edema, and eventually succumbed to the elevated intracranial pressure. Referring to the 1991 study by Brem et al., there were multiple incidences of carmustine wafer use in which reoperation was required for neurological decline two to three weeks postimplantation. In these cases, it was the necrosis induced by carmustine that was felt to be the culprit. This patient fits this scenario, and thus this is the likely mechanism of the edema and death.
The patient of Case 2 demonstrated symptoms one day postoperative, which fits well with the potential cytotoxic effects of carmustine. Repeatedly it has been shown that carmustine distributes widely, at least in the ipsilateral hemisphere, the first several days postimplantation. Thus the initial vasogenic edema induced by the surgery likely allowed for a situation of convective flow and greater distribution of carmustine, with subsequent cytotoxicity. While this patient eventually responded to therapy, initially the edema was refractory, producing profound disability and delay of further treatment modalities.
Qualitatively, the requirement of corticosteroids postimplantation is known to be important in reducing the degree of cerebral edema that is associated with Gliadel wafers. The patients presented here were subsequently on fairly high doses of dexamethasone at times, as is required; and whether even higher dosing would have benefited these individuals can be considered in retrospect. Yet, a search for corticosteroid use and dosing with Gliadel wafers in the published literature (as these authors were able to find) revealed no recommendations on the quantity of corticosteroids to be administered. This is apparently a very important issue and may need to be addressed in the near future if further complications such as these arise.
Conclusions
While the use of Gliadel wafers for treatment of malignant gliomas has been shown in multiple studies to be efficacious with minimal adverse effects, the two individuals described in this case review demonstrate significant ipsilateral cerebral edema and progressive neurological deficit. Prior to this case review, there had been only one report of malignant cerebral edema that was unresponsive to medical management (Subach et al., 1999). Case 1 here describes a second individual that likely succumbed to a detrimental effect of intracranial carmustine use. Both cases demonstrate notable morbidity, prolonged and repeated hospitalizations, and delays in other established treatment modalities. The literature supports Gliadel wafers having a role in the treatment of GBMs. Yet, it is important to recognize two things when using this product: first, the need for use of high-dose corticosteroids early and continued as needed to prevent cerebral edema, and second, the criticality of the time at which the cerebral edema presents clinically. Early presentation may require medical management only, while in the occurrence of late presentation, one may consider surgical management if initial medical management fails.
Footnotes
Abbreviations used are as follows: BCNU, carmustine; CSF, cerebrospinal fluid; GBM, glioblastoma multiforme.
References
- Brem H, Mahaley MS, Jr, Vick NA, Black KL, Schold SC, Jr, Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, Kenealy JN. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74:441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- Brem H, Tamargo RJ, Olivi A, Pinn M, Weingart JD, Wharam M, Epstein JI. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J Neurosurg. 1994;80:283–290. doi: 10.3171/jns.1994.80.2.0283. [DOI] [PubMed] [Google Scholar]
- Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC for the Polymer-Brain Tumor Treatment Group. Placebocontrolled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- Dang WB, Daviau T, Ying P, Zhao Y, Nowotnik D, Clow CS, Tyler B, Brem H. Effects of Gliadel wafer initial molecular weight on the erosion of wafer and release of BCNU. J Control Release. 1996;42(1):83–92. [Google Scholar]
- Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- Fung LK, Shin M, Tyler B, Brem H, Saltzman WM. Chemotherapeutic drugs released from polymers: Distribution of 1,3-bis (2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13:671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Grossman SA, Reinhard C, Colvin OM, Chasin M, Brundrett R, Tamargo RJ, Brem H. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg. 1992;76:640–647. doi: 10.3171/jns.1992.76.4.0640. [DOI] [PubMed] [Google Scholar]
- Olivi A, Grossman SA, Tatter S, Barker F, Judy K, Olsen J, Bruce J, Hilt D, Fisher J, Piantadosi S. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: A New Approaches to Brain Tumor Therapy CNS Consortium trial. J Clin Oncol. 2003;21:1845–1849. doi: 10.1200/JCO.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Reulen HJ, Graham R, Spatz M, Klatzo I. Role of pressure gradients and bulk flow in dynamics of vasogenic brain edema. J Neurosurg. 1977;46:24–35. doi: 10.3171/jns.1977.46.1.0024. [DOI] [PubMed] [Google Scholar]
- Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M, Schiff D. Morbidity and survival after 1,3-bis (2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: A retrospective case-matched cohort series. Neurosurgery. 1999;45:17–22. doi: 10.1097/00006123-199907000-00004. [DOI] [PubMed] [Google Scholar]
- Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsqaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: A randomized double-blind study. Neurosurgery. 1997;41:48–49. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


