Abstract
Histone modification has emerged as a promising approach to cancer therapy. We explored the in vivo efficacy of a butyric acid derivative, pivaloyloxymethyl butyrate (AN-9), for the treatment of gliomas. Relative to control and single-modality treatments, the combination of AN-9 and radiation significantly inhibited tumor growth and prolonged time to failure in mice bearing glioma xenografts. The enhanced response to radiation was accompanied by inhibition of cellular proliferation and by increased phosphorylation of H2AX, implicating DNA double-strand breaks in the antineoplastic effects of AN-9 and radiation. The data suggest that AN-9 in combination with radiation may be an effective therapy for malignant gliomas.
Keywords: AN-9, γ-H2AX phosphorylation, glioma, histone deacetylase inhibitor, radiation, temozolomide
Preclinical and clinical studies are evaluating several histone deacetylase inhibitors (HDACIs),4 including a prodrug of butyric acid (BA), pivaloyloxymethyl butyrate (AN-9) (Patnaik et al., 2002; Reid et al., 2004), the hydroxamic acids suberoylanilide hydroxamic acid (SAHA) and trichostatin A (Zhang et al., 2004), benzamide derivatives MS-275 and CI-994 (Prakash et al., 2001; Saito et al., 1999), cyclic peptides trapoxin, apicidin, and depsipeptide (Zhang et al., 2004), and valproic acid (Blaheta et al., 2005). Recent studies have revealed the radiosensitizing abilities of various HDACIs, including MS-275 (Camphausen et al., 2005), SAHA (Zhang et al., 2004), valproic acid (Camphausen et al., 2005), trichostatin A (Tang et al., 2004), and BA (Munshi et al., 2005), the last of which is the parental form of AN-9 (Nudelman et al., 1992). These studies highlight the potential for incorporating HDACIs into multimodality treatment of gliomas. We recently reported that AN-9 induces hyperacetylation, inhibits proliferation, and enhances apoptosis of glioblastoma multiforme cells in vitro. In addition, AN-9 enhances the cytotoxic effects of radiation in vitro (Entin-Meer et al., 2005).
In this study, we sought to assess the in vivo antineoplastic efficacy of AN-9 in combination with radiation for the treatment of high-grade gliomas grown as xenografts in athymic mice. Our data provide evidence that AN-9 enhances the in vivo therapeutic effects of radiation. Moreover, AN-9–mediated radiosensitization was associated with increased expression of phospho- γ-H2AX within glioma xenografts, suggesting that enhanced radiation-induced DNA damage plays a role in the combined effects of AN-9 and radiation.
Materials and Methods
Cells and Reagents
U251 MG human glioma cells (University of California, San Francisco brain tissue bank) were grown in a humidified incubator at 37°C and 8% CO2 in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (GIBCO-BRL/Invitrogen, Carlsbad, Calif.), penicillin (100 units/ml; GIBCO-BRL), and streptomycin (100 μg/ml; GIBCO-BRL). The BA derivative AN-9 was prepared as previously described (Nudelman et al., 1992). Temozolomide (TMZ) was kindly provided by the developmental therapeutic program of the National Cancer Institute/National Institutes of Health. Ketamine (80 mg/kg) and xylazine (10 mg/kg) were used to anesthetize mice during radiation treatments.
In Vivo Xenograft Administration
Xenografts of human glioma cell lines were established by subcutaneous inoculation of 5 × 106 U251 MG cells into the hind legs of BALB/c Nu/Nu athymic mice (Charles River, Wilmington, Mass.). Mice were monitored according to the protocol approved by the University of California, San Francisco Institutional Animal Care and Use Committee. When tumors reached 50–100 mm3, mice were randomly assigned to one of four treatment groups: vehicle control (10 animals), radiation alone (11 animals), AN-9 alone (11 animals), or combined AN-9 and radiation (12 animals). We randomized the animals unevenly among the groups in order to have more mice in the treatment groups than in the vehicle control group and thus add further significance to our statistical data.
AN-9, solubilized in mineral oil (Sigma, St. Louis, Mo.), was administered by oral gavage (50 mg/kg, three times per week) using 20-gauge curved needles (Pop-per and Sons, New Hyde Park, N.Y.) for a total of eight weeks. One week after AN-9 or vehicle (control) administration was initiated, mice were exposed to four fractions of 2.5 Gy, twice a week, with each treatment given 24 h after AN-9 administration.
For all radiation treatments, mice were anesthetized with ketamine/xylazine and then exposed to gamma irradiation (2.5 Gy or 5 Gy) directed at the tumor site, while the rest of the body was shielded by a lid jig. Tumor length (L) and width (W) were measured and tumor volume calculated as (L × W2/2), where L = longest diameter and W = shortest diameter, every four to seven days. Animals were euthanized (by CO2 inhalation followed by cervical dislocation) when tumors reached 2000 mm3 or became severely necrotic.
Staining and Immunohistochemical Analyses
Formalin-fixed, paraffin-embedded sections, 5 μm thick, were cut from tumors excised from the euthanized mice. Staining with hematoxylin and eosin and staining for Ki-67 and caspase-3 were carried out as previously described (Entin-Meer et al., 2005). The percentage of Ki-67–positive nuclei in a total of 1000 nuclei per section was calculated in each of two nonadjacent sections from each xenograft.
Histone Purification and Western Blot Analysis
Nu/nu athymic mice were inoculated with U251 MG cells. When tumors reached 100–200 mm3 in size, mice were treated with a single dose of AN-9 (50 mg/kg), radiation (2.5 or 5 Gy), or a combination thereof. Mice were then euthanized and the tumors excised. One portion of each tumor was prepared for paraffin-embedded sections, and the other portion was used for histone purification and Western blot analysis as previously described (Entin-Meer et al., 2005). Band intensities, visualized by enhanced chemiluminescence, were measured using Odyssey software (2002 version; Li-Cor Biosciences, Lincoln, Nebr.).
Cell Proliferation Assay
U251 MG cells (3000/well) were seeded in 96-well plates. Sixteen hours later, the medium was aspirated and fresh medium (100 μl) containing 120 μM AN-9 was added. Twenty-four hours later, cells were irradiated (4 Gy) and 100 μl of fresh medium containing 600 μM TMZ was added. Cells were incubated for 72 h, and then 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxmetho-xyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) reagent (Promega, Madison, WI) was added as previously described (Entin-Meer et al., 2005).
Data Analysis
Differences in tumor size between treatment groups were compared using a two-sided t-test (Excel for Windows 2002; Microsoft, Redmond, Wash.). The mean size of tumors receiving the combination treatment was compared to the mean size of tumors in mice from each of the other groups (receiving vehicle control, radiation alone, or AN-9 alone). The analysis was done on day 36 after treatment start, since this was the last day on which all animals were still alive. Time to treatment failure (TTF) was defined as the time from the initiation of treatment (experimental or control) to the time a tumor was severely necrotic or had reached ≥1000 mm3 in size. TTF was estimated by Kaplan-Meier curves, and differences in TTF between groups were assessed using the log-rank test (Statistica; StatSoft Ver. 6, Tulsa, Okla.). To be considered superior, the combination treatment needed to demonstrate significantly better (P < 0.05) antineoplastic effects than each of the other groups, and therefore no adjustment was necessary for multiple comparisons.
Results
We previously demonstrated that AN-9 augments the cytotoxic effects of radiation on glioma cells in vitro (Entin-Meer et al., 2005). To extend those findings, we investigated the in vivo radiosensitizing potential of AN-9 in U251 MG xenografts in mice. The xenografts were grown in the hind legs of Nu/Nu athymic mice and treated with vehicle control, AN-9 alone, radiation alone, or combined AN-9 and radiation. Each radiation fraction was administered 24 h after AN-9 treatment to maximize growth inhibition (Entin-Meer et al., 2005).
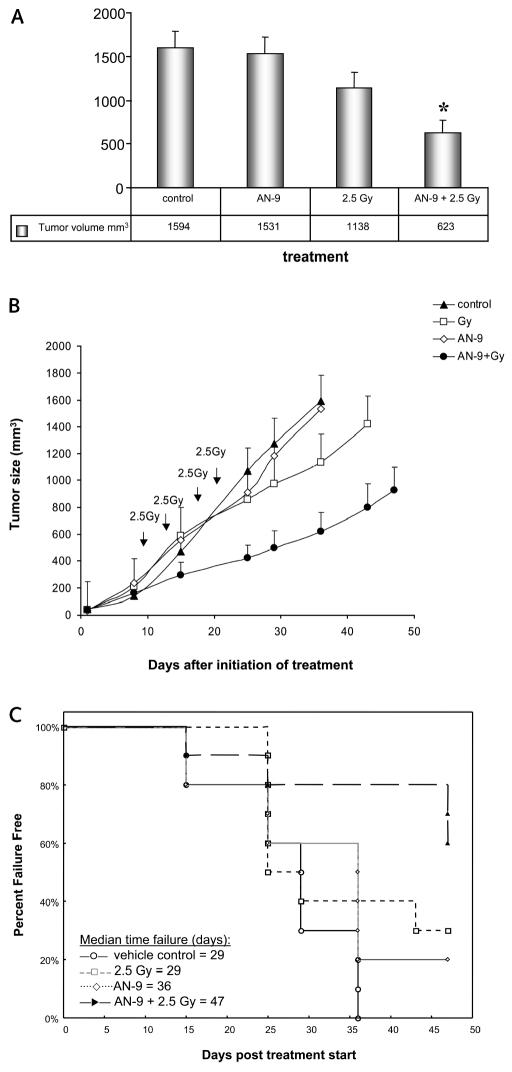
Thirty-six days after the start of treatment, all vehicle control and some AN-9–treated mice required euthanization because the tumors exceeded 2000 mm3 or had severe necrosis. At that time point, the mean tumor sizes were 1594 ± 193 mm3, 1531 ± 186 mm3, and 1138 ± 184 mm3 in mice receiving control, AN-9, and radiation treatments, respectively, whereas the mean tumor size in mice receiving the combination of AN-9 and radiation was 623 ± 148 mm3 (Fig. 1A). Fig. 1B shows mean tumor sizes ± SEM as a function of days after treatment start. Statistical analysis (two-sided t-test) revealed significantly smaller tumors in the group of mice receiving the combination treatment than in the three other groups (P = 0.037, P < 0.01, and P < 0.01, for comparison to control vehicle, AN-9 alone, and radiation alone, respectively), indicating a significant effect of the combined treatment on the tumor growth rate compared with each other treatment modality alone.
Fig. 1.
Combined in vivo treatment with AN-9 and radiation delays the tumor growth rate and prolongs TTF in mice bearing U251 MG xenografts. (A) Mean tumor volumes ± SEM on day 36 after treatment initiation in four cohorts receiving vehicle control, AN-9 alone, radiation alone, and AN-9 plus radiation. *Statistically significant difference between combined treatment and each of the other three conditions (two-sided t-test). (B) Tumor growth rate curves of the four experimental groups. Arrows indicate the days on which radiation was administered. The last tumor measurement was taken on day 36, the last day on which all animals were still alive. (C) TTF calculated for the first 47 days of treatment (log-rank test). Treatment failure was defined as tumor size greater than 1000 mm3 or development of severe necrosis requiring euthanasia.
TTF was calculated for the first 47 days after treatment start using the method of Kaplan and Meier, and treatment differences were assessed using the log-rank test. Twenty days later (67 days after treatment start), only two animals had nonnecrotic tumors smaller than 1000 mm3; both of these mice were in the combination treatment group, indicating inhibition of tumor growth in 16.7% of these animals. The data showed a trend toward prolonged TTF for mice in the combined treatment group, compared with the three other groups (P = 0.0017, P = 0.03, and P = 0.10 for comparisons with vehicle control, AN-9 alone, and radiation alone, respectively). Fig. 1C shows TTF as a function of experimental group. The median TTF was 29 days for vehicle control, 36 days for AN-9, 29 days for radiation alone, and 47 days for combined AN-9 and radiation treatment. Of note, no constitutional signs or toxicities were observed in any of the mice for the entire duration of the experiment.
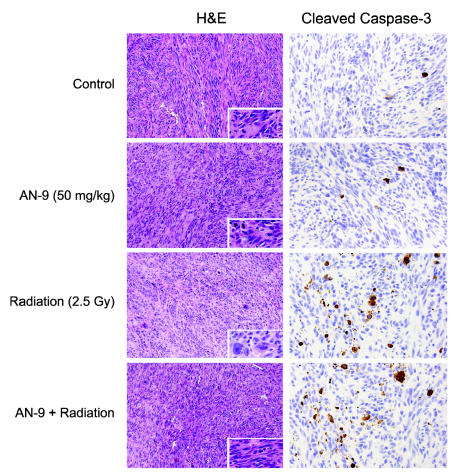
Tumors excised from two euthanized animals in each treatment group were assessed for morphology by hematoxylin and eosin staining, for apoptosis by immunohistochemical staining for cleaved caspase-3, and for proliferation by staining for Ki-67. Staining for Ki-67–positive cells, a common marker for cellular proliferation, revealed that the combination of AN-9 and radiation resulted in greater inhibition of cell proliferation than either treatment alone, consistent with the smaller volumetric measurements of the treated tumors (Table 1). Histopathologic examination of the xenografts demonstrated abundant mitotic figures, pleomorphic tumor cell populations, and rare apoptotic cells in all tumor sections (Fig. 2). Radiation treatment produced the most marked nuclear pleomorphism, with hyperchromasia, multinucleation, and abundant aberrant mitoses. Combined treatment with radiation and AN-9 reduced the degree of cytoplasmic and nuclear pleomorphism produced by radiation alone (Fig. 2, left column). Immunohistochemical staining for the cleaved form of caspase-3 revealed scattered clusters of apoptotic cells in irradiated tumors that were nearly absent from nonirradiated sections (Fig. 2, right column); however, absolute numbers were too low to enumerate. These immunohistochemical analyses suggest that the combined cytotoxic effects of AN-9 and radiation are due in large part to inhibition of tumor cell proliferation and are probably not mediated through the induction of aberrant mitoses.
Table 1.
Ki-67 staining after in vivo treatment of U251 MG xenografts
| Treatment | Ki-67–Positive Nuclei (%)* |
|---|---|
| Control | 56; 68 |
| AN-9 | 50; 46 |
| 2.5 Gy | 41; 45 |
| AN-9 + 2.5 Gy | 37; 35 |
Two animals per treatment group were analyzed. For each animal, two sections were analyzed (1000 nuclei per section) and the mean values are shown separately.
Fig. 2.
Analyses of tumor sections from mice bearing xenografts of U251 MG and treated with vehicle control, AN-9 alone, radiation alone, or combined AN-9 and radiation. (Left column) Hematoxylin and eosin staining. (Right column) Immunohistochemical staining for cleaved caspase-3.
We next sought to evaluate whether differences in DNA double-strand breaks (DSBs) contribute to the enhanced in vivo efficacy of combined AN-9 and radiation treatments. It was previously demonstrated that DSBs caused by ionizing radiation result in phosphor-ylation of H2AX on the γ-site of serine 139 to form γ-H2AX (Olive and Banath, 2004). Furthermore, several HDACIs, including MS-275, valproic acid, and sodium butyrate, have recently been reported to induce further phosphorylation of H2AX in irradiated cells, reflecting augmentation of DSBs (Camphausen et al., 2005; Munshi et al., 2005).
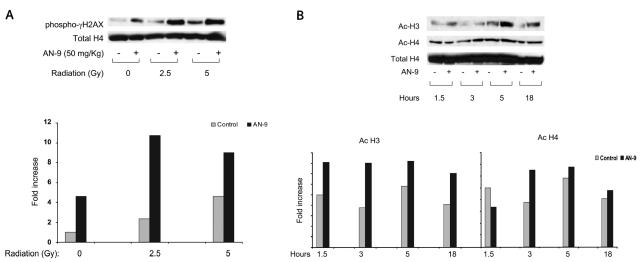
We quantitated the expression of γ-H2AX in tumors derived from mice in our four treatment cohorts and asked whether the generation of DSBs, as measured by phosphorylation of H2AX, would prove greatest in mice treated with both AN-9 and radiation. Two mice per group were treated with one dose of control vehicle, AN-9 alone (50 mg/kg), radiation alone (2.5 or 5 Gy), or AN-9 and then radiation 24 h later. All animals were euthanized 24 h after treatment, tumors were excised, and histones were purified. A single radiation dose of either 2.5 Gy or 5 Gy resulted in a 2.4-fold or 4.4-fold induction of γ-H2AX, respectively, relative to vehicle control. Interestingly, AN-9 given as a single agent also induced a 4.6-fold augmentation of this DSB marker. Regimens of AN-9 administered with radiation resulted in a robust further induction of γ-H2AX expression (8- to 12-fold increase relative to vehicle control) 24 h after irradiation (and 48 h after AN-9 pretreatment), paralleling the enhanced cytotoxicity of this combination treatment. Fig. 3A shows a representative experiment in which γ-H2AX expression was quantified, revealing the augmented DSBs generated by combining AN-9 and radiation treatments. An independent experiment showed equivalent results and confirmed these findings (data not shown).
Fig. 3.
(A) AN-9 and radiation cooperate to augment DNA DSBs, as reflected in increased expression of phospho-γ-H2AX. Established xenografts were treated with vehicle control, AN-9 (50 mg/kg), radiation (2.5 or 5 Gy), or AN-9 and then radiation 24 h later. Phospho-γ-H2AX levels were assayed 24 h after the last treatment. (B) AN-9 functions as an HDACI in vivo and results in enhanced H3 and H4 acetylation. At designated times following each treatment, mice were euthanized; histones were purified from resected xenografts and analyzed for H3 and H4 acetylation by Western blot analysis. Total H4 was used as a loading control for histone modification. Quantitation of total H4 was done with a lower exposure film.
To confirm the biologic activity of AN-9 as an HDACI in vivo, we examined acetylation of histones purified from xenografts established in mice treated with a single dose of AN-9 for 1.5, 3, 5, or 18 h. Two animals were included per time point. These tumors exhibited a broad peak of H3 acetylation 1.5–18 h after administration of 100 mg/kg AN-9. H3 acetylation levels remained higher than those of control xenografts even 18 h after a single drug dose. Acetylation of H4 was less pronounced and was most evident 3 h after treatment with AN-9. Two independent experiments yielded similar data under identical treatment conditions and time points; data from one of these experiments are shown in Fig. 3B.
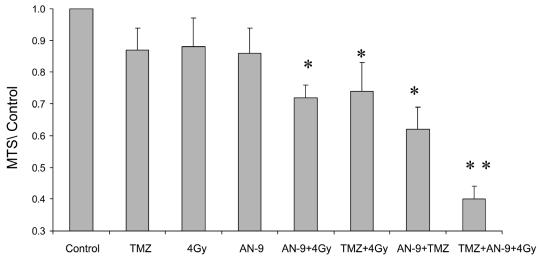
Given the increasingly prominent role of TMZ in the treatment of gliomas (Dehdashti et al., 2006), we sought to assess whether the combined therapeutic effects of AN-9 and radiation would be further enhanced by TMZ administration. MTS proliferation assays (Fig. 4) confirmed the effectiveness of combined AN-9 and radiation compared to either modality alone (P < 0.05, two-tailed t-test). As previously reported (Chakravarti et al., 2006), TMZ with concurrent radiation proved effective, as well (P = 0.04 and P = 0.02, compared with radiation and TMZ alone, respectively). Of note, AN-9 plus TMZ also displayed significantly improved efficacy compared with either agent alone (P < 0.01). However, the most potent antineoplastic activity was demonstrated by therapy using all three agents: AN-9, radiation, and TMZ. This trimodality treatment was significantly more effective than any combination of two agents (P < 0.01 compared with TMZ + AN-9, TMZ + 4 Gy, and AN-9 + 4 Gy). These data indicate that AN-9 may enhance the radiation response even more potently when TMZ is added to the regimen.
Fig. 4.
Trimodality therapy with AN-9, radiation, and TMZ displayed the greatest antineoplastic activity. Values from MTS proliferation assays are presented relative to untreated control. *Statistically significant difference (two-tailed t-test) between the combination treatment and each other modality alone. **Statistically significant difference between the trimodality therapy and any other bimodality treatment (AN-9 + 4 Gy, AN-9 + TMZ, and 4 Gy + TMZ).
Discussion
Despite advances in surgical approaches, radiotherapy technology, and chemotherapy options, the recalcitrant resistance of gliomas to treatment underlies their intractable clinical course. Even after aggressive therapy, glioblastoma multiforme inevitably recurs, most commonly in close proximity to the original tumor site. There is thus an urgent need to develop and test novel agents that demonstrate efficacy as single agents and cooperate with standard treatments to ultimately eradicate gliomas.
The promise of HDACIs for cancer treatment, as single agents and in combination with standard therapies, is supported by in vitro results, preclinical experiments, and clinical studies. Recent studies demonstrate that BA prodrugs that undergo intracellular esterase- catalyzed hydrolysis are orally bioavailable, reduce tumor growth, and inhibit metastases in xenograft models of prostate and lung cancers (Rephaeli et al., 2005, 2006). Furthermore, we have recently reported that these BA-prodrug HDACIs reduce the growth of human glioma xenografts in nude mice (Entin-Meer et al., 2005). In vivo data reported herein, in combination with results from other preclinical studies (Entin-Meer et al., 2005; Rephaeli et al., 2005) and observations from phase II clinical trials (Patnaik et al., 2002; Reid et al., 2004), reveal exceedingly limited toxicities and set these agents apart from other HDACIs, such as SAHA and MS-275 (Butler et al., 2000; Saito et al., 1999).
In this study, we showed that AN-9 enhances the antineoplastic effects of radiation in human glioma xenografts in vivo. The mean size of tumors exposed to both AN-9 and radiation was significantly smaller than the mean size of similar tumors treated with vehicle control, AN-9 alone, or radiation alone. This inhibition of tumor growth translated into a trend toward increased TTF in mice treated with the combination regimen. This augmentation of radiation-induced antitumor effects was accompanied by a significantly greater induction of H2AX phosphorylation, suggesting that DNA DSBs play a role in AN-9–mediated radiosensitization. Our data indicate that ultimately such DNA DSBs translate into reduced tumor cell proliferation, which is reflected in diminished Ki-67 staining. Our findings, however, do not exclude the possibility that AN-9–induced and/ or radiation-induced anticancer effects are mediated through apoptotic pathways that are independent of caspase-3. Such alternate apoptotic pathways have been previously documented in gliomas by studies that indicate a role for protein kinase C-η in UV-induced and gamma-radiation–induced apoptosis of U251 MG cells, the same line used in our in vivo studies (Hussaini et al., 2002).
A growing body of literature suggests that HDACIs are effective antineoplastic agents, and several different HDACIs are being scrutinized in clinical trials. BA pro-drugs in general, and AN-9 in particular, are unique in their combination of favorable characteristics, including very limited toxicities, oral bioavailability, ability to cross the blood–brain barrier, and synergy with chemotherapeutic agents (Cutts et al., 2001) and ionizing radiation. Furthermore, the effectiveness of AN-9 has been demonstrated not only in combination with radiation but also when administered with concurrent radiation and TMZ.
We have demonstrated in vitro and in vivo efficacy of novel BA-delivering prodrugs in the treatment of gliomas, and given these documented promising results, we hope to realize the potential of these HDACIs in the clinical treatment of glioma patients.
Footnotes
This research was supported in part by NIH-PO1 NS-42927-27A2 (D.A.H.-K., K.R.L.), NIH Brain Tumor SPORE grant P50 CA097257 (D.A.H.-K., K.R.L.), the Nancy and Stephen Grand Philanthropic Fund, and the Children’s Brain Tumor Foundation.
Abbreviations used are as follows: AN-9, pivaloyloxymethyl butyrate; BA, butyric acid; DSB, double-strand breaks; HDACI, histone deacetylase inhibitor; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxmethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; SAHA, suberoylanilide hydroxamic acid; TMZ, temozolomide; TTF, time to treatment failure.
References
- Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: Insights into the mechanism and clinical studies. Med Res Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;20:5165–5170. [PubMed] [Google Scholar]
- Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, Fine H, Tofilon PJ. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer. 2005;114:380–386. doi: 10.1002/ijc.20774. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Erkkinen MG, Nestler U, Stupp R, Metha M, Aldape K, Gilbert MR, Black PM, Loeffler JS. Temozolomide-mediated radiation enhancement in glioblastoma: A report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- Cutts SM, Rephaeli A, Nudelman A, Hmelnitsky I, Phillips DR. Molecular basis for the synergistic interaction of adriamycin with the formaldehyde-releasing prodrug pivaloyloxymethyl butyrate (AN-9) Cancer Res. 2001;61:8194–8202. [PubMed] [Google Scholar]
- Dehdashti AR, Hegi ME, Regli L, Pica A, Stupp R. New trends in the medical management of glioblastoma multiforme: The role of temozolmide chemotherapy. Neurosurg Focus. 2006;20:E6. doi: 10.3171/foc.2006.20.4.3. [DOI] [PubMed] [Google Scholar]
- Entin-Meer M, Rephaeli A, Yang X, Nudelman A, Vandenberg SR, Haas-Kogan DA. Butyric acid prodrugs are histone deacetylase inhibitors that show antineoplastic activity and radiosensitizing capacity in the treatment of malignant gliomas. Mol Cancer Ther. 2005;4:1952–1961. doi: 10.1158/1535-7163.MCT-05-0087. [DOI] [PubMed] [Google Scholar]
- Hussaini IM, Carpenter JE, Redpath GT, Sando JJ, Shaffrey ME, Vandenberg SR. Protein kinase C-eta regulates resistance to UV- and gamma-irradiation-induced apoptosis in glioblastoma cells by preventing caspase-9 activation. Neuro-Oncology. 2002;4:9–21. doi: 10.1093/neuonc/4.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, Ismail S, Stevens C, Meyn RE. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11:4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- Nudelman A, Ruse M, Aviram A, Rabizadeh E, Shaklai M, Zimrah Y, Rephaeli A. Novel anticancer prodrugs of butyric acid. J Med Chem. 1992;35:687–694. doi: 10.1021/jm00082a009. [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331–335. doi: 10.1016/j.ijrobp.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Patnaik A, Rowinsky EK, Villalona A, Hammond LA, Britten CD, Siu LL, Goetz A, Felton SA, Burton S, Valone FH, Eckhardt SG. A phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin Cancer Res. 2002;8:2142–2148. [PubMed] [Google Scholar]
- Prakash S. Foster, B.J. Meyer, M. Wozniak, A. Heilbrun, L.K. Flaherty, L. Zalupsky, M. Radulovic, L. Valdivieso, M. and, LoRusso PM. Chronic oral administration of CI-994: A phase 1 study. Invest New Drugs. 2001;19:1–11. doi: 10.1023/a:1006489328324. [DOI] [PubMed] [Google Scholar]
- Reid T, Valone F, Lipera W, Irwin D, Paroly W, Natale R, Sreedharan S, Keer H, Lum B, Scappaticci F, Bhatnagar A. Phase II trial of the histone deacetylase inhibitor pivaloyloxymethyl butyrate (Pivanex, AN-9) in advanced non-small cell lung cancer. Lung Cancer. 2004;45:381–386. doi: 10.1016/j.lungcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Rephaeli A, Blank-Porat D, Tarasenko N, Entin-Meer M, Levovich I, Cutts SM, Phillips DR, Malik Z, Nudelman A. In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. Int J Cancer. 2005;116:226–235. doi: 10.1002/ijc.21030. [DOI] [PubMed] [Google Scholar]
- Rephaeli A, Entin-Meer M, Angel D, Tarasenko N, Gruss-Fischer T, Bruachman I, Phillips DR, Cutts SM, Haas-Kogan D, Nudelman A. The selectivty and anti-metastatic activity of oral bio-available butyric acid prodrugs. Invest New Drugs. 2006;24:383–392. doi: 10.1007/s10637-006-6213-1. [DOI] [PubMed] [Google Scholar]
- Saito A, Yamashita T, Mariko Y, Nosaka Y, Tsuchiya K, Ando T, Suzuki T, Tsuruo T, Nakanishi O. A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci U S A. 1999;96:4592–4597. doi: 10.1073/pnas.96.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XX, Robinson ME, Riceberg JS, Kim DY, Kung B, Titus TB, Hayashi S, Flake AW, Carpentieri D, Ikegaki N. Favorable neuroblastoma genes and molecular therapeutics of neuroblastoma. Clin Cancer Res. 2004;10:5837–5844. doi: 10.1158/1078-0432.CCR-04-0395. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jung M, Dritschilo A, Jung M. Enchancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res. 2004;161:667–674. doi: 10.1667/rr3192. [DOI] [PubMed] [Google Scholar]