Abstract
Patients with malignancy, particularly patients with high-grade glioma (HGG; WHO grade III/IV), have an increased risk of venous thromboembolism (VTE). It has been suggested that VTE predicts survival in cancer patients. The aim of our study was to investigate the occurrence of symptomatic VTE and its impact on survival in patients with HGG. Consecutive patients (n = 63; 36 female, 27 male; median age, 58 years) who had neurosurgical intervention between October 2003 and December 2004 were followed after surgery until October 2005. Objectively confirmed VTE was recorded as an event. All patients had received thrombosis prophylaxis with low-molecular-weight heparin (LMWH) during the immediate postoperative period. Subsequently, 56 patients received radiochemotherapy, 6 radiotherapy, and 1 chemotherapy only. Patients were followed over a median time period of 348 days. Fifteen patients (24%) developed VTE. Pulmonary embolism was diagnosed in nine patients (60%) and was fatal twice. The cumulative probability of VTE was 21% after three months and 26% after 12 months. The highest frequency of VTE was observed in patients with biopsy and subtotal tumor resection (n = 37; multivariate hazard ratio, 3.58; 95% CI = 0.98–13.13; P = 0.054) compared with patients with total resection. Survival did not significantly differ among patients with and without VTE and was 53% after 12 months in both groups. Patients with HGG, particularly those with biopsy and subtotal resection, are at high risk to develop VTE postoperatively. Thrombosis was not associated with a significant reduction of survival.
Keywords: cancer, glioma, neurosurgery, pulmonary embolism, venous thrombosis
Patients with malignancy have an increased risk of venous thromboembolism (VTE)2 (Rickles and Edwards, 1983). The thrombosis incidence of different tumor types varies remarkably. An especially high risk of VTE has been found in patients with primary cerebral malignancy, in particular in those with high-grade glioma (HGG) (Brandes et al., 1997; Kayser-Gatchalian and Kayser, 1975).
Tissue factor (TF), a potent procoagulant factor, might play an important role in the pathophysiological mechanism of development of VTE, because its synthesis is up-regulated in a variety of neoplasms (De Cicco, 2004). In glioma cells, the amount of TF expression correlates with the histological grade (Hamada et al., 1996). However, inconsistent data on the frequency of VTE were given, for instance, 3%–25% during the first six postoperative weeks and 16%–28% during the first year (Marras et al., 2000). Differences in patient selection and in the applied regimen of treatment and prophylaxis might have contributed to heterogeneity of results. Patient selection is critical since tumor type, grade, and stage are associated with VTE (Sorensen et al., 2000). The importance of further parameters, such as tumor size, surgery, chemotherapy, paresis, thromboprophylaxis, age, and ABO blood group, is currently under discussion in glioma patients.
Only a limited number of studies investigated the importance of VTE for survival in cancer patients. Sorensen et al. (2000) described that cancer diagnosed at the same time or within one year after occurrence of VTE is associated with an advanced stage of cancer and poorer prognosis. In a case series that included patients with VTE, Prandoni et al. (1996) found that the presence of cancer increased the risk for death with a hazard ratio (HR) of 8.1 (95% confidence interval = 3.6–18.1).
Patients with HGG have a poor prognosis, and the occurrence of VTE, specifically pulmonary embolism, might affect the survival in these patients. Preliminary results of controlled trials support the idea that activation of coagulation plays a role in the natural course of tumor growth (Prandoni et al., 2005). A hypercoagulable state indicated by an increased baseline D-dimer is associated with decreased survival in colorectal cancer patients, and it might be hypothesized that cancer patients with overt thrombosis have an impaired prognosis (Blackwell et al., 2004). Alcalay et al. (2006) reported that VTE was a risk factor for a reduced survival among patients with local or regional colorectal cancer. In a large prospective glioma outcome study, risk factors for survival were investigated, but data on survival rates in patients with thrombosis were not given (Laws et al., 2003).
The primary objective of our study was to investigate symptomatic VTE in patients with HGG in the light of new therapeutic regimens. Second, we aimed to assess the influence of VTE on survival of glioma patients.
Materials and Methods
Design
A cohort of consecutive patients with HGG (WHO grade III/IV) was analyzed. All patients with newly diagnosed HGG that were seen at the oncology department of the University Hospital Vienna between October 14, 2003, and December 12, 2004, were included. Patients were included in the observational study when they were seen first at our hospital. In patients who had neurosurgical intervention at another hospital and who were seen at our hospital shortly after surgery (n = 16), the time period between this first visit at our department and surgery was evaluated retrospectively. Institutional research ethical approval was received for this study. In a subset of patients, a blood sample was also drawn; these patients signed an informed consent form. Patients that were only followed did not sign an informed consent form. The observation period started at the time of surgery, when diagnosis was confirmed histologically. The following parameters were recorded: patient height, weight, and blood group; type of surgery (complete tumor resection, partial resection, or biopsy in case of inoperable tumors); intensity and duration of postoperative thrombosis prophylaxis; the maximum tumor diameter; presence or absence of paresis of the upper and lower extremity; and, in cases of further radio- or chemotherapy, the applied regimen. To define the extent of resection and to detect intracranial hemorrhage, CT was performed the day after surgery. A prophylactic dosage of LMWH was given during the postoperative hospital stay, usually during the first 10 postoperative days; 40 mg enoxaparin, 2500 IU dalteparin, or 5000 IU dalteparin once daily was used. All patients received graduated compression stockings during the hospital stay. Corticosteroids and anticonvulsants were administered as medically indicated. The study was closed on October 1, 2005.
Follow-up
Regular visits at the outpatient department of patients were used for follow-up. When patients did not receive any further treatment or missed an appointment, we tried to reach them by phone or mail to get information about their clinical course. If they did not respond, their family doctors and relatives were contacted. No patient was lost to follow-up. At study closure, the Austrian Mortality Registry was searched for entries concerning study participants.
Thromboembolic Events
Symptomatic or fatal VTE was recorded as an event, regardless of the site (proximal or distal). There was no routine screening for VTE. When a patient developed symptoms of VTE, medical imaging was performed. Diagnosis of VTE was always confirmed by an objective method: duplex sonography for deep vein thrombosis (DVT) and CT for pulmonary embolism. Patients who developed VTE received long-term anticoagulation with therapeutic doses of LMWH. In cases where a patient died and an autopsy was performed, the written protocols were reviewed with regard to presence of VTE.
Statistical Methods
Kaplan-Meier analysis was applied to calculate the cumulative probability of VTE after various time points. In the statistical analysis, VTE patients who were free of events during the observation period were censored at the time of study closure, October 1, 2005, or at their death. In the statistical analysis for survival, patients were censored at October 1, 2005. Cox regression analysis was used to compare the probability of thrombosis and survival among different groups of patients. For the outcome variable VTE, two univariate models were calculated, for the parameter type of surgery (categorical variable, three levels: subtotal tumor resection, total tumor resection, biopsy) and for the parameter subtotal tumor resection including biopsy (dichotomous variable: true/false), respectively. For the outcome variable survival, the univariate analysis was calculated for the parameter VTE (time-dependent variable). In the multivariate Cox regression model for the outcome variable VTE, further parameters were included with the variable subtotal tumor resection including biopsy: paresis of an extremity (dichotomous variable: true/false), age greater than 60 years (dichotomous variable: true/false), glioblastoma multiforme (dichotomous variable: true/ false), and male gender (true/false). In multivariate Cox regression model for the outcome variable survival, we included the same parameters and also included the parameter VTE (time-dependent variable). A two-sided P value < 0.05 indicated statistical significance. Statistical analysis was performed with the SPSS statistical software package (version 12.0.1; SPSS Inc., Chicago, Ill.).
Results
Sixty-three patients (36 female, 27 male; median age, 58 years; range, 20–79 years; Table 1) were followed over a median time period of 348 days (range, 21–717 days). Histological diagnosis was glioblastoma multiforme in 51 patients, anaplastic astrocytoma in 6, oligodendroglioma grade III in 3, and ependymoma grade III, anaplastic glioma grade III, and anaplastic oligoastrocytoma in 1 patient each. Fifty-six patients were treated with combined radiochemotherapy, six with radiotherapy only, and one with chemotherapy only. Chemotherapeutic substances that were administered are given in Table 2. Thirty-seven patients died within the observation period, eight of them within the first 100 days.
Table 1.
Demographic and clinical characteristics of patients with HGG
| All Patients | Patients with VTE | Patients without VTE | PValue* | |
|---|---|---|---|---|
| Number | 63 | 15 | 48 | |
| Age, median (range), years | 57.7 (20.2–79.7) | 62.6 (20.2–74.6) | 57.6 (27.3–79.7) | 0.75 |
| Sex (female/male), n | 36/27 | 7/8 | 20/28 | 0.83 |
| BMI,** median (range) | 25.3 (18.6–45.1) | 24.5 (20.2–30.7) | 25.6 (18.6–45.1) | 0.25 |
| Tumor size,† median (range), cm | 4.0 (1.5–10.0) | 3.8 (2.6–6.0) | 4.0 (1.5–10.0) | 0.50 |
| Paresis, n (%) | 11 (18%) | 4 (27%) | 7 (15%) | 0.19 |
| Blood group O,‡ n (%) | 25 (40%) | 7 (47%) | 18 (38%) | 0.51 |
| Grade | ||||
| WHO III | 12 (19%) | 3 (20%) | 9 (19%) | |
| WHO IV | 51 (81%) | 12 (80%) | 39 (81%) | |
Abbreviations: BMI, body mass index; VTE, venous thromboembolism.
P values calculated with Cox proportional hazards model.
BMI not available in four patients.
Tumor size not available in 12 patients.
Blood group not available in seven patients.
Table 2.
Treatment modalities in patients with HGG
| All Patients | Patients with VTE | Patients without VTE | |
|---|---|---|---|
| Number | 63 | 15 | 48 |
| Surgery | |||
| Total resection | 24 (38%) | 3 (20%) | 21 (44%) |
| Resection (undefined) | 2 (3%) | 0 (0%) | 2 (4%) |
| Partial resection | 22 (35%) | 7 (47%) | 15 (31%) |
| Biopsy | 15 (24%) | 5 (33%) | 10 (21%) |
| Consecutive treatment | |||
| Radiochemotherapy | 56 (89%) | 13 (87%) | 43 (90%) |
| Radiotherapy only | 6 (10%) | 2 (13%) | 4 (8%) |
| Chemotherapy only | 1 (1%) | 0 (0%) | 1 (2%) |
| Substances* | |||
| Temozolomide | 38 (60%) | 11 (73%)** | 27 (56%) |
| Lomustine | 10 (16%) | 3 (20%) | 7 (15%) |
| Fotemustine/dacarbazine | 24 (38%) | 3 (20%) | 21 (44%) |
| Thalidomide | 5 (8%) | 3 (20% | 2 (4%) |
| Imatinib | 7 (11%) | 1 (2%) | 6 (13%) |
| Gefitinib | 1 (2%) | 0 (0%) | 1 (2%) |
| Rapamycin | 1 (2%) | 1 (2%) | 0 (0%) |
| Hydroxyurea | 2 (3%) | 0 (0%) | 2 (4%) |
Abbreviation: VTE, venous thromboembolism.
Percentage of substances does not add up to 100% because a number of patients received more than one substance.
Two patients developed VTE before treatment with temozolomide.
Fifteen of 63 patients (24%; 7 female, 8 male; median age, 63 years; range, 20–75 years) developed VTE. Six patients (40%) had DVT, which was located in the lower extremity in five patients and in the upper extremity in one patient. Nine patients (60%) had pulmonary embolism, three in combination with DVT. Two events were fatal (13%). Patients with an event had the following histological tumor types: glioblastoma multiforme in 12, anaplastic astrocytoma in 2, and oligodendroglioma in 1.
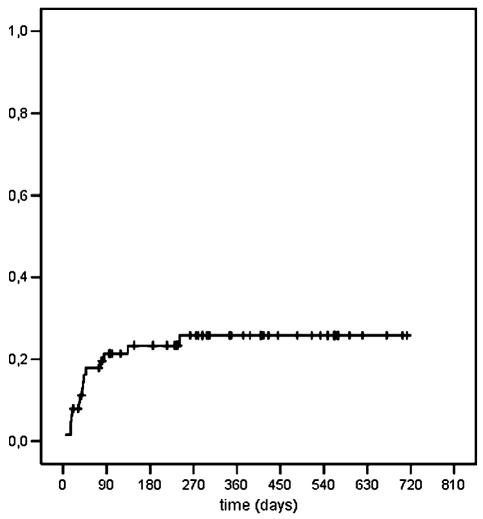
Two patients developed VTE during postoperative thrombosis prophylaxis, and two after cessation of prophylaxis but before consecutive treatment had started (n = 4). Ten events (67%) occurred during radio- or chemotherapy; one event occurred after cessation of radiochemotherapy but shortly after another neurosurgical intervention. Events occurred after a median time period of 40 days (range, 4–241 days). Eighty-seven percent of events (13 of 15) occurred during the first three months of observation. In Kaplan-Meier analysis, the cumulative probability of VTE was 21% after three months and 26% after one year (Fig. 1). No major bleeding complications were reported.
Fig. 1.
Cumulative probability of VTE.
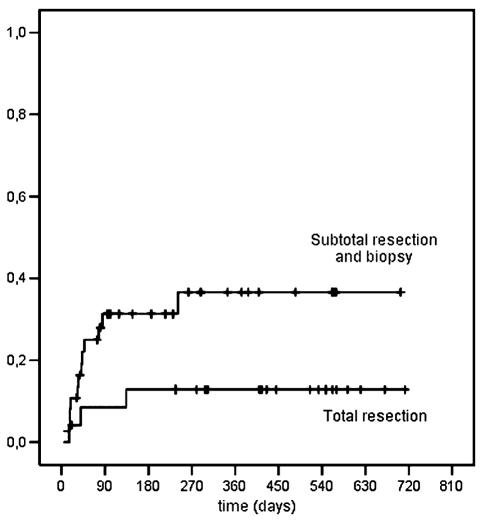
Compared with patients with total tumor resection (n = 24), a higher probability of VTE was found in patients who underwent biopsy (n = 15; univariate analysis: HR = 3.36, 95% CI = 0.80–14.15, P = 0.10; multivariate analysis: HR = 3.37, 95% CI = 0.73–15.68, P = 0.12) and in those who underwent a subtotal resection (n = 22; univariate analysis: HR = 3.07, 95% CI = 0.80–11.90, P = 0.11; multivariate analysis: HR = 3.73, 95% CI = 0.92–15.24, P = 0.07). Two patients in whom the extent of resection was not exactly defined were not included in the analysis. When patients who underwent subtotal resection or biopsy (n = 37) were compared with those who had total resection, the HR was 3.18 (95% CI = 0.89–11.33, P = 0.07) in univariate analysis and 3.58 (95% CI = 0.98–13.13, P = 0.054) in multivariate analysis (Fig. 2).
Fig. 2.
Cumulative probability of VTE, according to type of surgery.
A higher proportion of patients with paresis of the upper or lower extremity (n = 11, 18%) developed VTE (n = 4, 27%) compared with those without (n = 7, 15%), but this difference did not reach statistical significance in uni- or multivariate analysis.
No significant difference in body mass index, age, sex, and maximal tumor size was found between patients who stayed free of an event and those who developed VTE during the follow-up (Table 1).
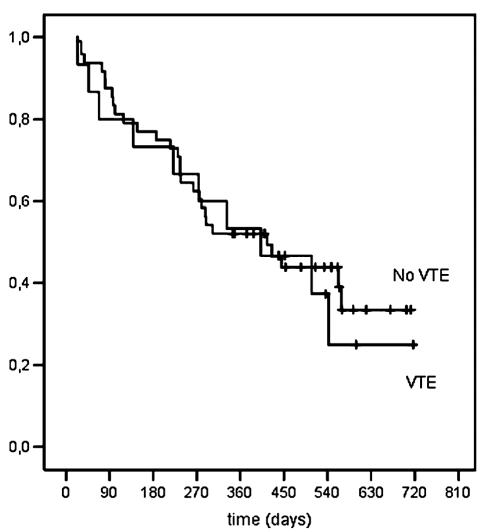
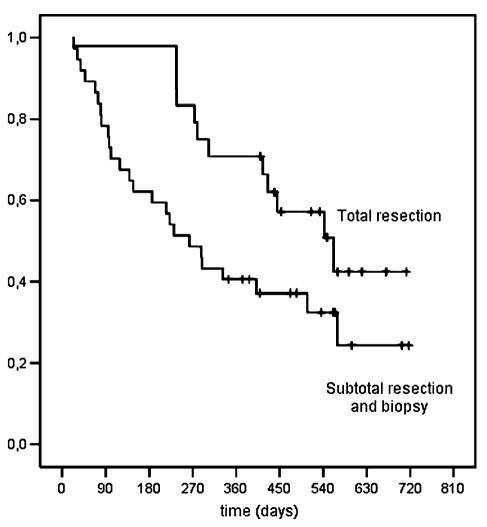
VTE was not significantly associated with survival (univariate time-dependent HR = 1.43, 95% CI = 0.68–2.97, P = 0.35; Fig. 3). Patients that received a total resection had a significantly better survival than did patients with subtotal resection and biopsy (univariate analysis: HR = 0.52, 95% CI = 0.26–1.04, P = 0.063; multivariate analysis: HR = 0.36, 95% CI = 0.17–0.77, P = 0.009; Fig. 4). Another significant risk factor for shorter survival was age greater than 60 years (HR = 2.16; 95% CI = 1.07–4.36, P = 0.032).
Fig. 3.
Cumulative probability of survival, according to VTE.
Fig. 4.
Cumulative probability of survival according to type of surgery.
Discussion
Despite improvements in antineoplastic treatment and regular postoperative thromboprophylaxis, the incidence of VTE in patients with HGG is still very high, especially in the first three months after neurosurgical intervention. In our cohort, biopsy and subtotal resection increased the risk for development of VTE; however, most probably because of the small number of patients, statistical significance was not reached. We observed a high frequency of pulmonary embolism, and two of these events were fatal. The incidence of VTE was comparable to data published by Brandes et al. (1997), who observed a cumulative probability of 21% at 12 months. However, the rate of pulmonary embolism was lower (4 of 20 thrombotic events) compared with our data (9 of 15 thrombotic events). Even though our follow-up was complete, it is possible that there were still some unreported events, most probably because not all patients who died underwent an autopsy.
Type of surgery seems to influence the incidence of thrombosis. Patients with biopsy and subtotal resection had the highest probability of developing VTE. Our data suggest that the combination of a neurosurgical intervention and a remaining tumor formation increases the thrombogenic potential. However, the relatively small number of patients limits the conclusions drawn from this study. This is especially true for the multivariate analysis. The general guideline for use of multivariate analysis is that there should be about 10 events per variable. With only 15 events, a multivariate model with five variables is questionable. We have performed multivariate analysis to confirm that the results are not substantially different from the univariate analysis. It might have been interesting to determine whether tumor progression was associated with an increased risk for VTE; however, because of the small number of patients who developed an event after three months postoperatively (n = 2), it is difficult to give information about such a correlation. Streiff et al. (2004) reported on an association between ABO blood group and the occurrence of VTE. Patients with blood group A or AB were reported to have a significantly shorter thrombosis-free survival than did patients with blood groups O and B. We could not confirm these findings; in our analysis, ABO blood group was not a significant risk factor.
Interestingly, only 2 out of 15 events in our patients occurred during thrombosis prophylaxis with LMWH within the first postoperative days. The risk rapidly increased after cessation of thrombosis prophylaxis. Goldhaber et al. (2002), who treated patients with prophylactic doses of LMWH or standard heparin in combination with intermittent pneumatic compression, observed no thromboembolic event within the first nine postoperative days. That might indicate that thromboprophylactic regimens are sufficient, but their duration might have been too short. In the 2004 guidelines from the consensus statement of the American College of Chest Physicians, prolonged prophylaxis was not recommended in brain tumor patients, in contrast to a suggestion for patients undergoing major orthopedic surgery and in elderly patients undergoing gynecological surgery because of malignancy (Geerts et al., 2004). Obviously, a reduction of the risk of VTE has to be balanced against an increased risk of hemorrhage. A considerable number of studies focused on the safety of postoperative thromboprophylaxis in neurosurgical patients and revealed that LMWH administered in a usual prophylactic dosage only slightly increased bleeding risks, while it effectively reduced VTE (Browd et al., 2004; Danish et al., 2004). A meta-analysis of studies in which LMWH was used for postoperative thrombosis prophylaxis in neurosurgery over 7 or 10 days reported a 38% relative risk reduction; an overall bleeding risk ranging from 4.1% to 11.8% (control patients, 1.2%–7.1%) and a risk of a major intracranial hemorrhage between 2.2% and 2.6% (control patients, 0.8%–2.6%) were observed (Iorio and Agnelli, 2000).
Data on prolonged prophylaxis in patients with other malignant diseases are available. In patients undergoing abdominal surgery for malignancy, prolonged anticoagulation with 5000 IU dalteparin for a total of four weeks reduced the risk of late-occurring VTE by 60% without increased bleeding complications (Rasmussen, 2002). The ENOXACAN II study investigated patients with abdominal and pelvic cancer and found the same risk reduction in patients receiving 40 mg enoxaparin subcutaneously (Bergqvist et al., 2002). Another study reported a thrombosis risk reduction by 85% under treatment with low-dose warfarin and an only slightly increased risk of bleeding (5.3% vs. 3.1%) in patients with advanced breast cancer (Levine et al., 1994).
The majority of events in our patients occurred immediately after cessation of prophylaxis and during the first three months after surgery when consecutive treatment was given. The extent to which new chemotherapeutic agents contributed to the risk of thrombosis is not known. However, thrombocytopenia is a well-known side effect of cytostatic drugs used in neuro-oncology (Stupp et al., 2005). In our study, the vast majority of patients received chemotherapeutic substances; thus, a comparison to patients without chemotherapy was not possible. Stupp et al. (2005) included a total of 573 patients with newly diagnosed glioblastoma who received radiotherapy alone or in combination with temozolomide; thromboembolic events were reported in 28 patients (5%): 16 in the radiotherapy group and 12 in the radiotherapy plus temozolomide group. Unfortunately, information on thromboprophylaxis and diagnostic procedures for thrombosis were not given. This relatively low prevalence of VTE might be explained partly by the fact that the observation of patients in their study started later than in our study; the median time interval between diagnosis and assignment to the different treatment arms was five weeks in their study. Gerber et al. (2006) summarized the findings of several studies investigating risk factors for VTE and the management of thrombotic events in patients with brain tumors. They concluded that anticoagulant treatment can be used safely and effectively but pointed out that anticoagulant treatment is most appropriate for postoperative and other hospitalized patients until data on primary prophylaxis in the long-term exist.
Treatment with thalidomide seems to increase the risk of VTE. In our patient cohort, three of five patients who received thalidomide developed VTE, two of them after subtotal resection and one after biopsy (Table 2). In a phase II trial, Fine et al. (2003) explored the clinical activity of thalidomide in combination with carmustine in 40 patients with recurrent HGG, including 38 patients with glioblastoma multiforme. Thromboembolic events occurred in 12 patients, including seven pulmonary emboli. This is in line with the data from our study; however, in our study the number of patients receiving thalidomide was too small to draw conclusions on the additional risk for development of VTE during treatment with this substance.
Few published studies have investigated the influence of VTE on survival in patients with cancer (Prandoni et al., 1996; Sorensen et al., 2000). Sorensen et al. (2000) found that the diagnosis of VTE in patients with various types of cancer is associated with an advanced stage of the disease and a poor prognosis. In our study, we did not find a difference in survival when we compared patients who had VTE with patients who did not. However, we have to consider that patients with glioma differ remarkably from other cancer patients, since they are usually at an advanced stage of cancer and generally have a poor prognosis. A large number of our patients developed pulmonary embolism, and two of these events were fatal. Despite this, the occurrence of VTE was not predictive for a shorter survival in our patient group.
Recent studies investigated whether prolonged treatment with LMWH leads to higher survival rates of cancer patients. Data from patients enrolled in the CLOT study, the FAMOUS study, and two other studies suggest that the overall survival of tumor patients could be improved by prolonged administration of LMWH (Altinbas et al., 2004; Kakkar et al., 2004; Klerk et al., 2005; Lee et al., 2005). Higher survival rates were found in patients with limited disease in three studies (Kakkar et al., 2004; Klerk et al., 2005; Lee et al., 2005). Altinbas et al. (2004) found a similar improvement in survival in patients with limited and extended disease. Today, no data from interventional trials investigating extended anticoagulation and its influence on VTE and survival in patients with HGG are available. A positive influence of LMWH on survival in our patients cannot be excluded, since patients who developed VTE were treated with LMWH long term.
We conclude from our data that thrombosis incidence in patients with HGG is still unacceptably high. Type of surgery seems to influence the risk of VTE. Survival does not seem to be impaired in patients who developed VTE during the course of disease. The strong incline of events after cessation of thrombosis prophylaxis indicates that patients might benefit from an extension of prophylaxis in the further postoperative weeks. Data from randomized clinical trials investigating incidence of VTE and survival in larger patient cohorts with HGG comparing standard treatment during the immediate postoperative period with prolonged thrombosis prophylaxis are urgently needed.
Footnotes
Abbreviations used are as follows: DVT, deep vein thrombosis; HGG, high-grade glioma; HR, hazard ratio; LMWH, low-molecular-weight heparin; TF, tissue factor; VTE, venous thromboembolism.
References
- Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: Incidence and effect on survival. J Clin Oncol. 2006;24:1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M, Soyuer S. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight-heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- Blackwell K, Hurwitz H, Lieberman G, Novotny W, Snyder S, Dewhirst M, Greenberg C. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101:77–82. doi: 10.1002/cncr.20336. [DOI] [PubMed] [Google Scholar]
- Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, Zampieri P, Baiocchi C, Fiorentino MV. Incidence of risk of thromboembolism during treatment high-grade gliomas: A prospective study. Eur J Cancer. 1997;33:1592–1596. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- Browd SR, Ragel BT, Davis GE, Scott AM, Skalabrin EJ, Couldwell WT. Prophylaxis for deep venous thrombosis in neurosurgery: A review of the literature. Neurosurg Focus. 2004;17:E1. doi: 10.3171/foc.2004.17.4.1. [DOI] [PubMed] [Google Scholar]
- Danish SF, Burnett MG, Stein SC. Prophylaxis for deep venous thrombosis in patients with craniotomies: A review. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.4.2. [DOI] [PubMed] [Google Scholar]
- De Cicco M. The prothrombotic state in cancer: Pathogenic mechanisms. Crit Rev Oncol Hematol. 2004;50:187–196. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fine HA, Wen PY, Maher EA, Viscosi E, Batchelor T, Lakhani N, Figg WD, Purow BW, Borkowf CB. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol. 2003;21:2299–2304. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: The Seventh ACCP Conference on on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- Gerber DE, Grossman SA, Streiff MB. Management of venous thromboembolism in patients with primary and metastatic brain tumors. J Clin Oncol. 2006;24:1310–1318. doi: 10.1200/JCO.2005.04.6656. [DOI] [PubMed] [Google Scholar]
- Goldhaber SZ, Dunn K, Gerhard-Herman M, Park JK, Black PM. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122:1933–1937. doi: 10.1378/chest.122.6.1933. [DOI] [PubMed] [Google Scholar]
- Hamada K, Kuratsu J, Saitoh Y, Takeshima H, Nishi T, Ushio Y. Expression of tissue factor correlates with grade of malignancy in human glioma. Cancer. 1996;77:1877–1883. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1877::AID-CNCR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: A meta-analysis. Arch Intern Med. 2000;160:2327–2332. doi: 10.1001/archinte.160.15.2327. [DOI] [PubMed] [Google Scholar]
- Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: The Fragmin Advanced Malignancy Outcome Study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kayser-Gatchalian MC, Kayser K. Thrombosis and intracranial tumors. J Neurol. 1975;209:217–224. doi: 10.1007/BF00312543. [DOI] [PubMed] [Google Scholar]
- Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G, Buller HR. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, Lillehei KO, Bernstein M, Brem H, Sloan A, Berger MS, Chang S. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, Kakkar AK, Prins M, Levine MN. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- Levine M, Hirsh J, Gent M, Arnold A, Warr D, Falanga A, Samosh M, Bramwell V, Pritchard KI, Stewart D, Goodwin P. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: An evidence-based review. Cancer. 2000;89:640–646. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–410. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen MS. Preventing thromboembolic complications in cancer patients after surgery: A role for prolonged thromboprophylaxis. Cancer Treat Rev. 2002;28:141–144. doi: 10.1016/s0305-7372(02)00043-9. [DOI] [PubMed] [Google Scholar]
- Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood. 1983;62:14–31. [PubMed] [Google Scholar]
- Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–1723. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]