Abstract
We conducted a phase II study to assess the efficacy of oral temozolomide (TMZ) in children with progressive low-grade glioma. Thirty eligible patients were enrolled on this study. Median age at enrollment was 10 years (range, 4–18 years). Eligible patients received TMZ (200 mg/m2 per day) by mouth for five days every four weeks. Patients received a median of nine cycles (range, 2–12 cycles) of treatment. Best responses in the 26 patients (86%) with optic pathway glioma (OPG)/pilocytic astrocytoma (PA) included partial response in 3 patients (11%), minor response in 1 (4%), stable disease in 10 (38%), and progressive disease in 12 (46%). Only one of four patients with fibrillary astrocytoma had stable disease for 29 months after TMZ. The overall disease stabilization rate in patients with OPG/PA was 54%, and disease control was maintained for a median interval of 34 months. Seventeen of 26 patients had progressive disease either on or off therapy, and three have died of disease. The two-year progression-free and overall survivals in patients with OPG/PA were 49% (95% CI, 30%–67%) and 96% (95% CI, 89%–100%), respectively. Worst toxicity related to TMZ in all 30 patients included grade 2–4 thrombocytopenia in seven patients, grade 2–4 neutropenia in seven, grade 2 skin rash in one, and intratumor hemorrhage in one. TMZ given in this schedule was successful in stabilizing disease in a significant proportion of the patients with OPG/PA, with manageable toxicity.
Keywords: low-grade glioma, phase II trial, responses, temozolomide
Low-grade gliomas (LGG)3 of diverse histopathology and location constitute 30%–40% of all CNS tumors diagnosed in pediatric patients. While the optimal initial treatment of such tumors is surgical resection either at diagnosis or at relapse (Watson et al., 2001), therapy for unresectable or residual progressive gliomas is controversial. Although radiotherapy is a reasonable therapeutic option, its use, particularly in young children, may result in severe neurocognitive, endocrine, and vascular sequelae (Duffner and Cohen, 1991; Grill et al., 1999). In recent years, chemotherapeutic agents (including vincristine, actinomycin-D, cyclophosphamide, carboplatin, lomustine, and etoposide) have been employed individually or in combination in patients with progressive LGG with the aim of stabilizing disease and either avoiding or delaying radiotherapy (Perilongo, 2005). Although single-agent carboplatin or carbopla-tin plus vincristine regimens are being used routinely in young children with recurrent LGG, there is a clear need for alternative chemotherapeutic agents in patients in whom this initial treatment is unsuccessful.
Temozolomide (TMZ) (Temodar; Schering-Plough Corporation, Kenilworth, N.J.) is an imidazole tetrazinone and an orally active alkylating agent that has been shown to be efficacious in preclinical models of various CNS tumor xenografts, including malignant glioma (Friedman et al., 2000). The drug is particularly useful in patients with brain tumors due to its excellent penetration into the CNS and almost 100% bioavailability and linear pharmacokinetics after oral administration (Ostermann et al., 2004). Phase I studies in adults and children with recurrent brain tumors have shown that the drug has an excellent safety profile (Brada et al., 1999; Nicholson et al., 1998).
We therefore performed a phase II trial of TMZ in adults and children with recurrent LGG to assess the efficacy of the drug in these patients. The data regarding responses in adults with recurrent LGG have been reported previously and included five children who also are included in the present report (Quinn et al., 2003). We now describe the responses and disease stabilization rates and toxicities after treatment with oral TMZ in children with recurrent or progressive LGG treated on this study.
Materials and Methods
The primary objective of this phase II trial was to determine the response rate to TMZ in children with recurrent or progressive LGG. The study was initiated at the Preston Robert Tisch Brain Tumor Center at Duke University Medical Center in June 1999. The study was subsequently opened in two other centers: Children’s Hospital of Philadelphia (Philadelphia, Penn.) and Beth Israel Medical Center (New York, N.Y.). The study was permanently closed for accrual in August 2005.
Eligibility criteria for this study (Quinn et al., 2003) included histologically confirmed recurrent or progressive primary LGG (optic pathway glioma [OPG], pilocytic astrocytoma [PA], fibrillary astrocytoma [FA], oligodendroglioma [OG], or oligoastrocytoma [OA]). Patients with OPG did not require biopsy confirmation of disease. In these patients, additional eligibility criteria included progressive loss of vision as shown by ophthalmologic examination, increase in proptosis of 3 mm, and increase in diameter of the optic nerve of >2 mm on neuroimaging or an increase in the distribution of tumor involving the optic tract or radiations using T1- or T2-weighted imaging on MRI of the brain. Subjects had to be between 4 and 18 years old, have a Lansky or Karnofsky status of ≥ 70%, and have a life expectancy of more than 12 weeks. An interval of at least three weeks from prior surgical resection and six weeks from prior radiotherapy or chemotherapy was required unless there was unequivocal evidence of tumor progression after any of these treatments. Required evidence of adequate organ function included absolute neutrophil count (ANC) of ≥ 1,500/ mm3, platelet count ≥ 100,000/mm3, hemoglobin ≥ 10 g/dl, blood urea nitrogen (BUN) and serum creatinine < 1.5× the upper limit of institutional normal (ULN), serum bilirubin < 1.5× ULN, and liver enzymes (alkaline phosphatase, serum glutamic oxalacetic transaminase/ prothrombin time) < 2.5× ULN. Signed informed consent by a patient or parent/guardian as approved by the local institutional review board was required.
Exclusion criteria included active infection, prior malignancies, pregnancy, and lactation.
Treatment Plan and Modifications
TMZ was administered orally to patients in a fasting state, once a day for five consecutive days (days 1–5) at a starting dose of 200 mg/m2 per day. Treatment cycles were repeated every 28 days after the first daily dose of TMZ from the previous cycle. The next cycle of treatment was not given until the ANC was ≥1,500/ mm3 and platelets were ≥ 100,000/mm3. As previously reported, dose modifications were made for grade 3–4 myelosuppression or nonhematologic toxicity (Quinn et al., 2003).
Required Observations Before, During, and After Protocol Therapy
Observations and laboratory tests obtained at baseline (within three days of starting treatment) included height, weight, body surface area, complete physical examination (including neuroophthalmologic examination in patients with OPGs), complete blood count with differential, electrolytes, BUN, liver function tests, MRI scan of brain with and without contrast, chest X-ray, and electrocardiogram. Subsequently, height, weight, physical examination, complete blood count with differential, electrolytes, BUN, creatinine, and liver function tests were obtained prior to each cycle, with MRI scan of the brain every eight weeks. In patients with optic pathway tumors and impaired vision, the frequency of ophthalmologic examinations was determined by the ophthalmologist but was usually every three to four months. Criteria for assessment of response are indicated in Table 1.
Table 1.
Criteria for assessment of response*
| Category | Criteria |
|---|---|
| Complete response (CR) | Disappearance of all enhancing or nonenhancing tumor on consecutive contrast-enhanced MRI scans at least one month apart and neurologically stable or improved |
| Partial response (PR) | At least 50% reduction in the size of enhancing or nonenhancing tumor maintained for at least one month and neurologically stable or improved |
| Minimal response (MR) | 25%–49% reduction in size of enhancing or nonenhancing tumor maintained for at least one month and neurologically stable or improved |
| Stable disease (SD) | Any changes in enhancing or nonenhancing tumor size that do not qualify for CR, PR, MR, or PD classification |
| Progressive disease (PD) | At least 25% increase in size of enhancing or nonenhancing tumor or any new tumor on MRI scan, or neurologically worse |
| For patients with OPG, one of the following was also applicable for assessment of PD: | Progressive visual loss, i.e., deterioration of visual acuity > 1 octave, or decline in vision to 20/400, or increase in papilledema, or increase in diameter of optic nerve of ≥ 2 mm, and/or increase in the distribution of tumor involving the optic tract or optic radiations as evidenced by MRI scan of brain (T1-weighted imaging with and without contrast and T2- weighted imaging) |
Abbreviation: OPG, optic pathway glioma.
Comparisons of objective assessments, excluding progressive disease, were based upon changes in tumor size on the gadolinium-enhanced MRI scan of brain compared with the baseline scan. Tumor size was measured as the product of the largest perpendicular diameters. Determination of progressive disease was based upon comparison with the previous scan with the smallest measurements.
Statistical Considerations
The statistical design for this study was previously reported from our center (Quinn et al., 2003). A two-stage design was used for this trial. We assessed the response rate to TMZ within each of the five histologic strata (PA, nonbiopsied OPG, FA, OG, or OA) after every two cycles of chemotherapy. If disease control (complete response [CR], partial response [PR], minimal response [MR], or stable disease [SD]) was not achieved in the first nine patients, we could infer with 95% confidence that the actual rate of response was less than 30% and the study would be closed to further accrual. Otherwise, a total of 25 evaluable patients would be enrolled in each stratum. With this number of patients, the disease control rate could be estimated with a standard error of 10% or less. Exact binomial CIs were computed for response rates. We expected to accrue six to eight patients per stratum per year, with the study completing in approximately three years. However, in contrast to the process with adult patients on this study (Quinn et al., 2003), accrual in children with recurrent LGG occurred mainly to the PA and OPG strata, as expected. Owing to slow accrual and opening of a competing national protocol for children with LGG using TMZ, it was decided to close the study after accrual of at least 25 patients with either OPG or PA.
Additional analysis was performed on collected data and included estimation of interval of disease control in patients with objective responses or SD (from date of enrollment in the study to date of disease progression or last follow-up), overall survival (OS), and progression-free survival (PFS). The latter two parameters were calculated by the Kaplan-Meier product-limit method using GraphPad Prism for Windows (version 4.00; GraphPad Software, San Diego, Calif.) for all patients and those with OPG/PA. PFS was determined from the date of study entry to date of disease progression, death from any cause, or last follow-up. OS was calculated from the time of study entry until death from any cause or last follow-up.
Results
Patient Characteristics
Between January 2003 and February 2005, 33 pediatric patients were enrolled from the three centers in this study. Three of these 33 patients were later found to be ineligible and excluded from analysis; reasons for exclusion included one patient with an ineligible histology, one patient with no documented disease progression, and one patient with an ineligible ANC at protocol entry. Patient characteristics are listed in Table 2.
Table 2.
Clinical characteristics in 30 pediatric patients with recurrent low-grade glioma treated with temozolomide
| Characteristic | Patient Data |
|---|---|
| Period of study | June 1999–August 2005 |
| No. of eligible enrolled patients | 30 (Duke 15, CHOP 9, and Beth Israel 6) |
| Median age at enrollment | 10 years (range, 4–18 years) |
| Sex ratio | 21 M/9 F |
| No. of patients who received prior focal radiotherapy and/or chemotherapy | 19 |
| Type of low-grade glioma | |
| Optic pathway glioma | 14 |
| Pilocytic astrocytoma | 12 |
| Fibrillary astrocytoma | 4 |
| No. of patients with NF-1 | 4 |
Abbreviations: CHOP, Children’s Hospital of Philadelphia; NF-1, neurofibromatosis type 1.
Responses to TMZ in Patients with OPG/PA
Details of prior therapy, toxicity, response, and final outcome for the 30 patients included in this study are given in Table 3. The 26 patients with OPG/PA received a median of nine cycles (range, 2–12 cycles) of TMZ. The best objective response observed after at least two cycles of treatment included PR in three patients (11%; 95% CI, 3%–30%; patients 5, 15, 17) and MR in one (4%; 95% CI, 0%–21%; patient 19). Stable disease (SD) was found in 11 patients (42%; 95% CI, 24%–63%), and progressive disease (PD) in 11 patients (42%; 95% CI, 24%–63%).
Table 3.
Types of low-grade glioma, prior therapy, toxicity, response, and final outcome in 30 patients with recurrent low-grade glioma treated with oral temozolomide
| Pt. No. | Age (Years)/ Sex | Diagnosis | Prior Therapy | No. of Cycles of TMZ | Best Response | Overall Response | Worst Toxicity Due to TMZ | Disease Course After Coming Off Study and Salvage Therapy | Final Outcome/ Duration of Survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7/M | OPG | PCV | 11 | SD | PD | Grade 2 rash | Oral VP-16 | SD/77+ months |
| 2 | 10/M | OPG | Carboplatin | 12 | SD | SD | None | PD, vinblastine, XRT | SD/53 months |
| 3 | 13/M | OPG/NF-1 | Carboplatin | 12 | SD | SD | Grade 4 plts | Stable | SD/42 months |
| 4 | 9/F | OPG/NF-1 | None | 5 | SD | PD | None | Carboplatin | SD/38 months |
| 5 | 13/F | OPG/NF-1 | None | 10 | PR | PD* | None | XRT, carboplatin | SD/24 months |
| 6 | 9/M | OPG | Surgery, carboplatin + VCR, TPCV, oral VP-16 | 9 | SD | PD | Grade 4 plts | Surgery, XRT | Dead/38 months** |
| 7 | 5/M | OPG | TPCV, carboplatin + RMP-7 | 6 | SD | PD | Grade 4 plts | NA | SD/41 months |
| 8 | 13/F | OPG | Surgery, TPCV, carboplatin + VCR, XRT | 3 | SD | PD | None | None | Dead/3 months |
| 9 | 5/M | OPG | Carboplatin + VCR | 12 | SD | SD | None | Stable | SD/41 months |
| 10 | 16/M | OPG | Carboplatin + VCR | 12 | SD | SD | Grade 1 fatigue | PD, XRT | SD/47 months |
| 11 | 5/F | OPG/NF-1 | Carboplatin + VCR | 9 | SD | PD | Grade 1 ANC | TPCV | SD/28 months |
| 12 | 8/M | OPG | Carboplatin + VCR | 12 | SD | SD | Grade 1 ANC | PD, vinblastine | SD/27 months |
| 13 | 12/M | OPG | Carboplatin + VCR | 9† | SD | SD | Grade 2 Plts | Stable | SD/20 months |
| 14 | 4/M | OPG | Carboplatin + VCR | 4‡ | SD | SD | Grade 4 ANC | Stable, TPCV | SD/21 months |
| 15 | 17/F | PA (tectal) | Carboplatin | 8 | PR | PR | Grade 3 hemorrhage into tumor | PD, carboplatin, XRT, vinblastine | SD/62+ months |
| 16 | 16/M | PA (cerebral) | Surgery | 9 | SD | PD | Grade 3 ANC | Surgery, XRT, CPT-11 | NED/48 months |
| 17 | 17/F | PA (hypothalamic) | Surgery | 12 | PR | PR | Grade 2 plts | Stable | SD/45 months |
| 18 | 11/F | PA (hypothalamic) | Surgery | 12 | SD | SD | None | Stable | SD/41 months |
| 19 | 16/M | PA (hypothalamic) | Surgery | 12 | MR | MR | Grade 2 ANC | Stable | SD/36 months |
| 20 | 10/F | PA (hypothalamic) | Surgery | 12 | SD | PD | Grade 2 plts | Carboplatin, TPCV | PD/38 months |
| 21 | 11/F | PA (thalamic) | Surgery | 6 | SD | PD | Grade 4 plts | Carboplatin, CPT-11 | Dead/26 months |
| 22 | 9/M | PA (hypothalamic) | Surgery | 6 | SD | PD | None | Carboplatin | SD/30 months |
| 23 | 14/M | PA (brainstem) | Carboplatin | 5 | SD | PD | None | Surgery | SD/9 months |
| 24 | 6/M | PA (brainstem) | Surgery, TPCV | 12 | SD | SD | None | Stable | SD/34 months |
| 25 | 5/M | PA | Surgery, carboplatin, TPCV | 12 | SD | SD | Grade 2 ANC | Stable | SD/33 months |
| 26 | 14/M | PA | Carboplatin + VCR, XRT | 12 | SD | SD | Grade 1 ANC | Stable | SD/40 months |
| 27 | 12/M | FA (cerebral) | Surgery | 12 | SD | PD | Grade 4 plts, ANC | Carboplatin, XRT | SD/35 months |
| 28 | 10/M | FA | Surgery | 2 | PD | PD | Grade 1 anemia | Carboplatin + VCR, XRT | SD/41 months |
| 29 | 10/M | FA | TPCV, estramustine + VP-16, XRT | 12 | SD | SD | Grade 1 fatigue, ANC | PD, carboplatin + VCR | SD/39 months |
| 30 | 10/M | FA | Carboplatin + VCR | 7 | SD | PD | None | XRT | SD/16 months |
Abbreviations: ANC, absolute neutrophil count; CPT-11, irinotecan; FA, fibrillary astrocytoma; MR, minimal response; NA, not available; NED, no evidence of disease; NF-1, neurofibromatosis type 1; OPG, optic pathway glioma; PA, pilocytic astrocytoma; PCV, procarbazine; PD, progressive disease; plts, platelets; PR, partial response; RMP-7, labridimil; SD, stable disease; TMZ, temozolomide; TPCV, thioguanine, procarbazine, chloroethyl cyclohexylnitrosourea (lomustine), and vincristine; VCR, vincristine; VP-16, etoposide; XRT, focal radiotherapy.
Patient had progressive disease based on change in visual acuity but MRI revealed stable disease.
Patient found on second biopsy to have elements of atypical teratoid/rhabdoid tumor.
Patient taken off study due to diagnosis of Lyme disease.
Patient taken off study due to prolonged neutropenia and started on TPCV chemotherapy eight months later.
Two patients with initial PR to TMZ (patients 5 and 15) had PD 13 and 8 months, respectively, after the initial response. Patient 5 (with neurofibromatosis type 1 [NF-1] and OPG) had a PR to TMZ after two cycles of treatment. However, eight months later, she developed significant decrease in visual acuity on a neuroophthalmologic examination even though MRI scan of her brain revealed SD. Patient 15 with a tectal plate PA had a PR to TMZ initially after eight months of treatment but was taken off therapy for noncompliance. Patients 2, 10, and 12, who completed therapy with SD, had PD 2, 24, and 29 months after coming off study.
The overall disease stabilization rate (PR + MR + SD) on study was 14 of 26 patients (54%; 95% CI, 34%–73%); disease control lasted a median of 34 months (range, 10–47 months) from beginning of treatment. Sixteen patients had PD either on or off treatment at a median of nine months (range, 2–29 months) after enrollment in the study. Patient 6, who had an initial diagnosis of PA by biopsy and who had PD on this study, was given focal radiotherapy as salvage therapy. A second biopsy on a subsequent recurrence revealed elements of atypical teratoid/rhabdoid tumor confirmed by immunohistochemical and genetic analysis (Allen et al., 2006). Patients 13, 14, and 15 were taken off study because of contracting Lyme disease, prolonged neutropenia, and poor compliance, respectively.
Responses in Patients with FA
The four patients with FA received a median of 12 cycles of TMZ (range, 2–12 cycles). Patient 29 had SD that lasted 29 months. The other three patients (patients 27, 28, and 30) had PD 12, 2, and 7 months, respectively, after enrollment in the study.
Disease Stabilization Based on Prior Therapy
Nineteen patients had received prior radiotherapy and/ or chemotherapy (seven patients had received alkylator-based therapy), and 11 had either no prior therapy or surgery alone prior to enrollment in the study (Table 3). Twelve (52%) of 23 patients who had no prior therapy, only surgery, or only carboplatin-based regimens had disease stabilization (PR + SD + MR), compared with only two (28%) of seven patients who had received prior alkylator therapy (procarbazine regimen, or thioguanine, procarbazine, chloroethyl cyclohexylnitrosourea [lomustine], and vincristine [TPCV] regimen) as part of their chemotherapy (Table 3).
PFS and OS for All Patients
With a median follow-up of 38 months (range, 3–77 months), three patients have died of PD. The two-year PFS and OS were 51% (95% CI, 34%–69%) and 97% (95% CI, 90%–100%), respectively. The corresponding four-year PFS and OS were 17% (95% CI, 1%–33%) and 71% (95% CI, 43%–100%), respectively.
PFS and OS for Patients with OPG/PA
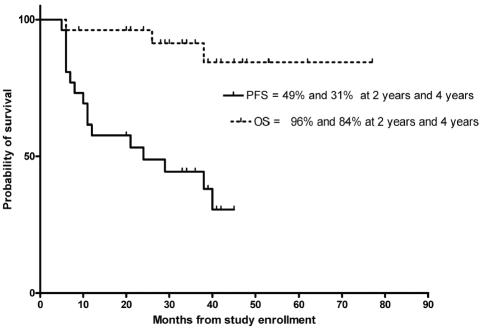
The two-year PFS and OS for the 26 patients with OPG and PA were 49% (95% CI, 30%–67%) and 96% (95% CI, 89%–100%), respectively (Fig. 1). The corresponding four-year PFS and OS were 31% (95% CI, 9%–51%) and 84% (95% CI, 69%–100%), respectively.
Fig. 1.
PFS and OS in 26 patients with recurrent OPG/PA treated with oral TMZ.
The two-year PFS for the 14 patients with OPG/PA who had disease stabilization (PR, MR, or SD) after TMZ therapy was 73% (95% CI, 51%–96%). The corresponding four-year PFS was 61% (95% CI, 33%–89%).
Toxicity Related to TMZ Therapy
Toxic effects related to TMZ in this study were typically myelosuppression and fatigue (Table 3). Only patient 14 was taken off study due to prolonged neutropenia after therapy; the neutropenia ultimately resolved, and the patient was able to start on TPCV chemotherapy. Patient 15 had hemorrhage into the tumor that was associated with shrinkage of tumor but was not related to thrombocytopenia. Patient 13 was taken off study after nine cycles of treatment due to a diagnosis of Lyme disease. There were no toxicity-related deaths.
Discussion
This phase II study of oral TMZ in children with recurrent LGG has demonstrated that despite a low rate of objective shrinkage of tumor, it is possible to stabilize disease in more than 50% of patients for a prolonged period without significant toxicity. This effect, combined with the relative ease of administration of oral chemotherapy in the home setting, makes TMZ one of the treatment options to consider in children with progressive LGG in the context of failure of first-line therapy.
An objective response (PR + MR) of only 12% was observed in these children, in contrast to results for adults with progressive LGG treated on the same study (Table 4; Quinn et al., 2003). Adult patients with LGG (predominantly FA, OG, or OA) have been reported to have higher rates of objective response (10%–60%), disease stabilization (>85%), seizure control, and quality of life (Brada et al., 2003; Hoang-Xuan et al., 2004; Pace et al., 2003; Quinn et al., 2003). In contrast to these reports, most patients (63%) in our study had disease progression on other chemotherapy regimens, including alkylators, prior to receiving TMZ, and this might explain the lower rates of observed objective responses. Only two of seven patients who had prior alkylator exposure experienced SD after TMZ treatment, compared with findings of PR or SD in 12 of 23 patients who had no prior therapy, surgery only, or a nonalkylator-based chemotherapy. Hence, it is likely that suboptimal responses in our study might be due to drug resistance caused by prior alkylator exposure, possibly related to overexpression of alkylguanine alkyl transferase (AGT).
Table 4.
Selected studies of chemotherapy treatment of progressive low-grade glioma in children and adults
|
Response to Chemotherapy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Drug | Study Population | Predominant Tumor | No. Pts. | Prior Therapy | CR | PR | SD | PD | Disease Stabilization Rate | Median Duration of Disease Stabilization | Reference |
| Carboplatin + vincristine | Children | OPG and PA | 78 | Surgery only | 4 | 22 | 47 | 5 | 94% | 36 months | Packer et al. (1997) |
| Carboplatin | Children | OPG and PA | 81 | Surgery, chemotherapy, XRT | 2 | 17 | 15 | 11 | 85% | 22 months | Gururangan et al. (2002) |
| TPCV + dibromodulcitol | Children | PA | 42 | Surgery only | 0 | 15 | 25 | 2 | 95% | 33 months | Prados et al. (1997) |
| Temozolomide | Children | OPG and PA | 13 | Surgery, chemotherapy, XRT | 0 | 3 | 6 | 4 | 70% | NA | Kuo et al. (2003) |
| Temozolomide | Children | OPG and PA | 26 | Surgery, chemotherapy, XRT | 0 | 3 | 11 | 12 | 52% | 34 months | Present study |
| Temozolomide | Adults | OG, FA, OA | 29 | Surgery only | 0 | 3 | 25 | 1 | 97% | NA | Brada et al. (2003) |
| Temozolomide | Adults | OG, FA, OA | 46 | Surgery, chemotherapy, XRT | 11 | 17 | 16 | 1 | 95% | 22 months | Quinn et al. (2003) |
| Temozolomide | Adults | OG, FA, OA | 43 | Surgery, chemotherapy, XRT | 4 | 16 | 17 | 6 | 86% | 9 months | Pace et al. (2003) |
| Temozolomide | Adults | OG, FA, OA | 60 | Surgery only. | 0 | 10 | 45 | 5 | 92% | NA | Hoang-Xuan et al. |
Abbreviations: CR, complete response; FA, fibrillary astrocytoma; NA, not available; OG, oligodendroglioma; OA, oligoastrocytoma; OPG, optic pathway glioma; PA, pilocytic astrocytoma; PD, progressive disease; PR, partial response; SD, stable disease; TPCV, thioguanine, procarbazine, chloroethyl cyclohexylnitrosourea (lomustine), vincristine; XRT, focal radiotherapy.
Bobola et al. (2005) observed elevated levels of AGT in LGG cell lines. The dose of TMZ that was lethal in 10% of these cell lines was about fourfold higher than the mean plasma concentration of TMZ that is clinically achievable (100 μmol/L). Prior exposure of these cell lines to O6-benzylguanine (O-6BG), an inhibitor of AGT, for 18–20 h restored sensitivity of these tumor cells to TMZ, suggesting that AGT overexpression was the predominant cause for initial TMZ resistance in these cell lines and possibly in the 46% of our patients who had PD during treatment. In contrast, Kuo et al. (2003), in a retrospective single-institution study, observed favorable responses in 13 children with progressive LGG treated with oral TMZ given either on a five-day schedule (150 mg/m2 per day, n = 4) or a prolonged 42-day schedule (75 mg/m2 per day, n = 9). PR was observed in four patients and SD in six patients. It should be noted, however, that nine patients in this study had no prior therapy except surgery or radiotherapy before treatment with TMZ. Also, it is likely that the prolonged schedule of TMZ might have had a better effect on tumor shrinkage due to increased AGT depletion in the tumor (Tolcher et al., 2003).
As expected, myelosuppression (mostly grade 2–4 thrombocytopenia) was the most common toxicity observed in our study, and the incidence was similar to what has been previously reported in the literature (Brada et al., 2003; Hoang-Xuan et al., 2004; Kuo et al., 2003; Pace et al., 2003; Quinn et al., 2003). Only one patient had prolonged neutropenia requiring discontinuation of TMZ. Myelosuppression from TMZ can be variable, and its severity depends on the level of AGT expression in the progenitor cells of the marrow, with low levels of AGT in CD34+ cells causing more severe myelosuppression, especially neutropenia and thrombocytopenia (Gerson, 2002). It has also been observed that low AGT expression in CD34+ cells in the marrow could predispose such patients treated with TMZ to a higher risk of developing secondary leukemia (Gerson, 2002).
Four (13.3%) of the 30 patients in our study had NF-1, and, as would be expected, all of them had OPG. NF-1 patients were enrolled on the study based on visual deterioration and/or change in the size of previously discovered optic pathway tumors. We did not exclude patients with NF-1 from this trial, although concerns have been raised regarding the long-term complications of using a DNA-methylating agent in such patients with an inherent genetic predisposition to tumors, especially leukemias. There have been recent reports of secondary acute leukemias after a short latency period following TMZ in adult patients who received TMZ concurrently with radiotherapy (De Vita et al., 2005). With follow-up periods of 24–48 months, none of our patients with NF-1 has had any undue ill effects from TMZ exposure.
Although disease stabilization after TMZ therapy in our patients with recurrent LGG lasted a median of 34 months, the PFS was only 17% at four years after enrollment for all patients and 31% for those with OPG/PA (see Fig. 1). Only nine patients (30%) have had SD and not required other modalities of treatment since completing TMZ therapy. Eighteen patients have undergone salvage chemotherapy, and nine of these patients have also required salvage radiotherapy after PD on or after TMZ treatment (Table 3). These results compare unfavorably with those obtained using carboplatin or TPCV-based regimens (Table 4; Gururangan et al., 2002; Packer et al., 1997; Prados et al., 1997). Therefore, further refinements are required in the chemotherapy of children with progressive LGG. On the basis of preclinical data confirming high AGT expression in LGG (especially PA) and the low rates of clinical objective responses, it might be prudent to consider alternative strategies of treatment that circumvent drug resistance.
O-6BG is a potent and irreversible inactivator of AGT in normal and tumor cells by serving as an alternative highly selective substrate for AGT (Friedman et al., 2002). A single intravenous dose of O-6BG (120 mg/m2) can deplete tumor cells of AGT for up to 24 h and can potentiate the cytotoxic effects of alkylating agents, including TMZ and nitrosoureas (Friedman et al., 2002; Warren et al., 2005). In a recently published phase I study of the use of oral TMZ plus O-6BG in children with recurrent brain tumors, complete response was seen in one patient with PA (after 12 cycles) and SD in two patients with OPG (Warren et al., 2005). Also, AGT depletion can be achieved by using a prolonged schedule of oral TMZ (Kuo et al., 2003; Tolcher et al., 2003). Future trials using TMZ in children with recurrent LGG should incorporate such novel strategies to improve disease control.
Footnotes
Presented in part at the Eleventh International Symposium on Pediatric Neuro-Oncology, June 2004, Boston, MA, USA.
Abbreviations used are as follows: AGT, alkylguanine alkyl transferase; ANC, absolute neutrophil count; BUN, blood urea nitrogen; FA, fibrillary astrocytoma; LGG, low-grade glioma; MR, minimal response; NF-1, neurofibromatosis type 1; OA, oligoastrocytoma; O-6BG, O6-benzylguanine; OG, oligodendroglioma; OPG, optic pathway glioma; OS, overall survival; PA, pilocytic astrocytoma; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; TMZ, temozolomide; TPCV, thioguanine, procarbazine, chloroethyl cyclohexylnitrosourea (lomustine), and vincristine; ULN, upper limit of institutional normal.
References
- Allen JC, Judkins AR, Rosenblum MK, Biegel JA. Atypical teratoid/rhabdoid tumor arising from an optic pathway ganglioglioma: Case study. Neuro-Oncology. 2006;8:79–82. doi: 10.1215/S1522851705000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola MS, Silber JR, Ellenbogen RG, Geyer JR, Blank A, Goff RD. O6-Methylguanine-DNA methyltransferase, O6-benzyl-guanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res. 2005;11:2747–2755. doi: 10.1158/1078-0432.CCR-04-2045. [DOI] [PubMed] [Google Scholar]
- Brada M, Judson I, Beale P, Moore S, Reidenberg P, Statkevich P, Dugan M, Batra V, Cutler D. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81:1022–1030. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brada M, Viviers L, Abson C, Hines F, Britton J, Ashley S, Sardell S, Traish D, Gonsalves A, Wilkins P, Westbury C. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715–1721. doi: 10.1093/annonc/mdg371. [DOI] [PubMed] [Google Scholar]
- De Vita S, De Matteis S, Laurenti L, Chiusolo P, Reddiconto G, Fiorini A, Leone G, Sica S. Secondary Ph+ acute lymphoblastic leukemia after temozolomide. Ann Hematol. 2005;84:760–762. doi: 10.1007/s00277-005-1093-6. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Cohen ME. The long-term effects of central nervous system therapy on children with brain tumors. Neurol Clin. 1991;9:479–495. [PubMed] [Google Scholar]
- Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- Friedman HS, Keir S, Pegg AE, Houghton PJ, Colvin OM, Moschel RC, Bigner DD, Dolan ME. O6-Benzylguanine-mediated enhancement of chemotherapy. Mol Cancer Ther. 2002;1:943–948. [PubMed] [Google Scholar]
- Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–2399. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- Grill J, Couanet D, Cappelli C, Habrand JL, Rodriguez D, Sainte-Rose C, Kalifa C. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gururangan S, Cavazos CM, Ashley D, Herndon JE, II, Bruggers CS, Moghrabi A, Scarcella DL, Watral M, Tourt-Uhlig S, Reardon D, Friedman HS. Phase II study of carboplatin in children with progressive low-grade gliomas. J Clin Oncol. 2002;20:2951–2958. doi: 10.1200/JCO.2002.12.008. [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Criniere E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broet P, Sanson M, Delattre JY. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- Kuo DJ, Weiner HL, Wisoff J, Miller DC, Knopp EA, Finlay JL. Temozolomide is active in childhood, progressive, unresectable, low-grade gliomas. J Pediatr Hematol Oncol. 2003;25:372–378. doi: 10.1097/00043426-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Nicholson HS, Krailo M, Ames MM, Seibel NL, Reid JM, Liu-Mares W, Vezina LG, Ettinger AG, Reaman GH. Phase I study of temozolomide in children and adolescents with recurrent solid tumors: A report from the Children’s Cancer Group. J Clin Oncol. 1998;16:3037–3043. doi: 10.1200/JCO.1998.16.9.3037. [DOI] [PubMed] [Google Scholar]
- Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- Pace A, Vidiri A, Galie E, Carosi M, Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B, Carapella CM. Temozolomide chemotherapy for progressive low-grade glioma: Clinical benefits and radiological response. Ann Oncol. 2003;14:1722–1726. doi: 10.1093/annonc/mdg502. [DOI] [PubMed] [Google Scholar]
- Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- Perilongo G. Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol. 2005;75:301–307. doi: 10.1007/s11060-005-6754-8. [DOI] [PubMed] [Google Scholar]
- Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32 :235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Provenzale JM, Mclendon RE, Gururangan S, Bigner DD, Herndon JE, II, Avgeropoulos N, Finlay J, Tourt-Uhlig S, Affronti ML, Evans B, Stafford-Fox V, Zaknoen S, Friedman HS. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21:646–651. doi: 10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KE, Aikin AA, Libucha M, Widemann BC, Fox E, Packer RJ, Balis FM. Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol. 2005;23:7646–7653. doi: 10.1200/JCO.2005.02.0024. [DOI] [PubMed] [Google Scholar]
- Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11:152–162. doi: 10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]