

Introduction
The refractive power of the human eye depends on three factors: the power of the cornea, the power of the lens, and the length of the eye. Following cataract surgery, only the power of the cornea and the length of the eye are relevant. If both of these variables are known, it is possible to calculate what lens power will give the best refraction. Biometry is the process of measuring the power of the cornea (keratometry) and the length of the eye, and using this data to determine the ideal intraocular lens power. If this calculation is not performed, or if it is inaccurate, then patients may be left with a significant refractive error.
On 8th February 1950, Harold Ridley implanted the first intraocular lens (IOL), following an earlier extracapsular cataract extraction. Post-operatively, the patient's refraction was −24.0/+6.0 × 30 degrees. Although Mr Ridley's choice of material was inspired, his patient did not enjoy the benefits of modern biometry.
Over fifty years later, despite sophisticated technology and intelligent software, one frequently encounters biometry mistakes or ‘surprises’. Most are avoidable and most are due to human error. This article will examine the steps involved in biometry and the ways in which mistakes can be minimised, based on feedback from ophthalmic staff in busy units.
NB Although accurate biometry represents the ideal, it is not always possible. In communities with a low prevalence of axial ametropia, an IOL of a standard power will give good results in at least half the population.1
Steps in selecting the correct IOL
1 Identifying the refractive needs of the patient
Emmetropia will be the goal for most patients, but some may benefit from being left intentionally myopic post-operatively (or, rarely, hypermetropic), depending upon their preference and the refraction of the other eye. Anisometropia should be kept below 3 dioptres. The need for reading glasses should be explained and the patient made aware of the options.
2 Measuring the axial length of the eye
The measurement of axial length measurement has the greatest potential for error in calculating IOL power. Traditionally, contact A-scan ultrasonography is used. This measures the time taken for sound to traverse the eye and converts it to a linear value using a velocity formula. Part of the ultrasound beam reflects back from each surface in the eye – cornea, anterior lens, posterior lens, and retina. The reflected beam is translated into an image showing lines (spikes) for each surface. The distance between the corneal and retinal spikes gives the axial length of the eye.
Figure 1.
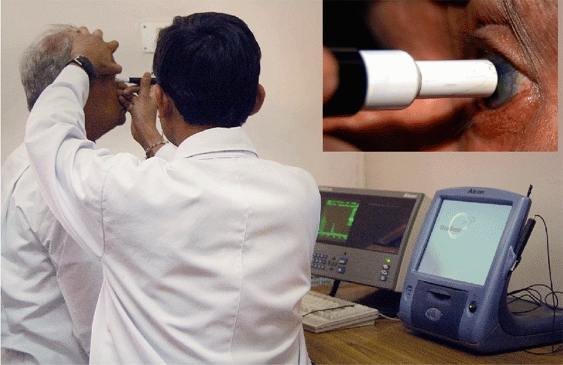
Photograph showing technique for applanation A-scan biometry
More recently, non-contact laser interferometry has been developed (IOL Master). It is more precise,2 combines axial length and keratometry, and enables different formulae to be used,3 but it may be inaccurate for patients with axial or dense cataracts or gross astigmatism. It is also expensive. However, this method is well suited to some special conditions. These include extremely short eyes, very long eyes with posterior staphylomata, eyes containing silicone oil, and pseudophakic eyes.
As a rule, biometry is done using an applanation probe in contact with the cornea, but the immersion method4 may also be used. In the immersion method, a scleral (Prager) shell is placed between the eyelids and centred on the cornea of the supine patient. This method avoids any corneal compression (and thus a falsely short axial length) and gives high quality, consistent spikes.
The alignment of the A-scan is vitally important. If the alignment is incorrect, the length of the eye will be underestimated. Most systems rely on the patient fixing on a target – usually a light in the probe. Patients with poor vision, whether from cataract or from some other pathology, are less likely to fix accurately, and are more prone to biometry errors.
Tips for accurate measurement of axial length (using applanation):
ensure the machine is calibrated and set for the correct velocity setting (e.g. cataract, aphakia, pseudophakia)
the echoes from cornea, anterior lens, posterior lens, and retina should be present and of good amplitude
misalignment along the optic nerve is recognised by an absent scleral spike
the gain should be set at the lowest level at which a good reading is obtained
take care with axial alignment, especially with a hand-held probe and a moving patient (as described above)
don't push too hard – corneal compression commonly causes errors
average the 5–10 most consistent results giving the lowest standard deviation (ideally < 0.06 mm)
errors may arise from an insufficient or greasy corneal meniscus due to ointment or methylcellulose used previously.
Take note of eyes that are very short (less than 22 mm) or very long (more than 25 mm). Axial length errors are more significant in short eyes and a posterior staphyloma may be present in a long eye. Look out for the unexpected result, for example an axial length of 27 mm in a patient with a +4.00 D refractive error. Always measure both eyes and repeat if the difference between eyes is greater than 0.3 mm, or if consecutive measurements differ by more than 0.2 mm.
3 Measuring the power of the cornea
Again, accuracy is essential, as an error of 0.75 D in the keratometry will result in a similar post-operative error. Keratometry may be carried out manually or using an automated or hand-held device.
Tips for accurate manual keratometry:
calibrate and check the accuracy of the keratometer
use a dedicated single instrument that is known to be accurate
don't touch the cornea beforehand and ensure a good tear film
adjust the eyepiece to bring the central cross-hairs into focus
make sure that the patient's other eye is occluded and that the cornea is centred
take an average of three readings, including the axes
if high or low results are encountered (< 40.00 D or > 48.00 D), it is advisable to have a second person check the measurements
repeat if the difference in total keratometric power between the eyes exceeds 1.50 D
in a scarred cornea, use the fellow eye or average the results.
4 Using the appropriate formula
The Hoffer Q, Holladay I, and SRK/T formulae are all commonly used, but the SRK I and II regression formulae are now regarded as obsolete.5 More recent formulae, such as the Holladay II or Haigis, are not currently built into biometry software. Where audit software is in use, the personalisation of calculation constants can increase accuracy.6 Table 1 indicates which formulae to use.
Table 1.
Range of axial length and preferred formula
| Axial length (mm) | Formula |
|---|---|
| <20 mm | Holladay II |
| 20–22 mm | Hoffer Q |
| 22–24.5 mm | SRK/T/Hoffer Q/Holladay (average) |
| >24.5–26 mm | Holladay I |
| >26 mm | SRK/T |
5 Difficult eyes
Extremely dense cataracts create difficulties, as they absorb sound as it passes through the lens. A higher gain setting may be necessary to achieve adequate spikes. Posterior staphylomata in myopic eyes not only cause an elongated globe, but often tilt the macula as well so that the ultrasound beam is deflected. In these cases, it may be necessary to add the A-scan anterior chamber depth measurement to vitreous depth taken from a B-scan.
Figure 2.
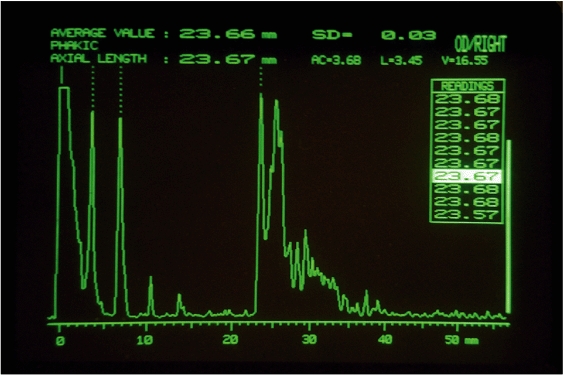
A-scan of the phakic eye
- initial spike (probe tip and cornea)
- anterior lens capsule
- posterior lens capsule
- retina
- sclera
- orbital fat
6 Why things go wrong
No matter how good the system, people will still make mistakes. Some reasons include:
people in a hurry
lack of training or accessible guidelines
reliance on others
technical failure (rarely)
human error (often).
Some common mistakes (collected from the UK and overseas departments):
wrong A-constant selected
wrong formula used
wrong K-readings entered by hand (90 degrees out)
biometry print-out stuck in wrong patient's notes
incorrectly labelled IOL
wrong patient in theatre
reversed IOL optic
wrong IOL implanted (25.5 D implanted instead of 22.5 D or +30 D instead of +3.0 D).
Some errors of omission include:
no biometry at all
no spectacle prescription or focimetry available
no IOL available on the day
not taking account of the other eye
not discussing the intended outcome with the patient.
Another factor to consider is the postoperative position of the IOL. Inadvertent placement in the sulcus will cause a 0.75 D myopic shift. If an anterior chamber IOL has to be used, the A-constant will be different. If all else fails, blame the machine! Different biometry machines may give different results, which can be confusing (e.g. A-scan biometry and IOL Master).
In some high-volume clinics, the time required for biometry exceeds the time taken for surgery. However, if you are going to do biometry, you have to do it properly and thoroughly. It is better to have a few well-trained and experienced members of staff who can get consistent results, than to have many people with limited training and experience.
Departments should aim for consistency in their biometry and audit their results. Mistakes are easy to make, but difficult (and sometimes expensive) to rectify. The following list sums up some lessons that can be learnt from others' mistakes:
slow down
train and certify your biometry staff
follow guidelines
don't rely on others
watch out for the unexpected
learn from mistakes, particularly any eyes with error greater than 2 dioptre
audit your outcomes.
If you are using biometry, 80 per cent of eyes should be within 1 dioptre of their intended refraction. Try to identify any issues that are leading to consistent errors.
Contributor Information
Nick Astbury, Consultant Ophthalmic Surgeon, Norfolk and Norwich University Hospital NHS Trust, Colney Lane, Norwich NR4 7UY, UK..
Balasubramanya Ramamurthy, Consultant, Cornea and Anterior Segment Services, LV Prasad Eye Institute, LV Prasad Marg, Banjara Hills, Hyderabad 500 034, India..
References
- 1.Connell B, Brian G, Bond MJ. A case-control study of biometry in healthy and cataractous Eritrean eyes. Ophthalmic Epidemiol. 1997;4(3):151–5. doi: 10.3109/09286589709115722. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth J, Fekete O, Pesztenlehrer N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg. 2003;29(1):85–8. doi: 10.1016/s0886-3350(02)01500-6. [DOI] [PubMed] [Google Scholar]
- 3.Kielhorn I, Rajan MS, Tesha PM, Subryan VR, Bell JA. Clinical assessment of the Zeiss IOLMaster. J Cataract Refract Surg. 2003;29(3):518–22. doi: 10.1016/s0886-3350(02)01819-9. [DOI] [PubMed] [Google Scholar]
- 4.Shammasa HJ. Slack Inc; 2004. Intraocular Lens Power Calculations. [Google Scholar]
- 5.Gale RP, Saha N, Johnston RL. National Biometry Audit II. Eye. 2006;20:25–28. doi: 10.1038/sj.eye.6701778. [DOI] [PubMed] [Google Scholar]
- 6.The Royal College of Ophthalmologists. Cataract Surgery Guidelines 2004. [(accessed October 2006)]. www.rcophth.ac.uk/docs/publications/CataractSurgeryGuidelinesMarch2005Updated.pdf.


