Abstract
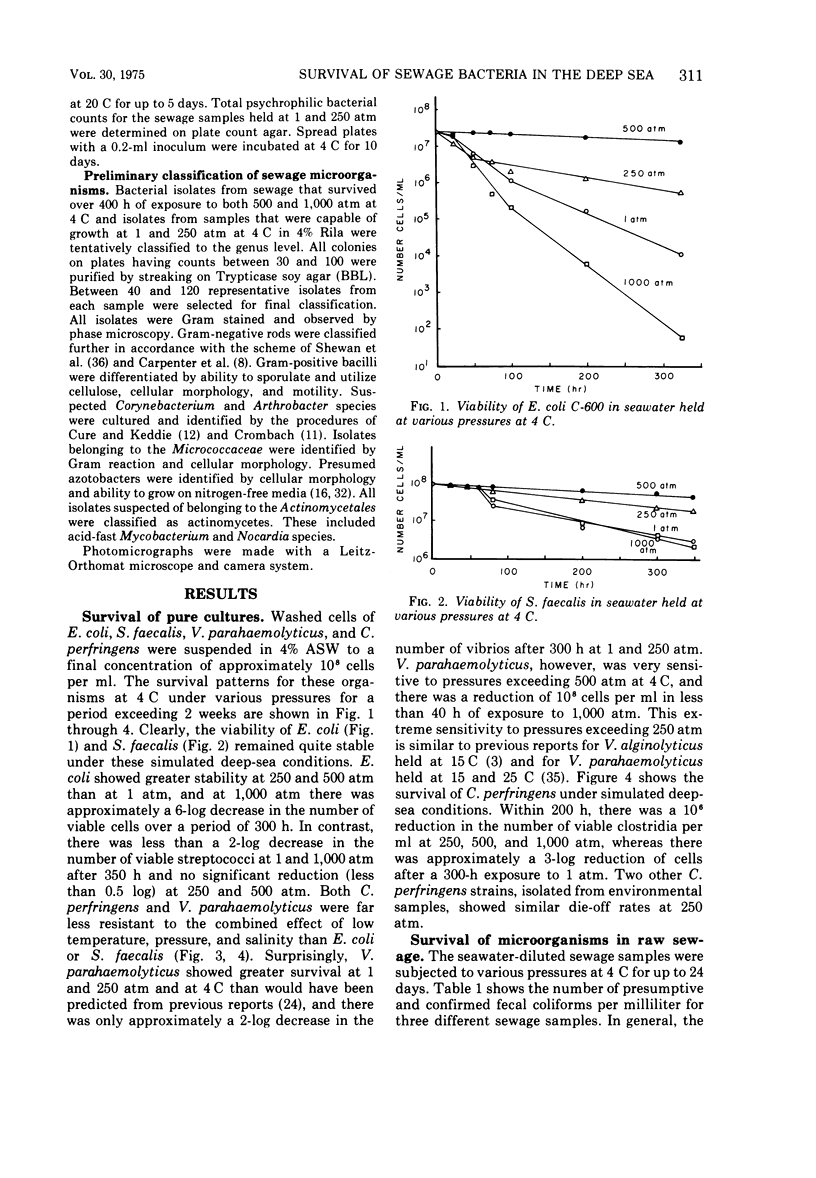
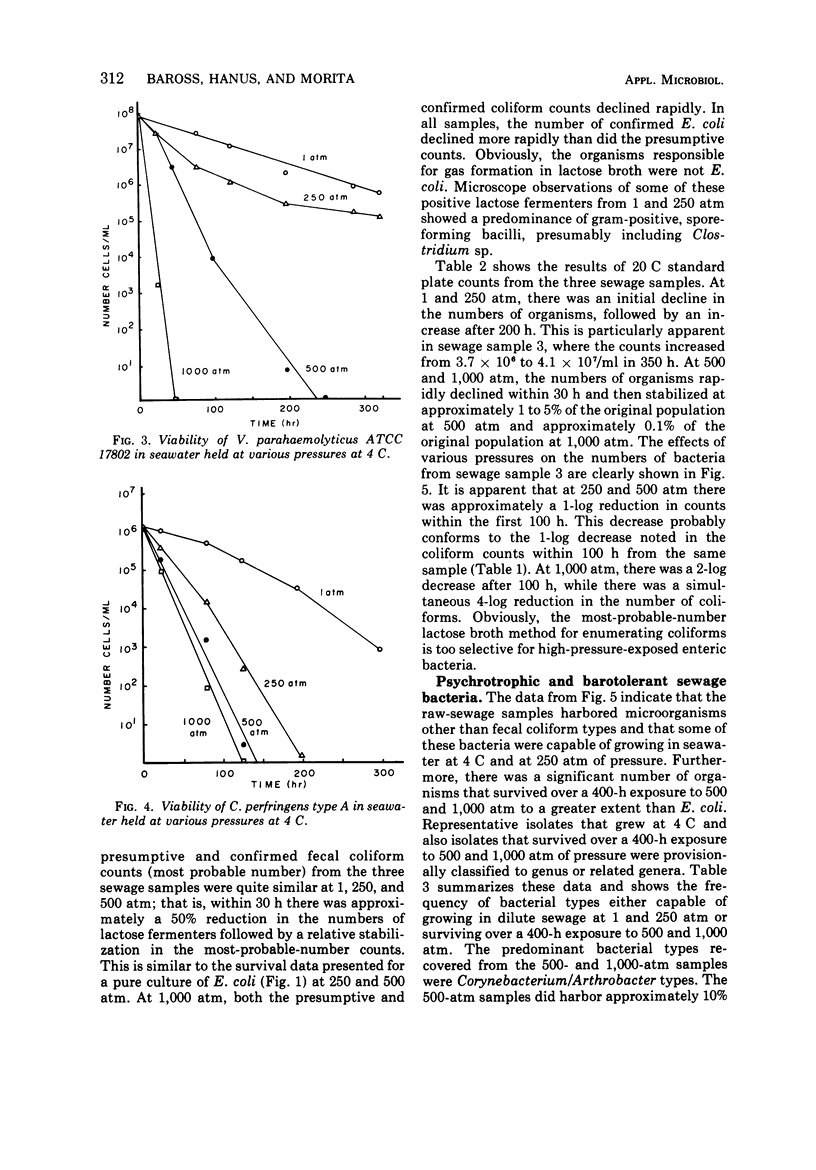
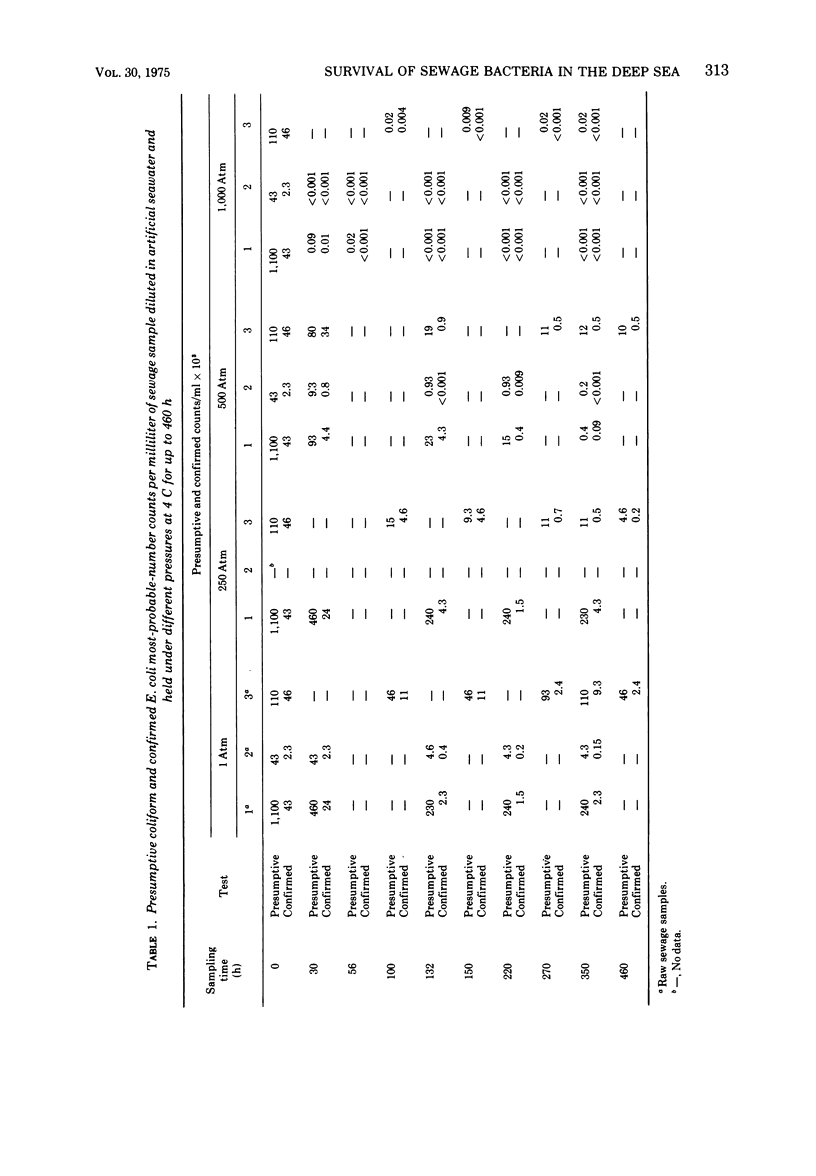
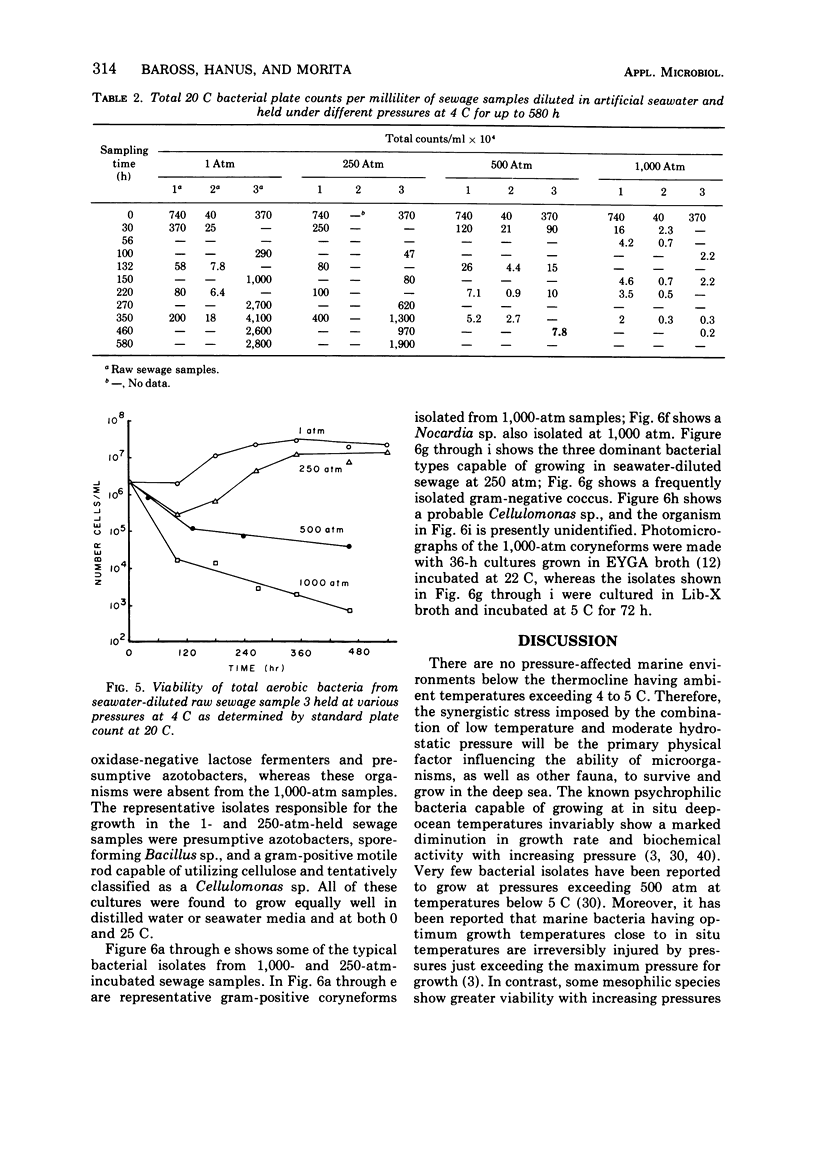
The survival of pure cultures of Escherichia coli, Streptococcus faecalis, Clostridium perfringens, and Vibrio parahaemolyticus under simulated deep-sea conditions of low temperature (4 C), seawater, and hydrostatic pressures ranging from 1 to 1,000 atm was determined over a period exceeding 300 h. The viability of E. coli and total aerobic bacteria in seawater-diluted raw sewage subjected to these deep-sea conditions was also measured. There was a greater survival of both E. coli and S. faecalis at 250 and 500 atm than at 1 atm at 4 C. S. faecalis was quite insensitive to 1,000 atm, whereas with E. coli there was a 10-fold die-off per 50-h exposure to 1,000 atm. In contrast, V. parahaemolyticus and C. perfringens were quite sensitive to pressures exceeding 250 atm, and with both of these species there was a total loss of viability of approximately 108 cells per ml within 100 h at 1,000 atm and within 200 h at 500 atm. The viability of the naturally occurring fecal coliforms in sewage exposed to moderate pressures at 4 C was found to be similar to the survival patterns demonstrated with pure cultures of E. coli. The total numbers of aerobic bacteria in these sewage samples, however, stabilized at 500 and 1,000 atm after 100 h, and at 1 and 250 atm there was significant growth of sewage-associated bacteria, which apparently utilized the organic compounds in the seawater-diluted sewage samples. A preliminary classification of some of these bacteria indicated that approximately 90% (160 isolates) of the organisms that survived over a 400-h exposure to 500 and 1,000 atm were Arthrobacter/Corynebacterium species, and the representative organisms capable of growing at 1 and 250 atm in seawater at 4 C were gram-positive cellulose digesters and an unidentified gram-negative coccus. The significance of these results with respect to the contamination of the deep ocean with human pathogens and the possibility of sewage-associated microorganisms growing and competing with indigenous marine microbial flora in situ is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew J. W., Rittenberg S. C. THERMOPHILIC BACTERIA FROM DEEP OCEAN BOTTOM CORES. J Bacteriol. 1949 Jun;57(6):658–658. doi: 10.1128/jb.57.6.658-658.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. C., Syzdek L. Mechanism for the water-to-air transfer and concentration of bacteria. Science. 1970 Nov 6;170(3958):626–628. doi: 10.1126/science.170.3958.626. [DOI] [PubMed] [Google Scholar]
- Bonde G. J. Pollution of a marine environment. J Water Pollut Control Fed. 1967 Oct;39(10 Suppl):R45–R63. [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. An evaluation of factors affecting the survival of Escherichia coli in sea water. I. Experimental procedures. Appl Microbiol. 1960 Jul;8:243–247. doi: 10.1128/am.8.4.243-247.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombach W. H. Morphology and physiology of coryneform bacteria. Antonie Van Leeuwenhoek. 1974;40(3):361–376. doi: 10.1007/BF00399348. [DOI] [PubMed] [Google Scholar]
- Halton J. E., Nehlsen W. R. Survival of Escherichia coli in zero-degree centigrade sea water. J Water Pollut Control Fed. 1968 May;40(5):865–868. [PubMed] [Google Scholar]
- JONES G. E. EFFECT OF CHELATING AGENTS ON THE GROWTH OF ESCHERICHIA COLI IN SEAWATER. J Bacteriol. 1964 Mar;87:483–499. doi: 10.1128/jb.87.3.483-499.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Eimhjellen K., Wirsen C. O., Farmanfarmaian A. Microbial degradation of organic matter in the deep sea. Science. 1971 Feb 19;171(3972):672–675. doi: 10.1126/science.171.3972.672. [DOI] [PubMed] [Google Scholar]
- Matches J. R., Liston J., Curran D. Clostridium perfringens in the environment. Appl Microbiol. 1974 Oct;28(4):655–660. doi: 10.1128/am.28.4.655-660.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. Role of predators in the reversal of imbalances in microbial ecosystems. Nature. 1971 Mar 26;230(5291):257–258. doi: 10.1038/230257a0. [DOI] [PubMed] [Google Scholar]
- Schwarz J. R., Colwell R. R. Effect of hydrostatic pressure on growth and viability of Vibrio parahaemolyticus. Appl Microbiol. 1974 Dec;28(6):977–981. doi: 10.1128/am.28.6.977-981.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. D. The clostridial flora of marine sediments from a productive and from a non-productive area. Can J Microbiol. 1968 Dec;14(12):1301–1304. doi: 10.1139/m68-218. [DOI] [PubMed] [Google Scholar]
- ZOBELL C. E., MORITA R. Y. Barophilic bacteria in some deep sea sediments. J Bacteriol. 1957 Apr;73(4):563–568. doi: 10.1128/jb.73.4.563-568.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E., Mathews H. M. A Qualitative Study of the Bacterial Flora of Sea and Land Breezes. Proc Natl Acad Sci U S A. 1936 Oct;22(10):567–572. doi: 10.1073/pnas.22.10.567. [DOI] [PMC free article] [PubMed] [Google Scholar]




