Abstract
Blood vessels are composed of two interacting cell types. Endothelial cells form the inner lining of the vessel wall, and perivascular cells—referred to as pericytes, vascular smooth muscle cells or mural cells—envelop the surface of the vascular tube. Over the last decades, studies of blood vessels have concentrated mainly on the endothelial cell component, especially when the first angiogenic factors were discovered, while the interest in pericytes has lagged behind. Pericytes are, however, functionally significant; when vessels lose pericytes, they become hemorrhagic and hyperdilated, which leads to conditions such as edema, diabetic retinopathy, and even embryonic lethality. Recently, pericytes have gained new attention as functional and critical contributors to tumor angiogenesis and therefore as potential new targets for antiangiogenic therapies. Pericytes are complex. Their ontogeny is not completely understood, and they perform various functions throughout the body. This review article describes the current knowledge about the nature of pericytes and their functions during vessel growth, vessel maintenance, and pathological angiogenesis.
Keywords: blood vessels, pathologic neovascularization, vascular endothelium
What Is a Pericyte?
Pericytes were described more than 100 years ago as perivascular cells that wrap around blood capillaries (peri, around; cyte, cell). They are also called Rouget cells after their discoverer, Charles Rouget, or referred to as mural cells or, because of their contractile fibers, as vascular smooth muscle cells (vSMCs)3 (Hirschi and D’Amore, 1996). Electron-microscope analyses first revealed the morphological character of pericytes. In general, pericytes possess a cell body with a prominent nucleus and a small content of cytoplasm with several long processes embracing the abluminal endothelium wall (Figs. 1A.a and b). They are embedded within the basement membrane of microvessels, which is formed by pericytes and endothelial cells (Mandarino et al., 1993). Pericytes do not serve solely as scaffolding as historically thought, but communicate with endothelial cells by direct physical contact and paracrine signaling pathways. Gap junctions provide direct connections between the cytoplasm of pericytes and endothelial cells, and they enable the exchange of ions and small molecules. Adhesion plaques anchor pericytes to endothelial cells, while peg-and-socket contacts enable the cells to penetrate through discontinuities in the vessel basement membrane and touch each other (Rucker et al., 2000). These junction complexes support transmission of mechanical contractile forces from the pericytes to the endothelium and contain N-cadherin, cell-adhesion molecules, β-catenin-based adherent junctions, and extracellular matrix (ECM) molecules such as fibronectin (Gerhardt and Betsholtz, 2003).
Fig. 1.
Pericytes A. Capillaries are composed of endothelial cells (ECs; green) that form the inner lining of the wall with a surrounding basal lamina and pericytes (PC; red) that extend long cytoplasmic processes over the surface of the vascular tube (a, b). Larger vessels have several layers of pericytes and smooth muscle cells. Arterioles have strong elastic vessel walls (c) with dense layers of concentrically formed smooth muscle cells (d) to withstand the blood pressure. B. Immunohistochemical detection of pericytes exemplified in murine brain (a–d; red) and pancreatic islet tissues (e–h; red). NG2 is expressed in pericytes (b; white arrow), but also in microglia cells (b; yellow arrow) in the brain, whereas NG2 appears to be solely specific for pericytes in the pancreas (f; white arrow). α-SMA+-pericytes (h) are less abundant in the pancreatic islets than NG2+ or desmin+ cells (e, f). Green arrows indicate EC and white arrows mark PC, while the yellow arrow points to microglia cells. Blood vessels in the tissues were visualized with FITC-labeled tomato lectin (green), and tissue sections were stained with red-labeled antibodies for desmin (A.c; B.a, e), NG2 (A.b; B.b, f), PDGFRβ (B.c, g), or α-SMA (A.d; B.d, h) to visualize pericytes and incubated with DAPI to identify nuclei. All pictures were captured on a confocal microscope at 63× magnification (A.b–d; B.a–h) with an additional 3× zoom (B.c, d) and additional 8× zoom (A.b). C. Brain capillaries consist of a continuous endothelium with complex tight junctions and interact with astrocytic foot processes and pericytes to constitute the BBB (a). Antibodies for glial fibrillary acidic protein (GFAP) detect astrocytes with their cytoplasmic processes in red (b–e), and antibodies for PDGFRβ identify pericytes in blue (white arrows; b, c). ECs are stained with both FITC-labeled tomato lectin (Lycopersicon esculentum) and FITC-labeled CD31 in green (b–e). Glioblastoma cells (in blue; cyan arrows) invade the brain parenchyma and migrate along blood vessels interrupting the interaction of normal astrocytes and the vasculature. Yellow arrows point to astrocytic foot processes; cyan arrows indicate glioblastoma cells and white arrows show pericytes. Pictures were captured on a confocal microscope at 63× magnification with an additional 4× zoom.
Pericytes exhibit long cytoplasmic processes that not only can contact numerous endothelial cells and thus integrate signals along the length of the vessel, but can also extend to more than one capillary in the vasculature. Interestingly, cell-cell contact appears necessary for the activation of the latent growth factor TGF-β1, which induces pericyte differentiation in vitro (Orlidge and D’Amore, 1987), supporting the notion that direct cell contact is a crucial communication tool for vessel maintenance and formation. Pericytes also exhibit a number of characteristics consistent with muscle-cell activity and express contractile smooth-muscle actin. In contrast to small blood vessels, which are composed of endothelial cells surrounded by a basal lamina and loosely covered by single pericytes, larger vessels are coated with multiple layers of smooth-muscle cells and elastic and collagenous fibers (Cleaver and Melton, 2003). Smooth-muscle cells in large vessels support and regulate blood flow (Fig. 1A.d). Veins are irregularly covered by smooth-muscle cells and pericytes and have valves to prevent the backflow of blood, whereas arteries have strong, elastic vessel walls with dense populations of concentrically formed smooth-muscle cells to withstand the higher blood pressures (Cleaver and Melton, 2003). It has been reported that smooth-muscle cells, in contrast to pericytes, are not embedded in the basement membrane and might not directly contact the endothelium (Gerhardt and Betsholtz, 2003). However, the possibility cannot be excluded that physical contacts between smooth-muscle cells and endothelial cells are more erratic, and therefore may go unnoticed. Because of all of this, it is still not clear whether pericytes are smooth-muscle cells or cells with smooth-muscle-cell characteristics that can turn into smooth-muscle cells, which would suggest that pericytes and smooth-muscle cells represent phenotypic variants of the same lineage, or whether these cells even have a distinct progenitor. It is therefore very common that pericytes are referred to as vascular smooth muscle cells.
Molecular Markers of Pericytes
The challenges of defining a pericyte have not been made easier by the facts that no general pan-pericyte molecular marker has been found, and that one will probably never be discovered because of the diverse characteristics, functions, and locations of pericytes in various organs. There are, however, a few dynamic molecular markers that are present in pericytes, albeit not exclusively, and are commonly used for their detection. The expression patterns of these markers can vary in a tissue-specific manner or be dependent on the developmental or angiogenic stage of a blood vessel. Desmin and alpha-smooth-muscle actin (α-SMA) are contractile filaments, and regulator of G protein signaling 5 (RGS-5) is a GTPase-activating protein; all three are intracellular proteins. Neuron-glial 2 (NG2), a chondroitin sulfate proteoglycan, and platelet-derived growth factor receptor beta (PDGFRβ), a tyrosine-kinase receptor, are cell-surface proteins. Antibodies against these proteins (except RGS-5) are commonly used to identify pericytes in tissue sections (Fig. 1B). Desmin is a muscle-specific class III intermediate filament found in mature skeletal, cardiac, and smooth-muscle cells. Desmin-deficient mice are viable and fertile, but they develop progressive muscle weakness and dystrophic alterations in both cardiac and skeletal muscles (Li et al., 1996; Milner et al., 1996).
Alpha smooth-muscle actin is one of the six mammalian isoforms of the cytoskeletal protein actin. The beta and gamma nonmuscle actins are present in all cells, whereas α-SMA is normally restricted to cells of the smooth-muscle lineages. Certain nonmuscle cells have been shown to transiently express α-SMA, specifically fibroblasts, which are then referred to as myofibroblasts (Ronnov-Jessen and Petersen, 1996). Interestingly, α-SMA appears to inhibit migration and mobilization of fibroblasts (Ronnov-Jessen and Petersen, 1996), which suggest that it can promote a differentiated phenotype.
RGS-5 is a member of the “regulator of G protein signaling,” or RGS, protein family, a category that includes more than 25 proteins. RGS proteins serve as GTPase-activating proteins for Gα subunits of heterotrimeric G proteins, and they negatively regulate G protein–coupled receptor signaling. RGS-5 acts as a GTPase-activating protein for Giα and Gqα and attenuates the signaling induced by angiotensin II, endothelin-1, sphingosin-1-phosphate, and PDGF in cultured cells (Bondjers et al., 2003; Cho et al., 2003). It was identified as a novel marker for developing pericytes when comparison was made of the gene expression profiles of wild-type embryos and PDGF-B-knockout embryos, in which developing blood vessels in the central nervous system are almost completely devoid of pericytes. RGS-5-positive cells in the wild-type embryos were identified as pericytes, and the expression pattern of RGS-5 strikingly overlapped with the expression pattern of PDGFR-β and NG2. In contrast, the brain tissue of PDGF-B-deficient mice lacked RGS-5 expression (Bondjers et al., 2003). Just recently, RGS-5 was discovered as a marker for sites of physiological and pathological angiogenesis in adults. Elevated levels of RGS-5 in pericytes have been observed during wound healing and ovulation, and RGS-5 expression was substantially increased concomitant with neovascularization during tumor progression in mouse models of pancreatic islet tumors and glioblastomas (Berger et al., 2005), indicating a strong correlation between RGS-5 expression and active vessel remodeling (Berger et al., 2005). However, RGS-5 is not found in every tissue or tumor, which indicates tissue-specific regulation of RGS-5 (R. Ganss, personal communication; S. Song, unpublished observation).
NG2 chondroitin sulfate proteoglycan (also called high-molecular-weight melanoma-associated antigen, or HMWMAA; the mouse derivate is called AN2) is expressed on the surface of pericytes during vasculogenic and angiogenic processes (Stallcup, 2002). Historically, NG2 was postulated to be characteristic of immature neural cells capable of differentiating into either glia or neurons and was therefore named neuron-glial 2. In support of this hypothesis, NG2 is found in the central nervous system in glial precursor O-2A cells, which give rise in vitro to either oligodendrocytes or type II astrocytes and lose NG2 expression during the differentiation process (Stallcup, 2002). NG2 exerts its biological activity partly by binding with high affinity to basic fibroblast growth factor (bFGF), PDGF-AA, and the kringle domains of plasminogen and angiostatin (Ozerdem and Stallcup, 2004). NG2 knockout mice are viable, but when pathological angiogenesis is induced in the adult mouse, such as ischemic angiogenesis in the mouse retina in response to hypoxia or bFGF-induced angiogenesis in the cornea, neovascularization is substantially reduced (Ozerdem and Stallcup, 2004).
The tyrosine-kinase receptor PDGFRβ is one of the most widely studied molecules expressed in pericytes and is discussed in detail in this review. Mice deficient in PDGFRβ or its ligand PDGF-B have severely reduced numbers of pericytes and subsequent hyperdilation of blood vessels, which causes edema formation and embryonic lethality. However, other cell types besides pericytes also produce PDGFRβ, such as fibroblasts, astrocytes, and certain tumor cells (Lindahl et al., 1997).
The Diverse Functions of Pericytes
Pericytes have been associated mainly with stabilization and hemodynamic processes of blood vessels. Their functions are, however, much more diverse. They can sense angiogenic stimuli, guide sprouting tubes, elicit endothelial survival functions, and even exhibit macrophage-like activities.
Pericyte Contractility and Regulation of Blood Flow
Similar to the smooth-muscle cells of larger vessels, pericytes can produce vasoconstriction and vasodilation within capillary beds to regulate vascular diameter and capillary blood flow (Rucker et al., 2000). The first line of evidence for this function came from the identification of contractile proteins such as α-SMA, tropomyosin, and myosin in pericytes. These filaments are also produced in smooth-muscle cells and therefore greatly contribute to the confusion between the definition of a smooth-muscle cell and a pericyte. Several molecules that regulate pericyte contractile tone have been identified. For example, pericytes possess receptors for both cholinergic and adrenergic (α-2 and β-2) receptors. The β-adrenergic response in pericytes leads to relaxation, whereas the α-2 response is antagonistic and produces contraction (Rucker et al., 2000). Other vasoactive substances that bind to pericytes are angiotensin II and endothelin-1. Interestingly, the expression of endothelin-1 is induced by endothelial cells, which also make nitric oxide, a potent vasodilator that promotes vessel relaxation by a cGMP-dependent mechanism. These molecules function as paracrine signals, regulate pericyte contraction and relaxation, and provide part of the evidence that endothelial cells and pericytes interact in the regulation of blood flow (Rucker et al., 2000). Finally, oxygen levels also regulate pericyte contraction. It has been shown that hyperoxia increases pericyte contraction in vitro, whereas elevated levels of carbon dioxide induce relaxation. These data suggest that vessels dilate when oxygen is needed but constrict if the levels are sufficient, thereby coupling the rate of blood flow to the metabolic state. Although pericyte contraction in vivo is more difficult to prove, ultrastructural morphometric techniques have demonstrated the compression of endothelial-cell membranes by apposing pericytes. Pericyte contraction was measured in response to vasoactive substances in skeletal muscle (Hirschi and D’Amore, 1996; Tilton et al., 1979).
Tissue-Specific Functions of Pericytes
Pericyte density differs in respect to the function of vessels and organs in which they are found. Pericytes are quite abundant on small venules and arterioles but are rather sparse on capillaries. Although it is unknown how pericytes choose their exact location on the vessel wall, they are not randomly located but functionally determined. It has been noted that they preferentially cover endothelial cell junctions, specifically during inflammation (Sims, 2000). Pericyte density is dependent on blood pressure levels, and it is an interesting observation that pericytes in humans are more abundant further down the torso and legs, at sites where increased pressure is necessary to pump the blood upward (Sims, 2000). Pericyte coverage of blood vessels also varies among the tissues. This is not necessarily surprising, because vascular cells acquire specialized characteristics in different organs, based on the functions of each organ. In several organs, such as the brain, liver, and kidneys, pericytes have been shown to perform specific functions and have therefore been given additional names in these organs.
Brain
The highest density of pericytes in the body is found in vessels of the neural tissues, such as the brain and the retinas. The reason for this is that endothelial cells in the brain form a continuous endothelium with complex, tight junctions, and they interact with astrocytic pedicels and with numerous pericytes to create the blood-brain barrier (BBB), which protects brain cells from potentially toxic blood-derived factors (Fig. 1C) (Ballabh et al., 2004; Cleaver and Melton, 2003). Pericytes play an essential role in the integrity of structural vessels and the BBB. Vessel degeneration is observed in hereditary cerebral hemorrhage with amyloidosis (Verbeek et al., 1997), and pericytes have been shown to protect hypoxia-induced BBB disruption in vitro (Hayashi et al., 2004). Most interesting is that pericytes in the brain can perform macrophage-like activities, thus providing an immunological defense. This phenotype has raised the hypothesis that pericytes can act as precursor cells of macrophages in the brain, and there are several observations to support this idea (Thomas, 1999). Like macrophages, pericytes take up small and soluble molecules by pinocytosis, thereby cleaning the extracellular fluid as part of the BBB. Pericytes also have phagocytic activity (Thomas, 1999). The first evidence of phagocytic activity in pericytes was revealed when, following histamine treatment, pericytes gradually took up carbon over a course of several months (Majno et al., 1961). Congruent with these observations, systemic injections of protein tracers into immature mice lead to an accumulation of the tracer in pericytes of the brain and spinal cord (Kristensson and Olsson, 1973). Pericytes display several types of phagocytotic activity. They express scavenger receptors, which have broad-ligand-binding specificity, and are crucial in routine scavenging of many different molecules (Thomas, 1999); pericytes in culture can ingest various macromolecules, including polystyrene beads (Balabanov et al., 1996). In addition, pericytes express Fc receptors, which are essential for antibody-antigen complex recognition to trigger antibody-dependent phagocytosis (Balabanov et al., 1996). Furthermore, pericytes express numerous macrophage markers such as CR3 complement receptor, CD4, class I and II major histocompability complex molecules, and ED-2 (Balabanov et al., 1996).
Liver
Pericytes also have specialized functions in the liver. Hepatic stellate cells (HSCs), also called Itoh cells (named after their discoverer, Toshio Itoh) (Suematsu and Aiso, 2001), are the pericyte equivalent in the liver (Sato et al., 2003). They are located between the parenchymal cell plates and the sinusoidal endothelial cells. In contrast to the continuous brain endothelium, liver endothelial cells are highly fenestrated and discontinuous. They line the hepatic sinusoids and mediate the exchange of metabolites between the portal blood, Kupffer cells, and hepatocytes and the processing of toxins (Abbott, 2002). Although a dense basement structure between hepatic epithelial cells and endothelial cells does not exist, HSCs have close contact with endothelial cells through incomplete basement-membrane components and interstitial collagen fibers. HSCs regulate the remodeling of the ECM by producing both ECM components and matrix metalloproteinases (Sato et al., 2003). HSCs are also involved in vitamin A metabolism and contain more than 80% of the total vitamin A in the body (Sato et al., 2003). Finally, HSCs are involved in the recruitment of inflammatory cells during hepatic-tissue repair and in fibrotic responses to liver diseases (Knittel et al., 1999; Sims, 2000).
Kidney
Pericytes of the glomerular capillaries in the kidney are called mesangial cells and account for approximately 30% of the glomerular cells. The cells are instrumental in the intussusceptive branching or splitting of a single invading vascular loop into several glomerular capillaries, which creates a significantly increased capillary surface area for blood ultrafiltration (Betsholtz, 2004). PDGF-B signaling appears to be critical for the development of mesangial cells, because mice deficient in PDGF-B or its receptor PDGFR-β lack mesangial cells and therefore form defective kidney glomeruli that have only one distended capillary loop (Betsholtz, 2004).
Pericyte Function in Vascular Development and Angiogenesis
Pericytes are not only involved in hemodynamic processes but also have an active role in vessel formation. Blood vessels develop early during embryogenesis and are derived from mesodermal precursors called angioblasts (Fig. 2A) (Carmeliet, 2004). Additionally, it is believed that endothelial cells share a common precursor with hematopoietic cells; this precursor is called a hemangioblast. This hypothesis of a common precursor is supported by the observations that developing hematopoietic and endothelial cells share common surface markers and that hematopoietic cells can bud from major embryonic blood vessels (Carmeliet, 2004; Cho et al., 2003; Ribatti et al., 2002).
Fig. 2.
Pericytes in vasculogenesis and angiogenesis A. Endothelial cells (ECs) and pericytes/vSMCs (PC) arise from different precursor cells. ECs develop from angioblasts or hemangioblasts in the embryo, while pericytes/vSMCs are derived form mesenchymal stem cells or neurocrest cells. In vitro data indicate that there exists a common vascular progenitor derived from embryonic stem cells that can give rise to EC in the presence of VEGF, and to PC in the presence of PDGF-B. In the embryo, endothelial cells first assemble into a simple capillary network. Vessels then sprout and prune (angiogenesis, B), become stabilized by pericytes/vSMCs that are recruited by PDGF-B-secreting endothelial cells, and segregate into the different vessel types. Arterioles exhibit a high density of circumferentially oriented SMCs and thicker EC walls to withstand the blood pressure. Venules, like capillaries, have irregularly arranged pericytes with multiple cytoplasmic processes and are composed of thinner EC walls with valves to prevent backflow of blood. B. New vessels are formed from existing blood vessels by endothelial cell bridging, intussusceptions, and/or sprouting. This is in general preceded by pericyte detachment from the vessel wall and subsequent vessel hyperdilation. When vessels form new sprouts, the vascular basement membrane is first degraded to enable EC to move into the ECM. This is accompanied by EC proliferation and migration toward an angiogenic stimulus. ECs can be either guided by EC tip cells expressing high levels of PDGF-B or by pericytes. Immature, newly formed vessels cease the proliferation, and ECs adhere to each other, form a lumen and become encircled by a basement membrane with recruited pericytes. ECs, and also PCs, can be recruited from the bone marrow, specifically in tumor angiogenesis. Green arrows indicate EC; white arrows point to PC.
Pericytes have a complex ontogeny, because they can develop from various cells, as a function of their location in the embryo. For example, pericytes can develop from the neurocrest in the forebrain and cardiac tracts (Bergwerff et al., 1998; Etchevers et al., 2002). Most commonly described and best understood, though, is their origin from mesenchymal stem cells (Creazzo et al., 1998). Under these circumstances, TGF-β1 appears to initiate differentiation of PDGFRβ+ pericyte progenitor cells that are then chemotactically attracted by PDGF-B-secreting endothelial cells in the capillary plexus (Hellstrom et al., 1999). TGF-β1 also appears to drive the differentiation of neurocrest- or mesenchyme-derived progenitors into smooth-muscle-like cells (Chen and Lechleider, 2004; Darland and D’Amore, 2001). Furthermore, in vitro analyses revealed that there exists a common VEGFR2+ vascular-progenitor cell type, derived from embryonic stem cells, which has the ability to differentiate into endothelial cells in the presence of VEGF or into vascular smooth-muscle cells when PDGF-B is added (Fig. 2A) (Carmeliet, 2004; Yamashita et al., 2000). Finally, there have been reports that pericytes can be generated from endothelial cells by transdifferentiation in a TGF-β3-dependent manner, as has been described in the dorsal aorta (Gittenberger-de Groot et al., 1999) and cardiac valves (Nakajima et al., 1997).
As soon as the primary capillary plexus has been assembled, it is refined into a functional network by angiogenesis—vessels undergoing extensive pruning and sprouting. Angiogenesis can include endothelial intussusception, endothelial cell bridging, vessel sprouting, or a combination of these processes (Fig. 2B). In vessel sprouting, angiogenic factors (e.g., VEGF) stimulate endothelial cells, which in turn start secreting several proteases to degrade the vessel basement membrane. This allows endothelial cells to invade the surrounding ECM and form a migration column that consists of proliferating and migrating endothelial cells. As demonstrated in the retina, this column is guided by a migrating endothelial cell at the very tip, which moves toward a VEGF gradient (Gerhardt et al., 2003). Studies of the corpus luteum have suggested that pericytes are also capable of guiding sprouting processes by migrating ahead of endothelial cells and expressing VEGF (Ozerdem and Stallcup, 2003; Ozerdem et al., 2001; Reynolds et al., 2000). Newly formed sprouts cease proliferation behind this migration zone, adhere to each other, and form a new, lumen-containing vessel. Endothelial cells then secrete growth factors, partly to attract pericytes that envelop the vessel wall, and promote vessel maturation. Although several factors like S1P-1 (sphingosine-1-phosphate-1) and the angiopoietins have been implicated in the recruitment of pericytes and in vessel maturation (Jain, 2003), it is PDGF-B that appears to be the crucial player in the recruitment of pericytes to newly formed vessels (Betsholtz, 2004). During angiogenesis in embryos and adults, PDGF-B is expressed by the sprouting capillary endothelial cells, whereas its receptor, PDGFRβ, is localized on pericytes, which suggests a paracrine signaling circuit between the two cell types (Fig. 2A) (Enge et al., 2002; Hellstrom et al., 1999; Hirschi et al., 1999). It is believed that pericytes, because of their vessel-embracing position, are able to transfer angiogenic signals along the vessel length by contacting numerous endothelial cells. Endothelial cells and pericytes communicate by either direct contact or paracrine signaling, thereby also affecting each other’s mitotic rate. Pericytes induce endothelial differentiation and growth arrest (Gerhardt and Betsholtz, 2003; Hirschi et al., 1998; Sims, 2000). These ideas on pericyte and endothelial cell interactions are primarily based on the views that pericyte recruitment lags behind endothelial sprouting and that a window of pericyte absence allows for vascular plasticity resulting in growth, survival, remodeling, or regression of the endothelium, dependent on the presence or absence of angiogenic growth factors such as VEGF (Bondjers et al., 2003).
Finally, vascular polarity and arteriovenous vessel specifications are established. Although blood flow and pressure have been described as major determinants of arterial or venous vessel generation, genetic mutation studies of the hedgehog, VEGF, and notch genes in zebrafish and quail indicate that the fate of an endothelial cell may already be determined before blood circulation is initiated (Carmeliet, 2004). For example, notch and gridlock genes are expressed in precursors of arterial endothelial cells but not venous cells in zebrafish embryos (Lawson et al., 2001; Zhong et al., 2000). Ephrinb2, an Eph family transmembrane ligand, is expressed in arterial endothelial cells and pericytes, while its receptor, the tyrosine kinase EphB4, is predominantly found on the corresponding venous cells. Blood flow and pressure are also important factors in smooth-muscle cell differentiation in coronary arteries during arteriogenesis, and differentiation of smooth-muscle cells is delayed in coronary veins because of their lower blood pressure (Carmeliet, 2004). Taken together, endothelial cells and pericytes regulate vessel formation, maturation, and specification, all of which require the orchestration of many tightly regulated molecules.
Molecular Regulators in Pericyte Biology
PDGF was originally identified in platelets and serum in vitro as a mitogen for fibroblasts, glial cells, and smooth-muscle cells (Betsholtz et al., 2001). The PDGF family is composed of four ligands. PDGF-A, -B, -C, and -D form homodimers, and PDGF-A and -B can also heterodimerize. Extracellular proteolytic removal of an N-terminus fragment (the CUB domain) is a prerequisite for PDGF-C and -D activation (Betsholtz et al., 2001). PDGFs bind to a membrane-bound receptor tyrosine kinase consisting of two subunits, alpha and beta, which can homo- and heterodimerize. In general, the alpha unit binds A, B, and C ligands, whereas the beta subunit binds B and D ligands. The response of a particular cell to PDGF is dependent on its specific complement of PDGF receptors and the bioavailability of the various PDGF dimers (Betsholtz et al., 2001). In the embryo, PDGF-B expression is restricted to endothelial cells and megakaryocytes (Lindahl et al., 1997) and is highest in sprouting, immature capillaries, whereas PDGFRβ expression is found on the mesenchymal pericyte progenitor cells, which indicates a paracrine signaling pathway between endothelial cells and pericytes. In agreement, genetic ablations of PDGF-B or PDGFRβ in mice produce identical phenotypes and reveal the significant role of PDGFRβ signaling in pericyte proliferation and recruitment to blood vessels. PDGF-B- or PDGFRβ-null mutants die during late gestation from cardiovascular complications that include widespread microvascular leakage and edema, arterial smooth-muscle cell hypoplasia, and abnormal kidney glomeruli (Hellstrom et al., 1999; Leveen et al., 1994, Lindahl et al., 1997, 1998; Soriano, 1994). Interestingly, the cause of the microvascular dysfunction in the mutant mice is severe pericyte deficiency (Lindahl et al., 1997). Blood-vessel dilation and microaneurysms in mutant embryos correlated with severe reduction or even total loss of pericytes on the affected vessels, most prominently in the brain and heart (Hellstrom et al., 1999). It has also been shown that PDGF-B expression in the endothelium is critical for proper pericyte coverage on vessels, because pericyte deficiency is still observed when PDGF-B is deleted only in the endothelial cells of mice (Enge et al., 2002). However, it is important to note that the development of pericytes still can be induced when PDGF-B/PDGFRβ signaling is disrupted, but the cells are unable to expand and spread along the newly formed vessels because of their reduced proliferative capability and, likely, reduced migratory capability (Betsholtz et al., 2001).
Tissue-culture experiments have revealed that contact between endothelial cells and pericyte precursors leads to activation of TGF-β1, which in turn inhibits endothelial cell proliferation and migration (Orlidge and D’Amore, 1987; Sato and Rifkin, 1989), reduces VEGF-receptor 2 (flk-1) expression on endothelial cells (Mandriota et al., 1996), and induces differentiation of perivascular cells into pericytes (Hirschi et al., 1998, Ramsauer and D’Amore, 2002). TGF-β1 acts as a multi-functional cytokine in vessel formation, because it also induces differentiation of mesenchymal stem cells and neuro crest cells into pericytes (Chen and Lechleider, 2004; Ding et al., 2004). The functional implications of TGF-β1 in this process are highlighted by experiments with endoglin-knockout mice, which are nonviable as a result of defective vascular remodeling and smooth-muscle cell differentiation (Li et al., 1999). Endoglin is a TGF-β-binding protein and is also referred to as TGFβ type III receptor. Another paracrine signaling pathway important in vessel-wall formation is that of the angiopoietins and their Tie receptors. The receptor tyrosine kinase Tie2 is expressed in endothelial cells (Sundberg et al., 2002; Suri et al., 1996) and is stimulated by angiopoietin-1 (Ang-1) or angiopoietin-2 (Ang-2), which is secreted by surrounding mesenchyme and perivascular cells (Fig. 2A). Ang-1 is believed to be involved in vessel maturation; this is supported by recent data that Ang-1 was able to mature pericyte-deficient blood vessels in the retina (Uemura et al., 2002). On the other hand, its sibling Ang-2 has been proposed as a destabilizing factor in blood vessels, loosening the pericyte-endothelial contact points. When VEGF is present, Ang-2 promotes blood-vessel growth and sprouting, whereas in the absence of VEGF, Ang-2 leads to endothelial cell death and vessel regression (Hanahan, 1997; Maisonpierre et al., 1997). Mouse mutants lacking Tie2 or Ang-1 (Suri et al., 1996; Vikkula et al., 1996) have severe vascular defects and are unable to recruit pericytes, but it is more likely that the primary defects still occur in endothelial cells and that the effects on the pericyte coat are secondary to a general derangement or dysfunction (Gerhardt and Betsholtz, 2003).
Pericytes in Vascular Disease
The vasculature is usually quiescent in the adult, and endothelial cell turnover can be measured in years in tissues that do not require ongoing angiogenesis. However, although angiogenesis is relatively rare, it does occur in wound healing and in the female reproductive system during the menstrual cycle and pregnancy. The neovascularization process resembles the embryonic process and involves the coordinated action of many molecules (Fig. 2). Angiogenesis also occurs under pathological conditions like diabetic retinopathy and tumor growth. The difference between physiological and pathological angiogenesis lies in the tightly regulated balance of pro-angiogenic and antiangiogenic factors. During physiological neovascularization, newly formed vessels rapidly mature, become stable, and cease proliferation, whereas blood vessel growth under pathological conditions loses the appropriate balance between positive and negative regulators. Vessels formed during pathological angiogenesis do not stop growing and are under constant reconstruction, leading to an aberrant vascular system.
Pericytes in Diabetic Retinopathy
The retina has the highest pericyte density in the body. Diabetic patients are prone to developing diabetic retinopathy, of which an early hallmark is the loss of pericytes in the retina (Cai and Boulton, 2002). Chronic hyperglycemia is believed to cause this “pericyte dropout.” This has severe consequences because the vascular walls weaken and generate microaneurysms (Hammes et al., 2002; Wilkinson-Berka et al., 2004). Indeed, ultrastructural analyses of retinal microaneurysms reveal a consistent absence of pericytes, suggesting that the loss of vessel integrity due to the absence of pericytes may render vessels vulnerable to aneurysms. When progressive vascular occlusions in the human diabetic eye lead to blindness, the retina responds with either a progressive increase of vascular permeability, leading to macula edema, or the formation of new proliferating, immature vessels (Campochiaro, 2004; Hammes et al., 2002; Miller et al., 1997). Congruently, increased expression of VEGF-A and its receptors has been demonstrated in diabetic retinas (Benjamin, 2001). VEGF-A is also very likely responsible for the induced vascular leakage, and antagonists of VEGF and its receptors have been shown to reduce retinopathy in animal models (Aiello et al., 1995; Benjamin, 2001; McLeod et al., 2002; Robbins et al., 1998). Two major rodent models have been preferentially used to elucidate the exact mechanism of pericyte degeneration: the streptozotocin-induced diabetic model of early retinal damage by hyperglycemia (Kondo and Kahn, 2004) and the model of oxygen-induced proliferative retinopathy (resembling human retinopathy of prematurity [ROP]). Both of these models undergo the same critical steps of retinal neovascularization as in proliferative diabetic retinopathy (Hammes et al., 2002; Smith et al., 1994). Although the underlying mechanisms of pericyte loss in the retina are still unknown, mice that show impaired PDGF-B signaling when they are adults (endothelial-specific PDGF-Bko/ko mice, PDGF-Bret/ret mice) also develop retinopathy concomitant with severe pericyte loss (Betsholtz, 2004). Pharmacological inhibition of PDGF signaling with Gleevec (STI57, imatinib) promoted pericyte apoptosis and exacerbated angiogenesis by further inducing VEGF and VEGFR2 in the rodent ROP model (Wilkinson-Berka et al., 2004). First, these data support the concept that PDGF is required for pericyte growth and viability. Second, pericytes elicit survival functions for endothelial cells because induction of the survival factor VEGF is likely to be a response to the loss of pericytes. Further, the correlation between lack of pericytes and onset of neovascularization in retinopathy, as well as the pericyte association with developing capillaries and cessation of vessel growth, supports the concept that pericytes have suppressive influence of capillary growth.
Pericytes in Tumors
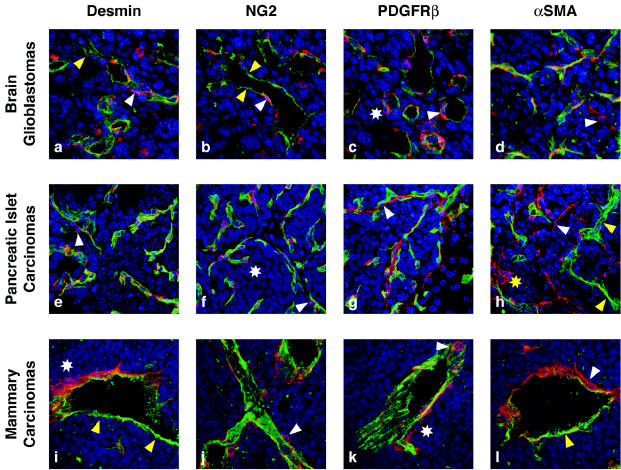
Angiogenesis in tumors leads to a chaotic, poorly organized vasculature with tortuous, irregularly shaped, and leaky vessels that are often unable to support efficient blood flow. Because of the imbalanced expression pattern of angiogenic factors, tumor vessels appear to be in a constant state of remodeling, which involves simultaneous formation and regression of vascular tubes (Bergers and Benjamin, 2003; Folkman, 2000). Just as tumor endothelial cells differ from the normal, quiescent endothelium, tumor pericytes also differ from normal pericytes. Figure 3 illustrates tumor pericytes, visualized with different markers, in three tumor types derived from transgenic or orthotopic mouse tumor models: glioblastomas, pancreatic islet carcinomas, and mammary carcinomas. In general, pericytes in tumors appear to be more loosely attached to the vasculature, and their cytoplasmic processes can extend into the tumor tissue (Fig. 3). They seem to be less abundant in some tumor tissues in comparison to the respective normal tissue (Fig. 1) and can change their pericyte expression profile (Fig. 3) (Benjamin et al., 1998; Morikawa et al., 2002). For example, pericytes in pancreatic islet tumors and glioblastomas appear to contain a higher number of α-SMA-positive pericytes than the respective normal tissue (Morikawa et al., 2002) (Figs. 1B and 3). In addition, tumor vessels differ among tumor types: The vasculature of mammary carcinomas is enormously enlarged and thickened, glioblastoma vessels appear thin walled and hyperdilated, and vascular tubes in islet carcinomas are more irregular but only slightly hyperdilated (Fig. 3). Pericyte coverage of blood vessels is also dependent on the tumor type. While islet carcinomas have reasonably dense pericyte coverage, glioblastomas and mammary carcinomas exhibit a more dramatic reduction in pericyte density when compared to the respective normal tissues (Fig. 3).
Fig. 3.
Pericytes in tumors. Tumor sections from three mouse models of tumorigenesis were used to visualize pericytes in glioblastomas (a–d), pancreatic islet carcinomas (e–h), and mammary carcinomas. SV40 Tag/H-ras transformed astrocytes intracranially injected into athymic mice, generate glioblastomas (Blouw et al. 2003). Transgenic mice expressing SV40Tag under the control of the insulin promoter develop pancreatic islet carcinomas (Hanahan, 1984). MMTV-neu mice overexpress the neu-oncogene under the control of the MMTV promoter and develop mammary adenocarcinomas (Guy et al., 1992). Blood vessels in the tumors were visualized by FITC-labeled tomato lectin (lycopersicon esculentum) and immunostaining with an FITC-labeled CD31 antibody. Tumor tissue sections were then stained with red-labeled antibodies for desmin (a, e, i), NG2 (b, f, j), PDGFRβ (c, g, k), or α-SMA (d, h, l) and incubated with DAPI to identify nuclei. Tumor vessels (green) are very distinct from their normal counterparts (see Fig. 1) because they become irregularly shaped, hyperdilated, and enlarged. In addition, tumor vessels differ among tumors. The vasculature of mammary carcinomas is enormously enlarged and thickened, GBM vessels are thin and hyperdilated, and vascular tubes in islet carcinomas are more irregular but only slightly hyperdilated. Pericyte coverage of blood vessels is also tumor dependent. Islet carcinomas have more pericyte coverage than GBMs or mammary carcinomas. Tumor pericytes are in general more loosely attached (c, d, f, h, i, k; white asterisks), and in some tumors like GBM less abundant (a, b) when compared to normal tissue. In addition, other tumors like mammary carcinomas contain clusters of pericytes that appear not to be distributed properly (i, l). Pericytes in tumors intend to bridge between blood vessels and to extend their cellular processes toward tumor cells (h, i, l).
For all of these reasons, pericytes are thought to be rather abnormal or dysfunctional in tumors and therefore, until recently, have been neglected as important contributors to tumor angiogenesis. The exact causes of abnormal pericyte behavior are still unknown, but may include imbalanced endothelial-cell/pericyte signaling circuits and/or a limited pool of recruitable pericytes (Abramsson et al., 2002). It is also notable that hematopoietic cells from bone marrow that expressed the pericyte marker NG2 were recently identified in close contact with blood vessels in xenograft Bl6-F1 melanoma tumor models (Rajantie et al., 2004) and in the transgenic model of pancreatic islet carcinomas (Song et al., 2005). This suggests that recruitment of bone-marrow-derived cells to sites of a growing vasculature is not limited to endothelial cells, but can also include pericytes.
It is surprising that normal vessels can apparently tolerate a substantial reduction in the density of pericytes, at least in mice; although a reduction in pericyte density produces functionally and structurally abnormal vessels, only pericyte reductions of >90% are lethal (Abramsson et al., 2003). This observation suggests that even small numbers of pericytes, as observed in tumors, can still be functional and important for vessel stability and endothelial-cell survival. Recent experiments targeting pericytes in tumors support this view. Glioblastomas contain a substantial fraction of blood vessels that are not covered by pericytes (see also Fig. 3). It has been demonstrated that these vessels are more dependent on VEGF as an endothelial survival factor, because they are selectively eliminated when VEGF is withdrawn from the tumors (Benjamin et al., 1999). In contrast, glioblastomas or fibrosarcomas that overexpress PDGF-B exhibited an increased pericyte density around blood vessels (Guo et al., 2003). Blocking PDGFR signaling in a transgenic mouse model of pancreatic islet carcinogenesis (Rip1Tag2) with the receptor tyrosine kinase inhibitor SU6668 caused regression of blood vessels, which was due to the detachment of pericytes from tumor vessels, and thereby restricted tumor growth and stabilized the cancer (Bergers et al., 2003). Similarly, SU6668 detached and diminished pericytes in xenotransplant tumors and thereby restricted tumor growth (Reinmuth et al., 2001; Shaheen et al., 2001). The functional significance of PDGFRβ signaling in tumor pericytes was confirmed by studies in PDGF-B retention mice. PDGF-Bret/ret mice lack the C-terminal retention motif in PDGF-B that mediates PDGF-B binding to proteoglycans at the cell surface and in the ECM. These mice are viable but have fewer pericytes, as they lack proper recruitment and integration of pericytes within the vessel wall, particularly in the retina and kidney (Lindblom et al., 2003). Implanted tumors in PDGF-Bret/ret mice are hemorrhagic and contain few pericytes around the tumor blood vessels that become hyperdilated. It is notable that ectopic expression of PDGF-B in those tumor cells was able to increase pericyte density but failed to cause pericytes to attach more firmly to blood vessels, which indicates that localized PDGF-B from the endothelium is essential for proper pericyte adhesion to the vessel wall (Abramsson et al., 2003). These data suggest that tumors use the same signal mechanisms that are used in developmental angiogenesis. These results also imply that tumor pericytes, albeit less abundant and more loosely attached than normal pericytes, still regulate vessel integrity, maintenance, and function. The fact that tumor vessels without pericytes appear more vulnerable suggests that they may be more responsive to antiendothelial drugs. Indeed, combinations of receptor tyrosine kinase inhibitors that target endothelial cells and pericytes by blocking VEGF and PDGF signaling, respectively, more efficiently diminished tumor blood vessels and tumors than any of the inhibitors individually (Bergers et al., 2003). The same effect was achieved when PDGF inhibitors were combined with an antiangiogenic chemotherapy regimen that targeted endothelial cells (Pietras and Hanahan, 2005). Targeting PDGFR signaling disrupted pericyte support, while the antiangiogenic chemotherapy targeted the sensitized endothelial cells, collectively destabilizing the preexisting tumor vasculature. When considered together, these data provide evidence that pericytes are potentially important and functional vascular-cell components in tumors that elicit survival mechanisms to establish and maintain tumor vessels.
Summary
Collectively, the data described above are far from complete, but should rather accentuate that pericytes are essential components of the microvessel wall, with important metabolic, signaling, and mechanical roles that support endothelial cells in a manner that depends on tissue and angiogenic stage. Pericytes are also proposed to be important contributors to the regulation of pathological angiogenic processes like diabetic retinopathy and tumor angiogenesis. During tumor propagation, pericytes, in spite of having structural abnormalities, still appear to provide functions necessary for vessel maintenance and endothelial-cell survival. These findings have led to a new concept of antiangiogenic therapy: combined targeting of endothelial cells and pericytes to more efficiently diminish blood vessels and halt subsequent tumor growth.
Acknowledgments
We thank Zena Werb, Laura Benjamin, and Scott VandenBerg for valuable discussions and advice, and we thank Sharon Reynolds for help with manuscript preparation.
Footnotes
This work was supported by grants from the National Institutes of Health (RO1 CA109390, RO1 CA99948) and by a grant from the V-Foundation.
Abbreviations used are as follows: α-SMA, alpha smooth-muscle actin; BBB, blood-brain barrier; bFGF, basic fibroblast growth factor; ECM, extracellular matrix; HSC, hepatic stellate cell; NG2, neuron-glial 2; PDGFβ, platelet-derived growth factor receptor beta; RGS, regulator of G protein signaling; ROP, retinopathy of prematurity; VEGF2, vascular endothelial growth factor 2; vSMC, vascular smooth muscle cell.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–1151. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King GL, Smith LE. Suppression of retinal neo-vascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52:127–142. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: An overview. Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Benjamin LE. Glucose, VEGF-A, and diabetic complications. Am J Pathol. 2001;158:1181–1184. doi: 10.1016/S0002-9440(10)64066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the pre-formed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal [see comment] J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. doi: 10.1182/blood-2004-06-2315. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland EB, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Boulton M. The pathogenesis of diabetic retinopathy: Old concepts and new questions. Eye. 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA. Ocular neovascularisation and excessive vascular permeability. Expert Opin Biol Ther. 2004;4:1395–1402. doi: 10.1517/14712598.4.9.1395. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res. 2004;94:1195–1202. doi: 10.1161/01.RES.0000126897.41658.81. [DOI] [PubMed] [Google Scholar]
- Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: Implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 2003;17:440–442. doi: 10.1096/fj.02-0340fje. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- Darland DC, D’Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- Ding R, Darland DC, Parmacek MS, D’Amore PA. Endothelial-mesenchymal interactions in vitro reveal molecular mechanisms of smooth muscle/pericyte differentiation. Stem Cells Dev. 2004;13:509–520. doi: 10.1089/scd.2004.13.509. [DOI] [PubMed] [Google Scholar]
- Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Le Douarin NM. Morphogenesis of the branchial vascular sector. Trends Cardiovasc Med. 2002;12:299–304. doi: 10.1016/s1050-1738(02)00178-0. [DOI] [PubMed] [Google Scholar]
- Folkman, J. (2000) Tumor angiogenesis. In: Bast, R.C., Jr., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Holland, J.F., and Frei, E., III (Eds.), Holland Frei Cancer Medicine. Hamilton, Ontario: B.C. Decker Inc., pp. 9–152.
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept. 2004;123:77–83. doi: 10.1016/j.regpep.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D’Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Knittel T, Dinter C, Kobold D, Neubauer K, Mehde M, Eichhorst S, Ramadori G. Expression and regulation of cell adhesion molecules by hepatic stellate cells (HSC) of rat liver: Involvement of HSC in recruitment of inflammatory cells during hepatic tissue repair. Am J Pathol. 1999;154:153–167. doi: 10.1016/s0002-9440(10)65262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kahn CR. Altered insulin signaling in retinal tissue in diabetic states. J Biol Chem. 2004;279:37997–38006. doi: 10.1074/jbc.M401339200. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Olsson Y. Accumulation of protein tracers in pericytes of the central nervous system following systemic injection in immature mice. Acta Neurol Scand. 1973;49:189–194. doi: 10.1111/j.1600-0404.1973.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- Li Z, Colucci-Guyon E, Pincon-Raymond M, Mericskay M, Pournin S, Paulin D, Babinet C. Cardiovascular lesions and skeletal myopathy in mice lacking desmin. Dev Biol. 1996;175:362–366. doi: 10.1006/dbio.1996.0122. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellstrom M, Kalen M, Betsholtz C. Endothelial-perivascular cell signaling in vascular development: Lessons from knockout mice. Curr Opin Lipidol. 1998;9:407–411. doi: 10.1097/00041433-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Majno G, Palade GE, Schoefl GI. Studies on inflammation: II. The site of action of histamine and serotonin along the vascular tree: A topographic study. J Cell Biol. 1961;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res. 1993;57:609–621. doi: 10.1006/exer.1993.1166. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Menoud PA, Pepper MS. Transforming growth factor beta 1 down-regulates vascular endothelial growth factor receptor 2/flk-1 expression in vascular endothelial cells. J Biol Chem. 1996;271:11500–11505. doi: 10.1074/jbc.271.19.11500. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2002;43:474–482. [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: A role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–952. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer M, D’Amore PA. Getting Tie(2)d up in angiogenesis. J Clin Invest. 2002;110:1615–1617. doi: 10.1172/JCI17326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the corpus luteum. Endocrine. 2000;12:1–9. doi: 10.1385/ENDO:12:1:1. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, Ria R, Dammacco F. Cross-talk between hematopoiesis and angiogenesis signaling pathways. Curr Mol Med. 2002;2:537–543. doi: 10.2174/1566524023362195. [DOI] [PubMed] [Google Scholar]
- Robbins SG, Rajaratnam VS, Penn JS. Evidence for upregulation and redistribution of vascular endothelial growth factor (VEGF) receptors flt-1 and flk-1 in the oxygen-injured rat retina. Growth Factors. 1998;16:1–9. doi: 10.3109/08977199809017487. [DOI] [PubMed] [Google Scholar]
- Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: Retardation of motility in fibroblasts. J Cell Biol. 1996;134:67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–369. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki S, Senoo H. Hepatic stellate cells: Unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105–112. doi: 10.1247/csf.28.105. [DOI] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: Activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE, Bucana CD, McMahon G, Ellis LM. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–1468. [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte diffentiation and survival. Nat Cell Biol. 2005 doi: 10.1038/ncb1288. [published online August 21], in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: Past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- Suematsu M, Aiso S. Professor Toshio Ito: A clairvoyant in pericyte biology. Keio J Med. 2001;50:66–71. doi: 10.2302/kjm.50.66. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: Phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Thomas WE. Brain macrophages: On the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Kilo C, Williamson JR. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc Res. 1979;18:325–335. doi: 10.1016/0026-2862(79)90041-4. [DOI] [PubMed] [Google Scholar]
- Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem. 1997;68:1135–1141. doi: 10.1046/j.1471-4159.1997.68031135.x. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Boon LM, Carraway KL, 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL, Babic S, De Gooyer T, Stitt AW, Jaworski K, Ong LG, Kelly DJ, Gilbert RE. Inhibition of platelet-derived growth factor promotes pericyte loss and angiogenesis in ischemic retinopathy. Am J Pathol. 2004;164:1263–1273. doi: 10.1016/s0002-9440(10)63214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]