Abstract
Various in vivo studies demonstrated a migration tendency of neural stem cells (NSCs) toward gliomas, making these cells a potential carrier for delivery of therapeutic genes to disseminated glioma cells. We analyzed which factors determine NSC migration and invasion in vitro. Conditioned media prepared from 10 different human glioma cell lines, as well as 13 different tumor-associated growth factors, were analyzed for their chemotactic effects on murine C17.2 NSCs. The growth factor receptor status was analyzed by reverse transcriptase–polymerase chain reaction. Invasion of NSCs into multicellular tumor spheroids generated from 10 glioma cell lines was quantified. NSCs displayed a heterogeneous migration pattern toward glioma spheroids as well as toward glioma-cell-conditioned medium. Chemotactic migration was stimulated up to fivefold by conditioned medium as compared to controls. In coculture assays, NSC invasion varied from single cell invasion into glioma spheroids to complete dissemination of NSCs into glioma spheroids of different cell lines. Among 13 different growth factors, scatter factor/hepatocyte growth factor (SF/HGF) was the most powerful chemoattractant for NSCs, inducing a 2.5-fold migration stimulation. An antibody against SF/HGF inhibited migratory stimulation induced by conditioned media. NSC migration can be stimulated by various growth factors, similar to glioma cell migration. The extent to which NSCs infiltrate three-dimensional glioma cell aggregates appears to depend on additional factors, which are likely to include cell-to-cell contacts and interaction with extracellular matrix proteins.
Keywords: glioblastoma, neural stem cell, chemotaxis, growth factor, spheroid
Because of their high rate of cell proliferation and diffuse invasion properties into the surrounding brain parenchyma, malignant gliomas are invariably fatal. Despite combined therapeutic strategies including surgery, radiation, and/or chemotherapy, the average life expectancy of patients suffering from a glioblastoma multiforme does not exceed 15 to 18 months (Black, 1991). Radical surgical resection is by definition impossible because of the disseminated invasion and growth beyond tumor boundaries visible even on modern neuroradiological imaging (Giese and Westphal, 1996). Therefore, selective targeting and treating the invading tumor cells, which are not amenable to complete surgical resection, at the same time protecting the functioning surrounding brain parenchyma, may be the goal for a new therapeutic approach.
Neural stem cells (NSCs)2 as well as neural progenitor cells have the biological potential to differentiate into different CNS cell subtypes, including neurons, astrocytes, and oligodendrocytes. These NSC cells have recently come to the forefront in neurobiology because of the capacity for CNS repair upon their transplantation into injured or malfunctioning brain tissue (Park et al., 2002; Vescovi et al., 1999; Villa et al., 2000). Similar to neural progenitor cells during CNS development, NSCs in the adult brain are able to migrate in a directed way toward pathologically altered tissues (Aboody et al., 2000; Benedetti et al., 2000; Ehtesham et al., 2002). Potential target tissues for NSC migration and therapy are ischemic areas, traumatized tissue, areas of inflammation, and metastatic or primary brain tumors (Park et al., 1999).
From the neuro-oncological perspective, NSC migration toward solid tumors and infiltrating tumor cells is of particular interest. Recent experiments showed that NSCs displayed a strong tendency to migrate toward gliomas and surround invading tumor cells in vivo (Aboody et al., 2000). This phenomenon makes these cells highly attractive as a vehicle to deliver therapeutic gene products into the immediate vicinity of the tumor cells, where they can function to inhibit glioma cell proliferation, migration, and angiogenesis (Flax et al., 1998; Ourednik et al., 1999).
During CNS development, precursor cell migration is a well-known and relatively well-characterized phenomenon; many different factors have been identified that guide the motility of these cells, including soluble factors, cell adhesion molecules, and extracellular matrix components. In contrast, very little is known about the specific mechanisms responsible for NSC migration in adult brain. It is currently unknown whether tumor cells themselves can attract NSCs or whether attraction of these cells is an indirect phenomenon, mediated, for example, by reactive astrocytes or activated microglial cells that are nonspecifically associated with different pathological lesions.
Elevated concentrations of soluble growth factors are a typical feature of malignant gliomas. The diffusion of soluble cytokines is likely to be involved in the chemotactic attraction of NSCs over long distances in the adult brain. In the present study, we therefore investigated the effects of 13 different tumor-associated growth factors on the directional motility of the murine C17.2 NSC line. We further analyzed the capacity of conditioned medium prepared from cultured glioma cells to stimulate C17.2 cell migration. In addition, we studied direct cell interactions in a three-dimensional spheroid coculture assay system.
Material and Methods
Cell Lines and Cell Culture Conditions
The murine NSC line C17.2, provided as a gift for this study (E. Snyder), was established from cells harvested from the outer granular layer of the cerebellum of a 4-day-old newborn mouse; it was immortalized by retroviral transfection with the c-myc oncogene and stably transfected with the lacZ gene (Snyder et al. 1992). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 5% horse serum, 1% l-glutamine, and 1% sodium pyruvate.
The human glioma cell line U87 was obtained from the American Type Culture Collection (Rockville, Md.). The human NCE (Neurochirurgie Eppendorf) glioblastoma cell lines G28, G44, G63, G96, G112, G123, G130, G169, and G350 were established from glioblastomas of patients treated at the Department of Neurosurgery, University Hospital Hamburg-Eppendorf, and propagated as described previously (Westphal et al., 1994). All glioma cell lines were cultured in Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% sodium pyruvate except for the U87 cell line, which was cultured in DMEM supplemented accordingly. These 10 glioma cell lines were chosen for their capacity to form three-dimensional spheroids in an agar overlayer culture system.
To generate conditioned medium, subconfluent glioma cell cultures were incubated in serum-free medium consisting of equal volumes of Ham’s F12 (Biochrom, Berlin, Germany) and DMEM with the addition of 10 μg/ml insulin, 10 μg/ml transferrin, 10−8 M selenium, 1% bovine serum albumin, and 1 mg/ml linoleic acid (all from Sigma, St. Louis, Mo.) for 5 days. The media were centrifuged to remove cellular debris, and the total protein concentration was determined by using the bicinchoninic acid protein assay (Pierce, Rockford, Ill.).
Modified Boyden Chamber Migration Assay
Chemotactic migration of C17.2 NSCs in response to conditioned media prepared from 10 human glioma cell lines and in response to human recombinant growth factors was assayed by using a modified Boyden chamber assay (Lamszus et al., 1998). For experiments, conditioned media with the same, normalized total protein concentration (1 μg/μl), undiluted, 50% diluted, and 10% diluted, were used.
The following recombinant human growth factors were assayed in concentrations ranging from 1 pM to 50 nM: scatter factor/hepatocyte growth factor (SF/HGF) (provided by R. Schwall); fibroblast growth factor-2 (FGF-2); pleiotrophin (PTN) (R&D Systems, Minneapolis, Minn.); epidermal growth factor; FGF-1; insulinlike growth factor I and II (IGF-I, IGF-II); midkine; platelet-derived growth factor-AA and BB (PDGF-AA, PDGF-BB); and transforming growth factor-α, -β1, and -β2 (TGF-α, TGF-β1, TGF-β2) (Peprotech, Rocky Hill, N.J.). For blocking experiments a neutralizing monoclonal antibody against SF/HGF (R&D Systems, Minneapolis, Minn.) was added to the conditioned media of U87, G28, and G44 cells at a concentration of 20 μg/ml.
Conditioned media or growth factors were added to the lower wells of a 96-well modified Boyden chamber (Neuroprobe, Cabin John, Md.), and wells were covered with a 8-μm-pore-size Nucleopore filter coated with Vitrogen 100 (Collagen Corp., Fremont, Calif.). C17.2 cells were suspended at 15,000 cells per 50 μl of serum-free medium containing 0.1% bovine serum albumin and seeded into the upper wells. After incubation for 5 h at 37°C, nonmigrated cells were scraped off the upper side of the filter, and filters were stained with Diff Quick (Dade, Munich, Germany). Nuclei of migrated cells were counted in 10 high-power fields by using a 40× objective with a calibrated ocular grid. Values were assessed in triplicate. Ten percent FBS was used as positive control and serum-free medium as negative control. All values are means ± SEMs of triplicate determinations.
Spheroid Coculture Assay
Tumor spheroids from 10 of the above-mentioned glioma cell lines were formed by using an agar overlayer culture method, as described previously (Yuhas et al., 1977). Briefly, spheroids were initiated by seeding 2 × 106 cells in 5 ml of media onto 25-ml culture flasks base-coated with 1% agar medium substrate in order to prevent cell attachment. After two to three days in culture, spheroids were formed. Spheroids with a mean diameter of 300 μm were selected for further experiments and transferred to a new 25-ml agar-coated culture flask. To the tumor spheroids, 5 × 106 C17.2 cells per flask were added and cocultured for 24 h. Fixation of the cocultures was performed with 1% formalin/0.125% glutaraldehyde solution for 20 min. Fixed cocultures were washed twice with phosphate-buffered saline. C17.2 cells stably expressing β-galactosidase (β-gal) were stained by using the 5-bromo-4-chloro-3-indolyl β-d-galactosidase (X-gal) histochemical reaction. Briefly, spheroids containing C17.2 cells were transferred onto uncoated 2.5-cm petri dishes and incubated in 2 ml of X-gal solution (2.2 ml ferri-ferro + 50 μl X-gal) for 12 h. After two washes in phosphate-buffered saline, the spheroids were embedded in Tissue-Tec (Sakura, Zoeterwonde, Netherlands). The embedded cocultures were shock-frozen in liquid nitrogen and stored at −80°C. Cryostat sections (thickness, 13 μm) were prepared, mounted onto glass slides, and counterstained with 0.1% eosin. All values are means ± SEMs of triplicate determinations.
Reverse Transcriptase–Polymerase Chain Reaction Analysis
The growth factor receptor status of the murine NSC line C17.2 was analyzed by reverse transcriptase–polymerase chain reaction (RT-PCR). Briefly, total RNA from the C17.2 cell line and control cell lines NIH/3T3 and PC12 was extracted by using TriSta (Hybaid GmbH, Heidelberg, Germany) and following the manufacturer’s instructions. Next, 3 μg of RNA was digested with DNase and reverse transcribed with You-Prime First-Strand Beads (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). PCR amplifications were performed by using 1 μl of cDNA in a total volume of 20 μl. Primer pairs (MWG-Biotech AG, Ebersberg, Germany) were used as listed in Table 1. PCR reactions contained 0.5 IU Taq polymerase, 0.5 mM dNTPs, and 20 pmol of each primer in 1× buffer (10 mM Tris-HCl, pH 8.3; 50 mM KCl; 1.5 mM MgCl2; 0.001% w/v gelatin). Reactions were initially denatured at 94°C for 3 min, followed by 35 cycles of 1 min at 94°C, 1 min at the respective annealing temperature (Table 1), and 1 min at 72°C in a thermal cycler (Biometra, Goettingen, Germany). In the final step, 9 μl of the PCR products was analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide.
Table 1.
Oligonucleotide primers used for RT-PCR analysis of C17.2 NSC receptor expression
| Target Receptor | Sense | Antisense | Product Size (bp) | Product Temp. (°C) |
|---|---|---|---|---|
| FGFR-1 | AGAAGCCTGAGACGCCGCCA | CCAGCACAGCCCAGAAGAGG | 443 | 55 |
| FGFR-2 | AAATACCAAATCTCCCAACC | GCCGCTTCTCCATCTTCT | 373 | 59 |
| FGFR-3 | ACTGTACTCAAGACTGCAGG | GTCCTTGTCAGTCGCATCAT | 635 | 55 |
| FGFR-4 | CTGTTGAGCATCTTTCAGGG | CGTGGAAGGCCTGTCCATCC | 550 | 53 |
| IGFR-1 | GACATCCGCAACGACTATCAG | GTAGTTATTGGACACCGCATC | 395 | 55 |
| IGFR-2 | CTGGAGGTGATGAGTGTAGCTCTGGC | GAGTGACGAGCCAACACAGACAG | 235 | 67 |
| TβR-1 | ATCCATCACTAGATCGCCCT | CGATGGATCAGAAGGTACAAGA | 824 | 55 |
| TβR-2 | CGTGTGGAGGAAGAACAACA | TCTCAAACTGCTCTGAGGTG | 560 | 55 |
| PDGFR-α | AAGAGACCCTCCTTCTACCACC | GTCGATGTCCTCGATGGTCT | 400 | 59 |
| PDGFR-β | AGCTACATGGCCCCTTATGA | GGATCCCAAAAGACCAGACA | 380 | 59 |
| MET | GAATGTCGTCCTACACGGCC | CAGGGGCATTTCCATGTAGG | 727 | 59 |
| ALK | CCACAACGAAGCTGGAAGAGA | GTCCCATTCCAACAAGTGAAGGA | 319 | 59 |
| EGFR | CTGTTGAGCATCTTTCAGGG | GTAGTTATTGGACACCGCATC | 454 | 53 |
| PTP-ζ intern | GGGAGAACGGGGACATACATT | CCTGTTACACTGCTTCAGGGC | 335 | 57 |
| PTP-ζ extern | ACCAGCCTTCTGGTCACATGG | CCAGGATTCAAGCCAGTGTCTTC | 361 | 57 |
Abbreviations: ALK, anaplastic lymphoma kinase; bp, base pairs; EGFR, epidermal growth factor; FGFR, fibroblast growth factor receptor; IGFR, insulinlike growth factor receptor; MET, hepatocyte growth factor receptor precursor; PDGFR, platelet-derived growth factor receptor; PTP-ζ, protein tyrosine phosphatase-ζ; TβR, transforming growth factor receptor-β.
Statistical Analysis
All data are expressed as means ± SEMs. Statistical differences between different test conditions (e.g., exposure to different growth factors or conditioned media) were compared by using an unpaired t-test. Correlation analysis was performed by using the Pearson product-moment correlation. Probability values <0.05 were considered statistically significant.
Results
Chemotactic Stimulation of NSC Migration
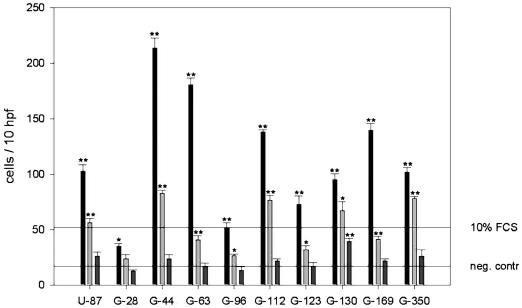
Directional migration of C17.2 NSCs toward conditioned media prepared from 10 different human glioblastoma cell lines was analyzed by using modified Boyden chamber migration assays. Significant chemotactic stimulation of C17.2 cell migration was obtained for all supernatants (Fig. 1). The effects were concentration dependent, and strongest motogenic responses were consistently observed at the highest protein concentration (1 μg/μl), whereas tenfold diluted conditioned media had no significant effect. For the majority of the supernatants (8 of 10), motogenic stimulation even exceeded that observed with 10% FBS. Stimulatory effects ranged from 2.5-fold stimulation (cell line G28) to 14.8-fold stimulation (cell line G44).
Fig. 1.
Chemotactic migration of C17.2 NSCs induced by conditioned media from 10 human glioma cell lines in three concentrations (undiluted, black column; 50% diluted, bright gray column; 10% diluted, dark grey column) in a modified Boyden chamber chemotaxis assay. Horizontal lines: negative control, serum-free medium; positive control, 10% fetal calf serum (FCS). Double asterisks indicate significant stimulation of migration compared to serum-free medium (unpaired t-test, probability value < 0.001); single asterisks indicate significance (unpaired t-test, probability value < 0.05). Values are means ± SEM of triplicate determinations.
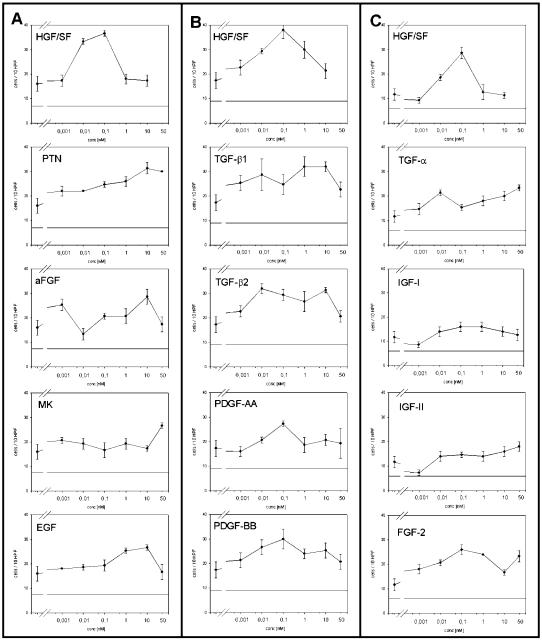
To identify specific factors responsible for NSC chemoattraction, migration of C17.2 cells was analyzed in response to 13 different recombinant growth factors. Modified Boyden chamber assays were used to assay all growth factors in concentrations ranging from 1 pM to 50 nM. Pilot experiments suggested that SF/HGF provoked a stronger migratory response of C17.2 cells than any other of the factors analyzed. Therefore, we included SF/HGF as a reference within each individual assay. In subsequent assays, we consistently observed significant stimulation of migration by SF/HGF, with bell-shaped stimulation curves and a maximally 2.5-fold effect obtained at 0.1 nM SF/HGF (Table 2, Fig. 2A–C).
Table 2.
Growth factor stimulation of C17.2 NSC migration*
| C17.2 NSC | ||
|---|---|---|
| Growth factor | Fold stimulation | Concentration (nM) |
| SF/HGF | 2.5* (± 0.28) | 0.1 |
| FGF-2 | 2.2* (± 0.16) | 0.1 |
| TGF-3 | 2.0* (± 0.18) | 50.0 |
| PTN | 2.0* (± 0.15) | 10.0 |
| TGF-31 | 1.8 (± 0.30) | 1.0 |
| TGF-32 | 1.8 (± 0.12) | 0.01 |
| FGF-1 | 1.8 (± 0.21) | 10.0 |
| PDGF-BB | 1.7 (± 0.24) | 0.1 |
| PDGF-AA | 1.6 (± 0.09) | 0.1 |
| EGF | 1.7 (± 0.09) | 10.0 |
| MK | 1.7 (± 0.10) | 50.0 |
| IGF-II | 1.5 (± 0.16) | 50.0 |
| IGF-I | 1.4 (± 0.21) | 0.1 |
Abbreviations: EGF, epidermal growth factor; FGF, fibroblast growth factor; IGF, insulinlike growth factor; MK, midkine; PDGF, platelet-derived growth factor; PTN, pleiotrophin; SF/HGF, scatter factor/hepatocyte growth factor; TGF, transforming growth factor.
Growth factor stimulation of cell migration was analyzed by using modified Boyden chamber chemotaxis assays. Values are maximum stimulation levels (fold stimulation ± SEM) obtained at the most effective growth factor concentration (nM) compared to unstimulated controls. Asterisks indicate significance (unpaired t-test, probability value < 0.05).
Fig. 2.
Chemotactic migration of C17.2 NSCs induced by different growth factors used in concentrations ranging from 1 pM to 5 nM in modified Boyden chamber chemotaxis assays (A to C represent different Boyden experiments). Values are means ± SEM of triplicate determinations.
In addition to SF/HGF, the only other growth factors that significantly stimulated NSC migration were FGF-2, with a maximally 2.2-fold effect, as well as TGF-α and PTN, both with maximally twofold stimulation (Table 2, Fig. 2A and C). The effects of all other growth factors were weak and variable and did not consistently reach significance in several repeat experiments.
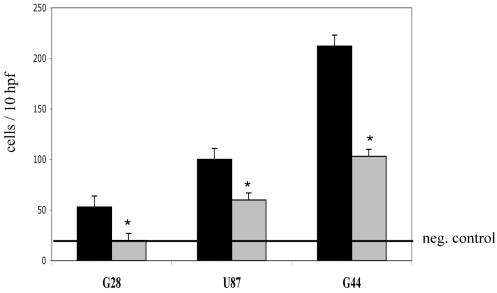
Since SF/HGF emerged as the most potent motogenic stimulant, we performed blocking experiments using conditioned media from glioblastoma cell lines. We selected three different cell lines known from previous ELISA experiments to secrete SF/HGF (not shown). Conditioned media from these cell lines induced a spectrum of minor (G28), moderate (U87), and very strong (G55) chemotactic stimulation of C17.2 NSC migration (Fig. 1, Table 3). Coaddition of neutralizing monoclonal antibodies against SF/HGF blocked the stimulatory effect of conditioned medium from G28 cells almost completely (97%), and it blocked the effects of U87-conditioned media by 48% and G44-conditioned media by 63% (Fig. 3).
Table 3.
Comparison of C17.2 NSC migration in Boyden assay and invasion in coculture assay
| Cell line | Coculture* | Boyden assay** |
|---|---|---|
| U87 | ++ | ++ |
| G28 | +++ | + |
| G44 | ++ | ++++ |
| G63 | − | ++++ |
| G96 | + | + |
| G112 | + | +++ |
| G123 | + | ++ |
| G130 | + | ++ |
| G169 | +++ | +++ |
| G350 | ++ | ++ |
Coculture: no invasion, −; little invasion, +; moderate invasion, ++; strong invasion, +++.
Boyden assay: twofold stimulation, +; threefold stimulation, ++; fourfold stimulation, +++; fivefold stimulation, ++++.
Fig. 3.
Chemotactic migration of C17.2 NSCs induced by conditioned media from G28, U87, and G44 glioma cells (black column). Reduction of C17.2 NSC migration by co-addition of an anti-SF/HGF antibody to the conditioned media (grey column). Negative control: serum-free medium (horizontal line). Asterisks indicate significance (unpaired t-test, probability value < 0.05). Values are means ±SEM of triplicate determinations. Abbreviation: hpf, high-power fields.
Stimulation of Stem Cell Invasion
Ten different glioblastoma cell lines were cultured as spheroids in order to mimic characteristics of the in vivo situation such as three-dimensionality, close cell-to-cell contact, and intercellular signaling. Spheroids of a diameter up to 300 μm resemble in vivo microtumors at a stage before angiogenesis occurs. Such spheroids were cocultured with C17.2 NSCs for 24 h, after which the migration pattern of the stem cells into the tumor spheroids was analyzed. The cocultures were prepared as 15-μm frozen sections, and C17.2 NSCs were distinguished from glioma cells by X-gal staining.
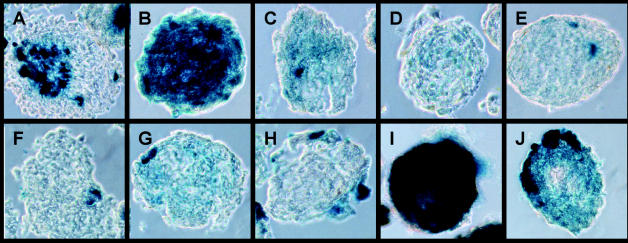
Invasion patterns of NSCs could be divided into four groups. Maximum invasion with almost completely X-gal stained spheroids indicating infiltration by C17.2 was observed for G28 and G169 spheroids (Fig. 4). Moderate invasion occurred into U87, G44, and G350 spheroids. Little invasion was observed for G96, G112, G123, and G130 spheroids. Virtually no invasion could be detected in G63 spheroids.
Fig. 4.
In vitro coculture assay of C17.2 NSCs and spheroids of 10 human glioma cell lines. Shown are 10-μm frozen sections after X-gal staining of lacZ-positive C17.2 NSCs (blue). Heterogeneous invasion characteristics are displayed by C17.2 cells. A: U87, B: G28, C: G44, D: G63, E: G96, F: G112, G: G123, H: G130, I: G169, J: G350. Representative results from three different experiments for spheroids derived from each of the 10 different glioma cell lines are shown.
Stem Cell Invasion versus Stem Cell Migration
Tissue invasion is determined by multiple factors, of which cell migration is one important component. Therefore, we compared the degree of invasion observed in the spheroid coculture assay with the degree of motogenic stimulation in the modified Boyden chamber assay (Table 3). No significant correlation was obtained between the stimulation of migration and invasion. Concordant results in both assays were obtained for conditioned media and spheroids from some cell lines (e.g., G169). However, discordant findings were obtained for several other cell lines, the most striking example being cell line G63, of which the supernatant strongly stimulated C17.2 cell migration, whereas G63 spheroids were not permissive for C17.2 cell invasion.
Growth Factor Receptor Expression
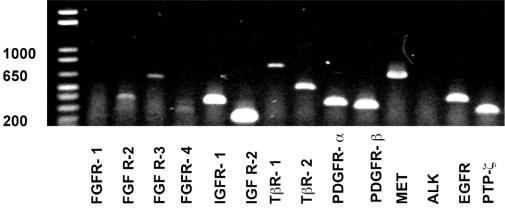
The expression of 14 different growth factor receptors, corresponding to the recombinant factors analyzed for motogenic stimulation of NSC, was analyzed in C17.2 cells by using RT-PCR. As positive controls, the murine cell lines NIH/3T3 and PC12 were analyzed in parallel. The majority of the receptors were found to be expressed by the C17.2 NSC line. Only two receptors, fibroblast growth factor receptor-1 (FGFR-1) and anaplastic lymphoma kinase, were not expressed by C17.2 NSCs (Table 4 and Fig. 5).
Table 4.
Growth factor receptor expression of C17.2 NSC*
| Receptor | Ligand | C17.2 | NIH3T3 | PC12 |
|---|---|---|---|---|
| FGFR-1 | FGF-1 | − | − | + |
| FGFR-2 | FGF-2 | (+) | + | − |
| FGFR-3 | FGF-3 | + | − | + |
| FGFR-4 | FGF-4 | (+) | (+) | (+) |
| IGFR-1 | IGF-1 | + | + | + |
| IGFR-2 | IGF-2 | + | + | + |
| TβR-1 | TGF-β1 | + | + | + |
| TβR-2 | TGF-β2 | + | + | + |
| PDGFR-α | PDGF-AA, -BB | + | + | + |
| PDGFR-β | PDGF-BB | + | + | − |
| MET | SF/HGF | + | + | − |
| ALK | PTN, MK | − | + | + |
| EGFR | EGF, TGF-α | + | + | + |
| PTPζ-intern | PTN, MK | + | + | − |
| PTPζ-extern | PTN, MK | + | + | + |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; IGFR, insulinlike growth factor receptor; MET, hepatocyte growth factor receptor precursor; PDGFR, platelet-derived growth factor receptor; PTP-ζ, protein tyrosine phosphatase-ζ; TβR, transforming growth factor receptor-β
RT-PCR analysis: + indicates presence of a PCR product; − indicates absence of a PCR product; (+) indicates faint band of a PCR product.
Fig. 5.
RT-PCR detection of growth factor receptor expression by C17.2 NSCs. Band sizes are listed in Table 1, and results are summarized in Table 4. The experiment was repeated twice with the same result.
Discussion
Transplanted NSCs have recently been recognized for their remarkable ability to migrate throughout the adult central nervous system and become normal constituents of the host cytoarchitecture (Hurelbrink et al., 2002; Snyder et al., 1995). It was further shown that the dissemination of bioactive molecules by genetically modified NSCs is feasible (Castellanos et al., 2002; Ethesham et al., 2002; Park et al., 2002). NSCs could therefore be useful to track invading glioma cells and inhibit their growth and expansion while preserving the surrounding brain tissue. However, the signals responsible for NSC migration in the adult CNS are unknown. We therefore analyzed the migratory and invasive properties of C17.2 NSCs in response to soluble tumor cell–derived factors and in direct confrontation with glioblastoma spheroids in vitro.
Conditioned media from 10 different human glioblastoma cell lines that we analyzed significantly stimulated the migration of C17.2 NSCs. However, the degree of stimulation varied by cell line, ranging from 2.5-fold to more than 14-fold. These findings suggest that soluble factors secreted by glioblastoma cells are potent inducers of NSC migration, but that the responsible factors are produced to a varying extent by the different cell lines. This heterogeneity likely reflects the heterogeneity of human glioblastomas in vivo, which differ in multiple respects, including their genetic backgrounds, growth factor expression profiles, and biological behavior.
Among the growth factors known to be expressed in gliomas are FGF-1 and -2; PDGF-AA and -BB; SF/HGF; IGF-1 and -2; TGF-α, -β1, and -β2; PTN; and midkine (Dunn et al., 2000; Hamel et al., 2000). Several of these factors have already been implicated in the stimulation of glioma cell migration (Brockmann et al., 2003). To determine which factors may serve as attractants also for NSCs, recombinant proteins were analyzed in chemotaxis assays. In all assays, SF/HGF consistently turned out to be the most potent attractant for C17.2 cells. Of the 13 other growth factors analyzed, only three had a significant motogenic effect, namely FGF-2, TGF-α, and PTN. In line with these biological effects, RT-PCR analysis revealed expression of the SF/HGF receptor MET as well as the receptors FGFR-2, -3, and -4; epidermal growth factor receptor; and protein tyrosine phosphatase-ζ (PTP-ζ) for FGF-2, TGF-α, and PTN, respectively.
Since SF/HGF emerged as the strongest motogen, we performed blocking experiments on supernatants from cell lines known to secrete SF/HGF. Coaddition of a neutralizing antibody against SF/HGF inhibited stimulation of C17.2 NSC migration induced by all three of the supernatants tested, indicating that the presence of SF/HGF is responsible for a significant part of the chemoattractant capacity of the conditioned media. In human gliomas, expression of both SF/HGF and MET correlates with increasing malignancy grade (Lamszus et al., 1999; Schmidt et al., 1999). This suggests that possibly, also, in vivo malignant gliomas might have a stronger chemotactic effect on NSCs than low-grade gliomas.
Interestingly, the growth-factor-dependent motility profile of neural stem cells is similar to that of glioma cells themselves; however, it is distinct from the profile of normal human cerebral microvascular endothelial cells (Brockmann et al., 2003). For glioblastoma cell lines also, SF/HGF turned out to be the most potent chemoattractant, followed by TGF-α, with weaker effects for FGF-2. In contrast, PDFGF-AA and -BB, which have no significant motogenic effect on glioma cells or C17.2 NSCs, have been found to be the most potent chemoattractant for cerebral microvascular endothelial cells in vitro. It is tempting to speculate that the common neuroectodermal origin of glioma cells and NSC might determine a similar responsiveness for environmental migratory cues, which is distinct from the response pattern of endothelial cells. Notably, SF/HGF has long been known to be predominantly an epithelial rather than mesenchymal cell stimulant (Rosen et al., 1997). The similar motile behavior of glioma cells and NSCs further suggests that glial tumor cells may reacquire properties characteristic of NSCs that may contribute to tumor cell dissemination and growth.
Gliomas growing in human brain usually consist of a solid component that can be distinguished from areas of diffuse infiltration. After demonstrating that soluble glioma-cell-derived factors can attract NSCs, we analyzed whether stem cells would also infiltrate a solid tumor mass, mimicked in vitro by tumor cell spheroids. Spheroids derived from two different glioblastoma cell lines became almost completely infiltrated by the stem cells. However, the majority (7 of 10) were moderately or little infiltrated, and to our surprise, spheroids from one cell line were not infiltrated at all. Thus, the secretion of soluble chemoattractants, which was a consistent property of all glioblastoma cell lines, is opposed by a variable permissiveness of spheroids for NSC infiltration. Several reasons may account for this unexpected discordance. In the spheroid invasion assay, unlike in the chemotaxis assay, tumor cells and NSCs are confronted directly, which adds the components of direct cell-cell contact and intercellular signaling and which also requires matrix degradation by NSC. Absence or only minor NSC invasion could therefore be due to either repellents presented by the tumor cells, or tight adhesion between tumor cells, or nonpermissiveness of the intercellular matrix, or a combination of these factors.
To conclude, the findings of this study show that despite their known heterogeneity, cells from a broad variety of glioblastomas all secrete soluble factors that attract NSCs. Apparently, NSCs can recognize signals from a broad spectrum of tumors as migration cues, which suggests that the potential usefulness of NSCs as therapeutic vehicles would not primarily be limited to tumors with a specific genetic background. However, it seems that the extent to which tumor cells will attract the stem cells might vary greatly in different patients, depending on the relative amounts of growth factors secreted. The extracellular matrix of the brain in that respect will be relatively consistent in all patients, and the behavior of NSCs that have to pass through this matrix to get to individual tumor cells will thus be relatively predictable. However, the extent to which tumor masses can be infiltrated by NSCs appears to be highly variable because the tumor extracellular matrix has a different and highly variable composition. Translated to a potential therapeutic scenario, it follows that only in some cases will stem cells be able to penetrate the major tumor mass also and not only reach the diffusely invading tumor cells. Nevertheless, the invading cells will in all cases be the major NSC target. Finally, only in vivo studies will show the extent to which the in vitro findings can be translated to the in vivo situation, in which a plethora of additional parameters such as the diffusion capacity, stability, and clearance of tumor-derived cytokines, as well as the reaction of the host tissue, will further modulate the motility of NSCs.
Acknowledgments
This article contains major parts of a doctoral thesis by Andreas Disko, to be submitted to the Fachbereich Medizin, University of Hamburg. We thank Evan Snyder (Burnham Institute, San Diego, Calif.) for providing us with the C17.2 murine neural stem cell line and Ralph Schwall (Genentech Inc., San Francisco, Calif.) for providing us with the recombinant human growth factor SF/HGF. We also appreciated the inspiration and discussion of this topic by Karen Aboody (City of Hope Cancer Center, City of Hope, Calif.) and Peter McL Black (Brigham and Women’s Hospital, Harvard Medical School, Boston, Mass.).
Footnotes
Abbreviations used are as follows: bp, base pairs; β-gal, β-galactosidase; CMEC, cerebral microvascular endothelial cell; DMEM, Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; IGF, insulinlike growth factor; NCE, Neurochirurgie Eppendorf; NSC, neural stem cell; PDGF, platelet-derived growth factor; PTN, pleiotrophin; PTP, protein tyrosine phosphatase; R, receptor; RT-PCR, reverse transcriptase–polymerase chain reaction; SEM, standard error of the mean; SF/HGF, scatter factor/hepatocyte growth factor; TGF, transforming growth factor; X-gal, 5-bromo-4-chloro-3-indolyl β-d-galactosidase.
References
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PM. Brain tumors. Part I. N Engl J Med. 1991;324:1471–1476. doi: 10.1056/NEJM199105233242105. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Brockmann MA, Ulbricht U, Gruner K, Fillbrandt R, Westphal M, Lamszus K. Glioblastoma and cerebral microvascular endothelial cell migration in response to tumor-associated growth factors. Neurosurgery. 2003;52:1391–1399. doi: 10.1227/01.neu.0000064806.87785.ab. [DOI] [PubMed] [Google Scholar]
- Castellanos DA, Tsoulfas P, Frydel BR, Gajavelli S, Bes JC, Sagen J. TrkC overexpression enhances survival and migration of neural stem cell transplants in the rat spinal cord. Cell Transplant. 2002;11:297–307. [PubMed] [Google Scholar]
- Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50:121–137. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Kabos P, Kabosova P, Neuman T, Black KL, Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Hamel W, Westphal M. Growth factors in gliomas revisited. Acta Neurochir. 2000;142:113–137. doi: 10.1007/s007010050015. [DOI] [PubMed] [Google Scholar]
- Hurelbrink CB, Armstrong RJ, Dunnett SB, Rosser AE, Barker RA. Neural cells from primary human striatal xenografts migrate extensively in the adult rat CNS. Eur J Neurosci. 2002;15:1255–1266. doi: 10.1046/j.1460-9568.2002.01959.x. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Schmidt NO, Jin L, Laterra J, Zagzag D, Way D, Witte M, Weinand M, Goldberg ID, Westphal M, Rosen EM. Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int J Cancer. 1998;75:19–28. doi: 10.1002/(sici)1097-0215(19980105)75:1<19::aid-ijc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Laterra J, Westphal M, Rosen EM. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int J Dev Neurosci. 1999;17:517–530. doi: 10.1016/s0736-5748(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Park KI, Snyder EY. Neural stem cells—a versatile tool for cell replacement and gene therapy in the central nervous system. Clin Genet. 1999;56:267–278. doi: 10.1034/j.1399-0004.1999.560403.x. [DOI] [PubMed] [Google Scholar]
- Park KI, Liu S, Flax JD, Nissim S, Stieg PE, Snyder EY. Transplantation of neural progenitor and stem cells: Developmental insights may suggest new therapies for spinal cord and other CNS dysfunction. J Neurotrauma. 1999;16:675–687. doi: 10.1089/neu.1999.16.675. [DOI] [PubMed] [Google Scholar]
- Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, Lachyankar MB, Redmond DE, Snyder EY. Global gene and cell replacement strategies via stem cells. Gene Ther. 2002;9:613–624. doi: 10.1038/sj.gt.3301721. [DOI] [PubMed] [Google Scholar]
- Rosen EM, Lamszus K, Laterra J, Polverini PJ, Rubin JS, Goldberg ID. HGF/SF in angiogenesis. Ciba Found Symp. 1997;212:215–226. doi: 10.1002/9780470515457.ch14. [DOI] [PubMed] [Google Scholar]
- Schmidt NO, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen EM, Lamszus K. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frölichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Villa A, Snyder EY, Vescovi A, Martinez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from human CNS. Exp Neurol. 2000;161:67–84. doi: 10.1006/exnr.1999.7237. [DOI] [PubMed] [Google Scholar]
- Westphal M, Hansel M, Hamel W, Kunzmann R, Holzel F. Karyotype analysis of 20 human glioma cell lines. Acta Neurochir. 1994;126:17–26. doi: 10.1007/BF01476489. [DOI] [PubMed] [Google Scholar]
- Yuhas JM, Li AP, Martinez AO, Ladman AJ. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977;37:3639–3643. [PubMed] [Google Scholar]