Abstract
Current regulations focus on the mandatory safety evaluation of functional foods before they come to market, but Nynke de Jongand colleagues argue that the effects of such foods should also be evaluated after they have been launched
Functional foods are modified foods that claim to improve health, quality of life, or wellbeing. These foods are intended for use in the context of a healthy lifestyle or as a means to compensate for an unhealthy one. From society's point of view, there are several potential problems—the medicalisation of our daily food intake, the long term safety and effectiveness of these foods, and the aggressive marketing and advertising of these highly profitable products.1 However, functional foods need to be fully evaluated to make sure they meet current scientific and regulatory standards.
EU regulations
Several European Union regulations and directives on functional foods are currently being developed. Current rules focus mainly on the mandatory safety evaluation of new foods before they come to market; minimum and maximum safe upper values for micronutrients used for fortification; lists of permitted substances for fortification; the registration of herbal products; and acceptable nutritional and health claims.2
One positive development has been the recent publication of regulations on nutrition and health claims. Nutritional claims can now be made only if the food fits a certain nutrient profile (such as below a predefined fat content if “low fat”). New claims can be issued only after being assessed and authorised by the European Food Safety Authority (EFSA) on the basis of good nutritional science.3 4 Most of the regulations and directives focus on evaluating safety before foods reach the supermarket, however—no regulations deal with aspects that arise after this point.
Market positioning of functional foods versus drugs
Similar to so called lifestyle drugs—drugs at the boundary between lifestyle wishes and health needs, such as erectile stimulants, appetite suppressants, and drugs to help people stop smoking5—functional foods are designed to meet consumers' needs and lifestyle wishes.6 7 Data on sales and market dynamics of functional foods are limited. An analysis of functional foods launched between January and April 2005 identified more than 200 new products.
Many functional foods are aimed at trying to improve gut health and heart health and are intended for people who have mild health problems or slight discomfort. The market for health drinks in the United Kingdom is fast growing, with a turnover of £316m (€464; $632) in 2005.8 Although some functional foods (table) might have beneficial effects on risk factors for various chronic and life threatening conditions, there is no proof that attacking these risk factors is good for general health in the free living population. Their main appeal may be particularly to worried consumers.
Some functional foods and drugs with identical targets available on the global market
| Food | Target | Drugs |
|---|---|---|
| Enriched with phytosterol-stanolesters | Low density lipoprotein cholesterol | Statins, ezetimibe |
| Containing bioactive peptides | Blood pressure | Antihypertensive drugs (such as thiazide diuretics) |
| Containing melatonin | Quality of sleep | Benzodiazepines |
| Containing omega 3 fatty acids | Depression | Antidepressants |
| Triglycerides | Fibrates | |
| Containing β glucan | Blood sugar values | Insulin, oral hypoglycaemic drugs |
| Low density lipoprotein cholesterol | Statins, ezetimibe | |
| Containing prebiotics | Bowel frequency | Laxatives |
| Containing probiotics | Immune functioning | |
| Diarrhoea (wet stools) | Loperamide | |
| Crohn's disease (under investigation) | Mesalazine, corticosteroids | |
| Containing extra calcium or vitamin D, or both | Bone health | Alendronate, calcitonin, oestrogens |
| Containing protein or bioactive peptides | Obesity and type 2 diabetes | Orlistat, rimonabant |
| Appetite |
The efficacy of these foods on their targets has not necessarily been confirmed.
Possible food and drug interactions
Functional foods may influence the effectiveness of drugs and patients' compliance. This can be illustrated by the example of phytosterol and stanol enriched products, which are intended for people with mildly raised cholesterol who do not take cholesterol lowering drugs.9 10 11 12 People in this group are often unaware of their cholesterol value. The enriched products may, therefore, be eaten only by those with substantially raised, and thus known, cholesterol values and associated higher cardiovascular morbidity, which inherently increases the potential for interactions with cardiovascular medication.
Phytosterols and stanols interact with statins to have an additive effect on reducing low density lipoprotein cholesterol values.10 13 The possible downside to this interaction is that serum phytosterol concentrations increase during long term statin treatment,11 and concern has been raised about the possible atherogenic effects of phytosterols.14 This is why Health Canada, the federal department responsible for helping Canadians maintain and improve their health, has not allowed these foods to be sold in Canada.15 16
Eating functional foods may also have detrimental effects on patient compliance with drug treatment; adherence to statins is known to be suboptimal.17 18 People who eat phytosterol or stanol enriched foods may alter the dose of their statins for various reasons, without consulting a doctor. A lower dose of statins can never be compensated for by the intake of functional foods.
Limited postlaunch scientific data
Once functional foods come to market, limited data are available about their impact on the community. We have little understanding of the circumstances under which these foods are eaten, whether target groups are reached, and if targeted education programmes or health policies should be recommended. Very little is also known about exposure, long term or otherwise, and safety under free conditions of use,19 and whether and how functional foods interfere with drugs designed for the same target.20 21 These problems have not been addressed even in the best studied of these foods—phytosterol and stanol enriched foods. There is no evidence that functional foods cause harm, but the data are limited to five to six years of use and a restricted number of users.
Scientific developments at the interface between food and pharmacology are ongoing, so data supported assessments of these foods are now possible. The development of a structured postlaunch monitoring system has been suggested but not yet implemented in Europe.22
The case for postlaunch monitoring
Despite two recent reports on the subject,23 24 we are still uncertain about how many people buy and consume functional foods and about any benefit they may have, so more thorough investigations are needed. If these foods are found to be of net benefit to public health, targeted education programmes and supportive government policy should be considered. If no effect or an adverse effect is identified, the associated health claims should be re-evaluated. New regulations may also be needed.
In addition to questions about user compliance, problems such as the impact of compensating behaviour, the possible alternative use of drugs or more traditional foods, and the effect size of these approaches should also be evaluated and compared.
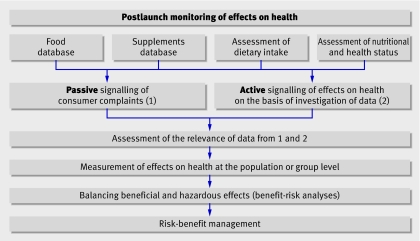
Postlaunch monitoring, which consists of several phases spread over time—starting with signalling and ending with a risk-benefit analysis (figure)—could deal with these uncertainties. Signalling could be performed transparently by manufacturers under supervision of and in collaboration with national food safety authorities. An EFSA committee could be mainly responsible for distributing monitoring tasks to national food safety bodies or nutrition research institutes, with national policymakers and risk managers as important intermediaries. Each monitoring question would determine which institute is assigned a particular task. Such a strategy would also mean close collaboration with those responsible for pharmacovigilance.
Postlaunch monitoring programme
Summary points
Functional foods are designed to meet consumers' needs and lifestyle wishes and may be used as self medication
EU regulations focus on the warrant of safety before a functional food reaches the market
Postlaunch monitoring is needed to assess whether functional foods are safe and effective under customary conditions of use
The ultimate result of postlaunch monitoring could be a decision support tool that helps policymakers and others such as health insurance companies evaluate the impact of health promoting strategies (traditional foods, drugs, functional foods). Intermediaries, such as doctors and dietitians, should be informed about the results so they can educate and help consumers. Practical and unbiased information on when and how to use functional foods and potential side effects and interactions could be conveyed in instruction leaflets, reference books, and on websites. In turn, the intermediaries could provide feedback on consumer experiences to the monitoring team. This would make the best possible use of the results of monitoring and extend their coverage, so that funding for postlaunch monitoring might come not only from manufacturers, but also from national and international governments and private sources.
Until now, postlaunch monitoring for functional foods has been erratic.25 26 27 The first attempt at such monitoring (for phytosterol enriched foods) found that people who buy the product eat less than was anticipated and that no serious adverse effects have been reported to manufacturers' consumer care lines.19 27 Data were gathered at the household level only, however, so users were not characterised and it was not possible specifically to estimate exposure.
Our unit has been mapping the effectiveness of phytosterol and stanol enriched margarine eaten by the Dutch population. The maximum effect seen over five years was stabilisation of total cholesterol values rather than the slight increase usually seen with age. Although this effect is modest, it can still reduce the risk of coronary heart disease and provide health benefits in the general population.24 These observations support the inclusion of effectiveness in the postlaunch monitoring programme. A future topic for research would be to evaluate the effectiveness of adding functional foods to a traditional diet compared with altering the total diet according to dietary guidelines. This single example suggests that we need to invest more in finding out what functional foods can contribute to individual and public health in relation to the promises made by manufacturers.
Contributors and sources: The authors are experts in postlaunch monitoring of functional foods (NdJ, MCJW, MCO, HV) and medicines (OHK, HGML). This draft was written by NdJ, OHK, and MCJW, with comments by HV, MCO, and HGML. Background studies that underpin the views presented in this paper were carried out by all authors. NdJ is guarantor.
Funding: The Netherlands Organisation for Health Research and Development (ZonMW) is acknowledged for funding the PLM research and supporting MCJ Wolfs (grant number 014-12-010).
Competing interests: None declared.
Provenance and peer review: Non-commissioned; peer reviewed.
References
- 1.Pletscher W. Functional food: a growing and not clearly controlled market with a risk potential ranging from a food to a drug. Swiss Med Wkly 2004;134:367-8. [DOI] [PubMed] [Google Scholar]
- 2.European Commission. Novel foods legislation 2007. http://europa.eu.int/comm/food/food/biotechnology/novelfood/legisl_en.htm
- 3.Aggett PJ, Antoine JM, Asp NG, Bellisle F, Contor L, Cummings JH, et al. PASSCLAIM: consensus on criteria. Eur J Nutr 2005;44(suppl 1):i5-30. [DOI] [PubMed] [Google Scholar]
- 4.European Commission. Health and nutrition claims. http://ec.europa.eu/food/food/labellingnutrition/claims/index_en.htm
- 5.Gilbert D, Whalley T, New B. Lifestyle medicines. BMJ 2000;321:1341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pariza MW. Functional foods: technology, functionality, and health benefits. Nutr Today 1999;34:3 [Google Scholar]
- 7.Zureik M, Courbon D, Ducimetiere P. Serum cholesterol concentration and death from suicide in men: Paris prospective study I. BMJ 1996;313:649-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leatherhead Food International. The international market for functional foods. Market report leaflet June 2006. www.leatherheadfood.com/lfi/pdf/ffoods2.pdf.
- 9.Hendriks HFJ, Weststrate JA, van Vliet T, Meijer GW. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 1999;53:319-27. [DOI] [PubMed] [Google Scholar]
- 10.Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, et al. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 2003;78:965-78. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen TA, Gylling H. Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann Med 2004;36:126-34. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med 1995;333:1308-12. [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Capuzzi DM, Gottlieb SO, Nguyen T, Morgan JM, Cater NB. Incremental reduction of serum total cholesterol and low-density lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52. [DOI] [PubMed] [Google Scholar]
- 14.Patel MD, Thompson PD. Phytosterols and vascular disease. Atherosclerosis 2006;186:12-9. [DOI] [PubMed] [Google Scholar]
- 15.Ratnayake W, Vasavour E. Potential health risks associated with large intakes of plant sterols. In: Dutta PC, ed. Plant sterols: analytical, nutritional and safety aspects as functional food components. New York: Marcel Dekker, 2004:365-96.
- 16.Home Health Canada. Health Canada advises that Becel Pro-activ not approved for sale. Oct 2001. www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/2001/2001_106_e.html.
- 17.Mantel-Teeuwisse AK, Goettsch WG, Klungel OH, de Boer A, Herings RM. Long term persistence with statin treatment in daily medical practice. Heart 2004;90:1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantel-Teeuwisse AK, Klungel OH, Schalekamp T, Verschuren WM, Porsius AJ, de Boer A. Suboptimal choices and dosing of statins at start of therapy. Br J Clin Pharmacol 2005;60:83-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scientific Committee on Food. Opinion of the Scientific Committee on Food on a report on post launch monitoring of “yellow fat spreads with added phytosterol esters.” Brussels: SCF, 2002
- 20.Izzo AA, Di Carlo G, Borrelli F, Ernst E. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol 2005;98:1-14. [DOI] [PubMed] [Google Scholar]
- 21.Ohnishi N, Yokoyama T. Interactions between medicines and functional foods or dietary supplements. Keio J Med 2004;53:137-50. [DOI] [PubMed] [Google Scholar]
- 22.de Jong N, Fransen HP, van den Berg SW, Ocké MC. Postlaunch monitoring of functional foods—methodology development (III), report number 350030006 Bilthoven: RIVM, 2005
- 23.de Jong N, Zuur A, Wolfs MCJ, Wendel-Vos GCW, van Raaij JMA, Schuit AJ. Exposure and effectiveness of phytosterol- and stanol-enriched margarines. Eur J Clin Nutr 2007 Feb 14[Epub ahead of print]. [DOI] [PubMed]
- 24.Wolfs M, de Jong N, Ocke MC, Verhagen H, Verschuren WMM. Effectiveness of customary use of phytosterol/-stanol enriched margarines on blood cholesterol lowering. Food Chem Toxicol 2006;44:1682-8. [DOI] [PubMed] [Google Scholar]
- 25.Allgood GS, Kuter DJ, Roll KT, Taylor SL, Zorich NL. Postmarketing surveillance of new food ingredients: results from the program with the fat replacer olestra. Regul Toxicol Pharmacol 2001;33:224-33. [DOI] [PubMed] [Google Scholar]
- 26.Butchko HH, Stargel WW. Aspartame: scientific evaluation in the postmarketing period. Regul Toxicol Pharmacol 2001;34:221-33. [DOI] [PubMed] [Google Scholar]
- 27.Lea LJ, Hepburn PA. Safety evaluation of phytosterol-esters. Part 9: results of a European post-launch monitoring programme. Food Chem Toxicol 2006;44:1213-22. [DOI] [PubMed] [Google Scholar]