Abstract
Mandibular ramus morphology on a recently discovered specimen of Australopithecus afarensis closely matches that of gorillas. This finding was unexpected given that chimpanzees are the closest living relatives of humans. Because modern humans, chimpanzees, orangutans, and many other primates share a ramal morphology that differs from that of gorillas, the gorilla anatomy must represent a unique condition, and its appearance in fossil hominins must represent an independently derived morphology. This particular morphology appears also in Australopithecus robustus. The presence of the morphology in both the latter and Au. afarensis and its absence in modern humans cast doubt on the role of Au. afarensis as a modern human ancestor. The ramal anatomy of the earlier Ardipithecus ramidus is virtually that of a chimpanzee, corroborating the proposed phylogenetic scenario.
Keywords: hominins, phylogeny, ramus
In all primates, the superior end of the mandibular ramus terminates in two processes: posteriorly, the condylar process, which articulates with the base of the skull, and anteriorly, the coronoid process, which is the insertion site of the temporalis muscle. An indentation, the mandibular (or sigmoid) notch, separates these two processes.
Among extant higher primates, each species shows species-specific characteristics of the ramus. Nevertheless, the ramal configurations in those primates that we studied clearly fall into two groups: one consists of gorillas, and the other consists of modern humans, two chimpanzee species, and orangutans. In the latter group, which shares a pattern of ramal morphology with many other primates that we examined, the coronoid process is typically lower than the condylar process; the base of the coronoid constitutes a relatively small percentage of the ramal width and tapers into a slender, pointed, superiorly directed tip (Fig. 1). This tapering produces a spacious mandibular notch between the two processes; hence, the deepest point of the notch is situated anteriorly. In gorillas, on the other hand, the coronoid process is usually higher than the condylar process. The broad base of the coronoid constitutes much of the ramal width, moving the deepest point of the mandibular notch closer to the condylar process than in what we interpret to be the more primitive (common) configuration. With the tip of the coronoid pointing posteriorly, the superior edge of the process in gorillas assumes a flat contour; in many cases, the tip overhangs the mandibular notch, lending the process a hook-like appearance, and the notch, a narrow, deep, confined appearance. As a consequence, the notch occupies a smaller portion of the total ramal area than in the more common ramal morphology.§
Fig. 1.
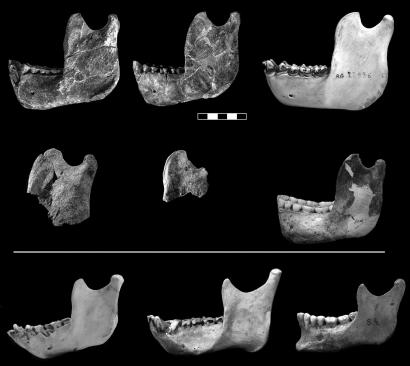
Ramal morphology in Au. afarensis and extant primates. (Top) Left mandibular ramus and right mandibular ramus (horizontally flipped) of Au. afarensis specimen A. L. 822-1 and left mandibular ramus of a gorilla. (Middle) Left mandibular ramus of Au. afarensis MAK-VP 1/83 specimen; fragment of left mandibular ramus of Au. afarensis specimen A. L. 333-100; and mandibular ramus of Au. robustus specimen SK 23. (Bottom) Mandibular ramus of a chimpanzee, an orangutan, and H. sapiens. (Scale bar: 5 cm.) Note that the upper end of the ramus in all of the specimens above the white line resembles that of a gorilla (particularly in the shape of the coronoid, the great percentage that the coronoid base constitutes of the ramal width, the confined appearance of the mandibular notch, and the small percentage that the notch area constitutes of the ramal area). The limited reconstruction of the coronoid process on the left ramus of A. L. 822-1 is based on the corresponding preserved area on the right ramus and vice versa.
The ramus of an Australopithecus afarensis specimen discovered in 2002, A. L. 822-1 (Fig. 1), closely matches that of the gorilla. The specimen is a fragmentary but well preserved skull of an adult individual found in the Unda Hadar, a tributary of the Awash River running parallel to the Kada Hadar. Discovered ≈2.5 km east of A. L. 288 (Lucy's site), the specimen was recovered from the lower Kada Hadar member and is ≈3.1 million years old. Its calvarial, facial, mandibular, and dental morphologies demonstrate that the specimen belongs to Au. afarensis.¶
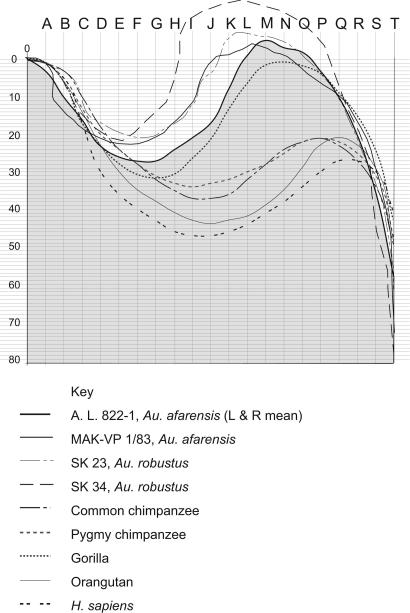
To quantify the contour of the essentially two-dimensional structure that lies between the posterior ramal margin and the parallel, anterior ramal margin, we plotted each ramus in our fossil and extant primate samples on a fixed, specifically constructed system of coordinates. In this procedure, the contour of each ramus is expressed as 20 numeric values referenced to the coordinate system [supporting information (SI) Table 1; see Materials and Methods for details] from which a mean contour could be calculated for each taxon (Fig. 2). This procedure enabled us to perform statistical analyses of ramus shape.
Fig. 2.
The mean contour of the superior margins of the left and right mandibular ramus in A. L. 822-1 compared with the mean contours of other primates. The A. L. 822-1 contour constitutes the border of the shaded area. Note that the contours fall into two distinct groups. Each contour was plotted with the posterior margin of the ramus vertically oriented. The tip of each condylar process lies at the upper left corner of the coordinate system (the zero point), and the anterior margin of the ramus lies at the far right, at the vertical line T. These contours do not express the posteriorly directed tip of the coronoid process (see Fig. 1) exhibited by A. L. 822-1 and many gorillas; the resolution of the grid does not capture the full extent of the tip's morphology.
We performed a discriminant function analysis (1, 2) on the 20 ramal shape variables. The Kolmogorov–Smirnov normality test showed that all of the variables, except for the first two, were distributed normally. On the basis of posterior probabilities, we classified all of the mandibles to taxon and identified misassigned cases. A classification matrix summarizes these results (SI Tables 2 and 3).
Results
The analysis of variance reveals that group centroids of the extant great ape species differ significantly [Wilk's λ = 0.048, F(80, 483), = 7.04, P < 0.0001]. Post hoc tests among group means show a significant difference between all pairwise combinations [F(20, 122) ≥ 1.89, P < 0.02 in all comparisons]. The least different were the two species of chimpanzees, Pan troglodytes and Pan paniscus [F(20, 122) = 1.89, P < 0.02]. [The other 14 pairwise comparisons yielded F(20, 122) ranging from 3.18 to 15.52; P < 0.005 in all comparisons.] The greatest differences were observed between gorillas and all of the other species.
The a priori classification probability was determined on the basis of group size (that is, the calculation took into account the differences between the sample sizes of the species). The final classification matrix shows an overall classification success of 82%. Modern humans were correctly assigned in 93% of the cases. Not unexpectedly, the highest percentage of assignment errors (31%) occurred with common chimpanzees, with a classification success of only 69%; most of the misassigned individuals were assigned as pygmy chimpanzees.
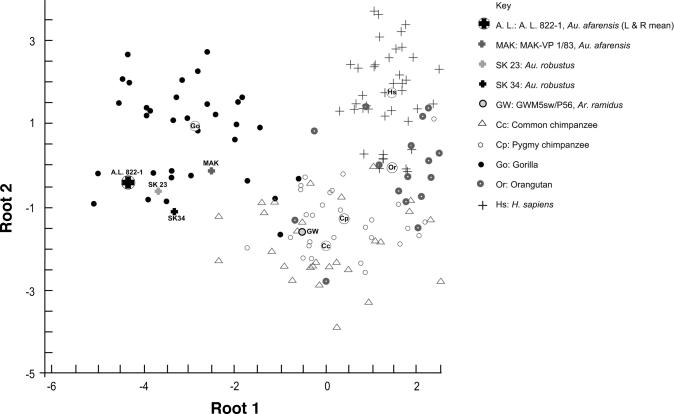
The first two canonical variables account for 89% of the variance (50% and 39%, respectively). The variables N and G constitute the highest loads in the first and second factor, respectively. These two variables roughly correspond to the high point of the coronoid process and the low point in the mandibular notch in the ramal contour (see Fig. 2). Fig. 3 presents a scatterplot of the first two canonical roots. Au. afarensis was initially considered an unknown in the analysis, so its position in the plot and its probability of assignment were calculated subsequently, according to the predetermined classification functions. The posterior probability for Au. afarensis is highest with gorillas, at 99.9% for A. L. 822-1 and 92.7% for another Au. afarensis mandible, MAK-VP 1/83 (Fig. 1), a specimen from the Maka sands in the Middle Awash, Ethiopia (3). Although less complete, this specimen retains sufficient morphology for quantification. The visual resemblance between the ramal anatomy of Au. afarensis and that of gorillas is confirmed by the morphometric analysis, as are the anatomical differences between this species pair and the other extant species in our sample. Only a small overlap exists between the gorilla cluster and the chimpanzee cluster (Fig. 3 shows the canonical scores of individual specimens); nevertheless, the two Au. afarensis specimens fall within the gorilla area, clearly outside the area of overlap.
Fig. 3.
Canonical scores of root 1 versus root 2. Individual scores are indicated by species-specific symbols, and group centroids are indicated by encircled species-specific abbreviations. Roots 1 and 2 account for 50.2% and 38.8% of the variance, respectively.
Although only A. L. 822-1 and MAK-VP 1/83 are complete enough to be analyzed by the method used here, the same ramal anatomy is evident on other Au. afarensis specimens that are more fragmentary: A. L. 333-100, from the Hadar site in Ethiopia (4) (Fig. 1); A. L. 333w-15, a small fragment that is probably pathological; and the meager remains of the mandibular notch in A. L. 438-1g, A. L. 333-108, and A. L. 288–1i (Lucy). Until the discovery of A. L. 822-1, the resemblance of these specimens to gorillas went unnoticed even though the specimens have been known of for many years.
Even very young Au. afarensis individuals, such as A. L. 333-43b (SI Fig. 4) and the newly discovered, less complete A. L. 333n-1, exhibit a similar morphology to that of gorillas of comparable age and differ from young individuals of the other primate species that we studied. Thus, no significant ontogenetic change is evident in the shape of the superior portion of the mandibular ramus.
The observed interspecific differences in ramal morphology are not due to the relative size and orientation of the temporalis muscle, which can be deduced from differences in the osteological calvarial landmarks related to the origin of the temporalis: the height and length of the sagittal crest, the extent and size of the temporal/nuchal (T/N) compound crest, and the size of the “bare area” between the temporal and nuchal lines (5). Male and female gorillas, for example, differ considerably in these indicative exocranial landmarks but display the same ramal morphology. Female gorillas and male and female chimpanzees have a similar cranial cresting pattern, yet they differ substantially in ramal morphology. Although Au. afarensis resembles chimpanzees (and female gorillas) in its cresting pattern (5), the Au. afarensis mandibular ramal morphology resembles that of gorillas, not chimpanzees.
The similarity between gorillas and Au. afarensis in ramal morphology does not appear to be the outcome of similar selective pressures, because these two species differ in habitat (6–9) and diet. [Evidence of the latter derives from studies of differences in tooth enamel thickness, the topography of the occlusal surfaces, and the shape of the dental arcade in gorillas and in Au. afarensis (3, 4, 9–13).]
We note again that orangutans, the outgroup in the analysis, fall within the generalized group. The contour of the superior portion of the ramus in orangutans is much more similar to that of modern humans and chimpanzees than to the gorilla contour (Figs. 1–3).
Significantly, the ramal anatomy observed in Au. afarensis and gorillas is present also in the only two Au. robustus specimens that are complete enough to permit examination of the ramus: SK 23 (Figs. 1–3) and SK 34 (Figs. 2 and 3 and SI Fig. 4). The posterior probability of SK 23 is highest with gorillas, at 97.4%, as is that of SK 34, at 55.2% (SI Table 3). Even more importantly, when we assign the Au. robustus specimens as the sixth (known) classification group, the posterior probabilities for A. L. 822-1 (99.9%) and the Maka mandible (97.9%) are highest for Au. robustus. In other words, the superior ramal contours of Au. afarensis and Au. robustus are virtually identical. Before the analysis of Au. afarensis reported here, this ramal morphology went practically unnoticed in Au. robustus (but see Robinson, ref. 14, page 226).
Discussion and Conclusions
Given a phylogeny in which chimpanzees and modern humans are sister groups, parsimony dictates that we view the similarity in ramal morphology between Au. afarensis and gorillas as a homoplastic character, a character that appears independently and as such has no phylogenetic value.
The ramal morphology of modern humans and chimpanzees clearly represents the primitive condition, because this morphology closely matches that of orangutans, the outgroup in this analysis, and is shared by many other primates, as can be observed visually.
Thus, the Au. afarensis ramal morphology is a novelty that appeared independently in gorillas and hominins. In the latter, this morphology constituted at first an autapomorphic (unique) trait and eventually became a synapomorphic (shared derived) trait that unites Au. afarensis and Au. robustus into a single clade (which possibly includes all of the robust australopiths, although no Australopithecus boisei or Au. aethiopicus specimens exhibiting this region are available at present). Nonetheless, the morphology of the Au. afarensis face (5, 15) and dentition (4) still represents the most generalized state in the robust morphocline of these characters. For those who advocate the inclusion of Au. africanus in the robust clade (15–17), it is significant that Sts 7, the only Au. africanus specimen that permits the relevant observation, although still embedded in breccia, exhibits the same ramal morphology as Au. robustus and Au. afarensis (see figure 21 on Plate 5 in ref. 18).
The Au. afarensis ramal morphology can be added to other traits that this species shares with Au. robustus (5) and casts doubt on the postulated role of Au. afarensis as the common ancestor of later hominins (15, 17, 19). Au. afarensis is simply too derived to occupy a position as a common ancestor of both the Homo and robust australopith clades. Claims that Au. afarensis is too derived to fulfill this role have, indeed, been voiced sporadically ever since this species was recognized (20–23).
If one accepts the gorilla–Au. afarensis ramal morphology as homoplasy, one may legitimately ask why we consider the resemblance between Au. afarensis and Au. robustus a synapomorphy rather than homoplasy. The answer is that, in the gorilla–hominin case, we are equipped with all of the genetic evidence supporting the claim of homoplasy, whereas such evidence is unavailable in the fossil hominins that exhibit the gorilla-like ramal morphology. Hence, claiming synapomorphy within the latter group is a simpler solution. Furthermore, synapomorphy aside, even if the presence of similar ramal morphology in Au. afarensis and Au. robustus did, indeed, represent homoplasy, the Au. afarensis ramal anatomy would still exclude this taxon from our ancestry.‖
Additional support for the phylogenetic hypothesis proposed here comes from another early hominin, Ardipithecus ramidus, whose ramus was recently unearthed at an Ethiopian site dated at 4.51–4.32 million years ago (ref. 24 and figure 3 therein). In our analysis, the specimen's posterior probability is highest with chimpanzees, at 98% (Fig. 3 and SI Tables 2 and 3). In other words, the Ar. ramidus ramal morphology is almost identical to that of a chimpanzee and thus constitutes further evidence that this morphology is primitive for the chimpanzee and human clade.**
Materials and Methods
We examined a total of 146 extant primate specimens: 41 modern Homo sapiens specimens, 31 gorillas (Gorilla gorilla), 29 pygmy chimpanzees (P. paniscus), 29 common chimpanzees (P. troglodytes), and 16 orangutans (Pongo pygmaeus). All of the specimens were mature individuals. The modern human specimens come from varied regions: Australia, India, the Levant, and northern Canada (Eskimos).
To convey the anatomical differences in the upper ramal contour, we adopted a method based on Rak et al. (27), which consisted of capturing a digital image of the mandibular ramus with the camera centered at the vertical level of the mandibular notch and held perpendicular to the lateral surface of the ramus. Using FreeHand 9.0 for Macintosh (Adobe Systems, Seattle, WA), we traced the digital image of each ramus from the tip of the condylar process to the anterior margin of the ramus. (This step represents a slight modification of the original method, in which the contour extended only as far as the coronoid tip.)
With the aid of the FreeHand software, we stretched the contour proportionally on the vertical and horizontal axes by dragging the contour's lower right corner until it occupied the entire width of the area of the fixed coordinates in the background template. This part of the procedure eliminated differences in size in the analysis. The posterior margin was aligned with the vertical line at 0, and the anterior margin was aligned at T. The posterior ramal margin in the entire sample exhibits a slight concavity between the posterior end of the condyle and the insertion site of the posterior fibers of the masseter and medial pterygoid muscles; using these two posteriorly protruding structures, we were able to orient the posterior margin on a vertical line throughout the sample. The intersection of the ramal contour with each of the vertical lines, A through T, yielded 20 numeric variables for each ramus (SI Table 1).
The decision to position the mandible with the posterior margin of its ramus oriented vertically was based on our observation that this orientation varies the least in reference to the Frankfurt horizontal (the ground) within and between species (SI Fig. 5). In addition, this choice permits us to include two important fragmentary fossils that lack all landmarks except the posterior margin of the ramus: the Au. afarensis MAK-VP 1/83 mandible and the Ar. ramidus mandible.
One author, A.G., carried out the entire measurement procedure. To evaluate the accuracy of the method, we repeated the procedure on 20 randomly selected mandibles from the modern H. sapiens sample and compared the two sets of readings. The second set differed insignificantly from the first, with a discrepancy of 1.7% between the sums of the values of the two full data sets.
We performed the discriminant and variance analyses with STATISTICA for Macintosh (version 4.0; StatSoft, Tulsa, OK).
Supplementary Material
Acknowledgments
We thank the Authority for Research and Conservation of Cultural Heritage (of the Ethiopian Ministry of Youth, Sports, and Culture) and its head, Mr. Jara Haile Mariam, for permission to carry out fieldwork at the Hadar site; the Culture and Tourism Bureau of the Afar Regional State government for local permissions and assistance; and Mrs. Mamitu Yilma and the National Museum of Ethiopia for permitting and facilitating our laboratory research in Addis Ababa. W. H. Kimbel and D. J. Johanson shared the original fossil with us and provided constructive comments and suggestions throughout the study. We also thank H. Odwak, A. Barash, and D. Deflandere for assisting in gathering the data; the Canadian Museum of Civilization (Hull, QC, Canada) and Royal Museum for Central Africa (Tervuren, Belgium) for permitting access to their collections; and W. Hylander, C. Lockwood, and E. Hovers for their thorough reading of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606454104/DC1.
Although the height of the gorilla coronoid can be considerable, it is the constellation of the noted features (the width of the base of the coronoid process, the flatness of its superior contour, and the location of the highest point of the process and the lowest point in the notch relative to the width of the ramus) that determines the shape of the superior ramal contour in our comparative primate sample and differentiates the two morphologies.
Kimbel, W., Rak, Y., Johnson, D. (2003) Am J Phys Anthropol Suppl 36:129 (abstr.).
A much less likely scenario is that the Au. afarensis ramal morphology was apomorphic (newly derived) for all hominins and many other primates and that a reversal to the chimpanzee-like condition occurred in the Homo clade.
In light of the debate about which modern chimpanzee species is more representative of the common ancestor (“prototype”) of modern humans and chimpanzees (25, 26), we note that the posterior probability for the Ar. ramidus mandibular ramus in our analysis is highest for common chimpanzees (twice as high as for pygmy chimpanzees).
References
- 1.Afifi AA, Clark V. Computer-Aided Multivariate Analysis. New York: Chapman & Hall; 1995. [Google Scholar]
- 2.Bernstein IH. Applied Multivariate Analysis. Berlin: Springer; 1988. [Google Scholar]
- 3.White TD, Suwa G, Simpson S, Asfaw B. Am J Phys Anthropol. 2000;111:45–68. doi: 10.1002/(SICI)1096-8644(200001)111:1<45::AID-AJPA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.White TD, Johanson DC. Am J Phys Anthropol. 1982;57:501–544. [Google Scholar]
- 5.Kimbel WH, Rak Y, Johanson DC. The Skull of Australopithecus afarensis. Oxford: Oxford Univ Press; 2004. [Google Scholar]
- 6.Andrews P, Humphrey L. African Biogeography, Climate Change, and Human Evolution. In: Bromage TG, Schrenk F, editors. Oxford: Oxford Univ Press; 1999. pp. 282–300. [Google Scholar]
- 7.Foley R. In: African Biogeography, Climate Change, and Human Evolution. Bromage TG, Schrenk F, editors. Oxford: Oxford Univ Press; 1999. pp. 328–348. [Google Scholar]
- 8.Reed KE. J Hum Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- 9.Uchida A. Craniodental Variation Among the Great Apes, Peabody Museum Bulletin 4. Cambridge, MA: Harvard Univ Press; 1996. [Google Scholar]
- 10.Johanson DC, White TD, Coppens Y. Am J Phys Anthropol. 1982;57:545–603. [Google Scholar]
- 11.Swindler DR. Dentition of Living Primates. New York: Academic; 1976. [Google Scholar]
- 12.Grine FE, Martin LB. In: Evolutionary History of the “Robust” Australopithecines. Grine FE, editor. New York: Aldine de Gruyter; 1988. pp. 3–42. [Google Scholar]
- 13.Martin LB. Nature. 1985;314:260–263. doi: 10.1038/314260a0. [DOI] [PubMed] [Google Scholar]
- 14.Robinson JT. Early Hominid Posture and Locomotion. Chicago: Univ of Chicago Press; 1972. [Google Scholar]
- 15.Rak Y. The Australopithecine Face. New York: Academic; 1983. [Google Scholar]
- 16.Aguirre E. Crónica del XI Congreso Nacional de Arqueología (Mérida) 1970;1969:98–124. [Google Scholar]
- 17.Johanson DC, White TD. Science. 1979;203:321–330. doi: 10.1126/science.104384. [DOI] [PubMed] [Google Scholar]
- 18.Broom R, Robinson JT. Further Evidence of the Structure of the Sterkfontein Ape-Man Plesianthropus (Part I) Johannesburg: Voortrekkerpers; 1950. Transvaal Museum Memoir No. 4, Pretoria. [Google Scholar]
- 19.White TD, WoldeGabriel G, Asfaw B, Ambrose S, Beyene Y, Bernor RL, Boisserie JR, Currie B, Gilbert H, Haile-Selassie Y, et al. Nature. 2006;440:883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 20.Falk D, Conroy G. Nature. 1983;306:779–781. [Google Scholar]
- 21.Arsuaga JL, Martinez I. The Chosen Species. Oxford: Blackwell; 2006. [Google Scholar]
- 22.Rak Y. J Hum Evol. 1991;20:283–290. [Google Scholar]
- 23.Olson TR. In: Aspects of Human Evolution. Stringer CB, editor. London: Taylor and Francis; 1981. pp. 99–128. [Google Scholar]
- 24.Semaw S, Simpson SW, Quade J, Renne PR, Butler RF, McIntosh WC, Levin N, Dominguez-Rodrigo M, Rogers MJ. Nature. 2005;433:301–305. doi: 10.1038/nature03177. [DOI] [PubMed] [Google Scholar]
- 25.Zihlman A, Cronin J, Cramer D, Sarich VM. Nature. 1978;275:744–746. doi: 10.1038/275744a0. [DOI] [PubMed] [Google Scholar]
- 26.Latimer BM, White TD, Kimbel WH, Johanson DC, Lovejoy CO. J Hum Evol. 1981;10:475–488. [Google Scholar]
- 27.Rak Y, Ginzburg A, Geffen E. Am J Phys Anthropol. 2002;119:199–204. doi: 10.1002/ajpa.10131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.