Abstract
Here, we report the identification of a metastasis promoting factor by a forward genetic screen in mice. A retroviral cDNA library was introduced into the nonmetastatic cancer cell line 168FARN, which was then orthotopically transplanted into mouse mammary fat pads, followed by selection for cells that metastasize to the lung. The genes encoding the disulfide isomerase ERp5 and β-catenin were found to promote breast cancer invasion and metastasis. Disulfide isomerases (thiol isomerases), which catalyze disulfide bond formation, reduction, and isomerization, have not previously been implicated in cancer cell signaling and tumor metastasis. Overexpression of ERp5 promotes both in vitro migration and invasion and in vivo metastasis of breast cancer cells. These effects were shown to involve activation of ErbB2 and phosphoinositide 3-kinase (PI3K) pathways through dimerization of ErbB2. Activation of ErbB2 and PI3K subsequently stimulates RhoA and β-catenin, which mediate the migration and invasion of tumor cells. Inhibition of ErbB2 and PI3K reverses the phenotypes induced by ERp5. Finally, ERp5 was shown to be up-regulated in human surgical samples of invasive breast cancers. These data identify a link between disulfide isomerases and tumor development, and provide a mechanism that modulates ErbB2 and PI3K signaling in the promotion of cancer progression.
Keywords: breast cancer invasion and metastasis, disulfide isomerase ERp5, ErbB2/phosphoinositide 3-kinase, forward genetic screen, orthotopic animal model
Metastasis remains one of the most poorly understood processes in cancer biology despite extensive study (1). Metastasis occurs by a multistep process requiring the coordinated action of many genes (2–5): the escape of cancer cells from the primary tumor and entry into the blood stream (intravasation), survival in the circulation, exit from capillaries into surrounding tissues at a new site (extravasation), initiation of growth to form micrometastases, and development of new blood vessels to form secondary tumors (2–5). Although a number of mechanisms have been identified that induce tumor cell migration and invasion, including the activation of cell surface receptors and small GTPases of the Rho family (6–13), the systematic identification and characterization of additional genes that promote or suppress tumor invasion and metastasis should advance our understanding of the metastasis process and ultimately provide new therapeutic targets for the treatment of cancer.
Forward genetic screens provide an unbiased approach to the identification of genes that contribute to a phenotype of interest (14–16). Arrayed cDNA and RNAi libraries provide powerful tools for both gain-of-function and loss-of-function cell-based screens at the genome-wide level (17–21). However, due to the complexity of metastasis, no cell-based screen accurately recapitulates the entire tumor dissemination process from the primary tumor to the growth of metastatic tumors in secondary organs. Thus, the application of animal models of metastasis to such genetic screens should facilitate the systematic identification of genes that play critical roles in this process. In this study, an in vivo selection system in the mouse was used to identify genes from a large cDNA library that complement a cell line defective in the early steps of metastasis. Two gene products, β-catenin and the disulfide isomerase ERp5, were found to cause metastasis to the lung in an orthotopic breast cancer model. Additional experiments demonstrated that ERp5 promotes breast cancer cell migration and invasion through activation of the EGF receptor ErbB2/PI3K pathway and downstream signaling molecules, including Akt and RhoA. In addition, ERp5 was found to be up-regulated in invasive clinical breast cancer samples. These experiments define a role for the disulfide isomerase ERp5 in tumor invasion and metastasis and provide an additional mechanism for modulation of ErbB2/PI3K signaling.
Results and Discussion
A Forward Genetic Screen in an Orthotopic Mouse Model for Metastasis Promoting Genes.
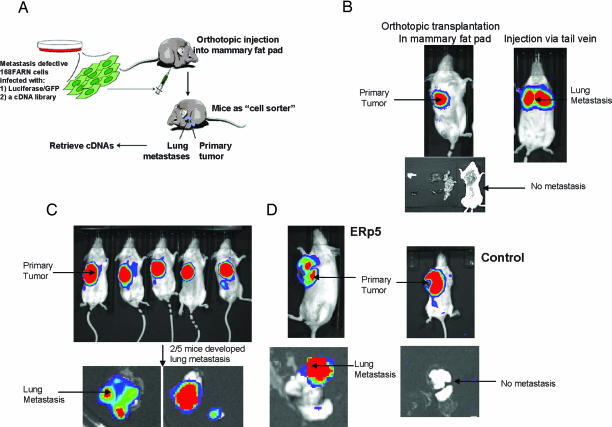
Our genetic screen for metastasis promoting genes is based on orthotopic transplantation of tumor cells infected with a retroviral cDNA library into the mouse mammary fat pad, subsequent selection for metastasis to secondary organs, and finally retrieval of genes that promote this process (Fig. 1A). An orthotopic transplantation mouse model was used (instead of a tail vein injection model) because transplanted tumor cells in the mammary fat pad must complete all of the steps of metastasis to spread to the lung. 168FARN cells, originally isolated from a single mouse mammary tumor, which arose spontaneously in a wild type BALB/cJ mouse (22), were chosen for the initial screen. 168FARN cells are able to grow at the primary site but are not able to develop metastatic nodules when transplanted into the mammary fat pad (Fig. 1B). However, 168FARN cells develop lung metastasis when injected directly into the blood stream through the tail vein (Fig. 1B), indicating that they are defective in the early steps of metastasis (i.e., tumor migration and invasion), but have full metastatic potential once they reach the blood stream and lung. Therefore, this cell line is especially suitable for the isolation of genes that promote the early steps of tumor spread. Any gene that can complement the metastatic defects in this cell line should cause cells to metastasize from the mammary fat pad to the lung, which serves as a positive selection system.
Fig. 1.
Identification of ERp5 as a metastasis-promoting gene. (A) The scheme for the forward genetic screen. (B) Model establishment: 168FARN tumor cell growth in the primary site after transplantation (1 × 106 cells) to mammary fat pad in BALB/cJ mice (B Upper Left). Dissection of mice 7 weeks posttransplantation shows no metastasis in secondary organs (B Lower). 168FARN cells are able to form lung metastasis after tail vein injection (1 × 106 cells) (B Upper Right). (C and D) Screening and validation: Tumor cell growth in the primary site after injection of 168FARN cells (1 × 106 cells) containing a cDNA library in mammary fat pad. (C) Two of five mice developed lung metastasis. Transplantation of 168FARN cells stably overexpressing ERp5 in mammary fat pad leads to lung metastasis. (D) Mice were imaged 6–8 weeks after transplantation.
To carry out the screen, a retroviral cDNA library consisting of cDNAs prepared from day 13.5 embryonic stage mice and expressed behind the LTR promoter was used (23). The incorporation of cDNAs into the host genome after retroviral infection makes it possible to easily retrieve cDNAs from the positively selected cells by PCR (23). 168FARN cells (1 × 106) that contain the cDNA library were transplanted into the mouse mammary fat pad (Fig. 1A). A proof-of-concept experiment using five BALB/cJ mice was performed. Of these five mice, two subsequently developed lung metastasis in 7 weeks (Fig. 1C). Genes encoding β-catenin and ERp5 were retrieved from the metastatic cells of these mice. β-Catenin is a major downstream effector of the Wnt pathway (24, 25) that impairs epithelial cell differentiation and induces the epithelial-mesenchymal transition (26–29). In addition, nuclear β-catenin activity is up-regulated in various types of aggressive cancers (30–32). The isolation of a gene that is known to play an important role in tumor metastasis validates the ability of this approach to identify bona fide metastasis promoting genes.
ERp5 is a member of the thiol isomerase family which is responsible for the formation of native disulfide bonds in cell surface and secreted proteins (33–37). These enzymes ensure the correct pairing of cysteine (Cys) residues in proteins by catalyzing the formation and rearrangement of disulfide bonds. This process ensures the correct folding of their substrates into native conformations (33–37). Recent studies have demonstrated that in addition to their localization in the endoplasmic reticulum, some thiol isomerases are also located on the cell surface where they function in receptor activation and remodeling (38). For example, it has been shown that ERp5 is recruited to the cell surface and plays a major role in platelet aggregation in response to platelet agonists (39). Despite extensive studies of the thiol isomerase family, little is known regarding their roles in cellular signaling pathways and tumor progression.
To validate the metastasis-promoting activity of ERp5, the gene was cloned into a retroviral vector behind the LTR promoter and introduced into 168FARN cells to generate a cell line that stably expresses ERp5. Overexpression of ERp5 was confirmed by immunoblot with an ERp5 antibody (data not shown). These cells were then transplanted into the mammary fat pad using the same procedure as in the initial screen. Lung metastasis nodules developed after transplantation, demonstrating that ERp5 promotes tumor metastasis in vivo, whereas 168FARN cells containing a control vector do not cause metastasis (Fig. 1D).
Two considerations may account for the lack of isolation of other metastasis-promoting genes in this proof-of-concept study. The cDNA library that was used had a titer of 107 to 108 cfu, which is much larger than the coverage of cDNAs that can be obtained with five mice. Many genes, especially genes with low copy numbers, might be found using larger numbers of mice. The other possibility is that the cDNA library was prepared from day 13.5 embryonic mice and contains only genes that are expressed during this stage. An ideal cDNA library for this type of study is one that contains all genes in the genome, where each gene has equal representation in the library. Such a library is now being generated for future studies.
ERp5 Promotes Tumor Cell Migration and Invasion in Vitro.
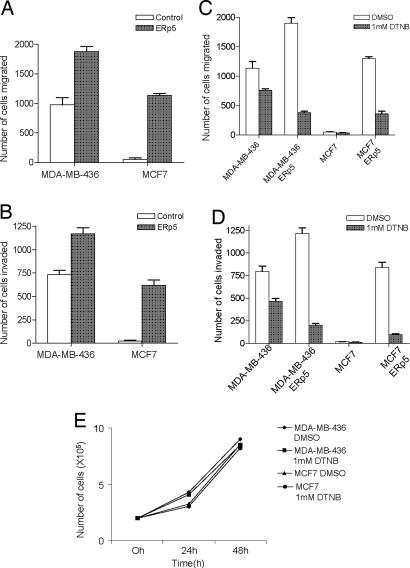
Because 168FARN cells are defective in the early steps of tumor metastasis, it is likely that ERp5 plays a role in initial tumor migration and invasion into neighboring tissues. The effect of ERp5 on these initial steps was therefore evaluated in a transwell cell migration assay in vitro. Human ERp5 was cloned into a retroviral vector with its expression driven by the LTR promoter and was subsequently introduced into human breast cancer cell lines MCF7 and MDA-MB-436 to establish cells stably expressing ERp5. Overexpression of ERp5 in both cell lines was confirmed by immunoblot with an ERp5 antibody (data not shown). Overexpression of ERp5 in MCF7 cells results in a migratory phenotype, whereas cells containing a control vector are nonmigratory. In addition, MDA-MB-436 cells that stably express ERp5 show more than an 80% increase in the number of migrated cells compared with the cells containing a control vector (Fig. 2A). Similar results were obtained in an invasion assay in which cells must penetrate Matrigel during migration. The phenotype of MCF7 cells that overexpress ERp5 changes from noninvasive to invasive, whereas MDA-MB-436 cells overexpressing ERp5 display more than a 60% increase in the number of invasive cells (Fig. 2B). Similar results were obtained in 168FARN cells as well. Taken together, these results demonstrate that ERp5 promotes tumor cell migration and invasion.
Fig. 2.
ERp5 promotes tumor cell migration and invasion in vitro. MDA-MB-436 and MCF7 cells were serum-starved for 24 h before the transwell assay. Complete medium was added to the bottom wells of the transwell chambers. Cells that migrated to the lower surface of the filters were fixed and counted 12 h after they were added to the upper chamber. Stable ERp5 overexpression in human breast cancer MDA-MB-436 cells causes 100% more migrated cells (A) and 60% more invaded cells (B) in a transwell assay compared with these cells with a control vector. The expression of ERp5 in human breast cancer MCF7 cells causes a phenotype change from nonmigratory and noninvasive to significant migration (A) and invasion (B). Suppression of cell surface disulfide exchange blocks cell migration and invasion. Treatment of cells with 1 mM DTNB reduces migration of MDA-MB-436 cells by 40%, reduces migration of MDA-MB-436 cells overexpressing ERp5 by >80%, and reduces migration of MCF7 cells overexpressing ERp5 by >60% compared with DMSO treatment as a control (C). DTNB (1 mM) has a similar effect on cell invasion: MDA-MB-436 cell invasion is inhibited by 30%; invasion by MDA-MB-436 cells overexpressing ERp5 is inhibited by 80%; and invasion by MCF7 cells overexpressing ERp5 is inhibited by >80% (D). DTNB (1 mM) has no effect on cell growth (E).
It has been shown that some disulfide isomerases regulate cell surface protein function through disulfide exchange at the cell surface (38–41). The role of disulfide exchange in cell migration and invasion was examined by treatment of cells with 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), an activated disulfide which reacts with free thiols and thereby inhibits cell surface thiol exchange (38–41). Treatment with 1 mM DTNB reduces migration of MDA-MB-436 parent and ERp5-overexpressing cells by 40% and 80%, respectively. Furthermore, migration of MCF7 cells that overexpress ERp5 is reduced by >60% upon treatment with 1 mM DTNB (compared with DMSO-treated cells) (Fig. 2C). Similarly, 1 mM DTNB inhibits invasion of MDA-MB-436 parent and ERp5 overexpressing cells by 30% and 80%, respectively, and inhibits invasion of MCF7 cells overexpressing ERp5 by >80% (Fig. 2D). In contrast, 1 mM DTNB has no effect on cell growth (Fig. 2E). The effect of inhibiting cell surface disulfide exchange is more prominent in cells that overexpress ERp5, which may reflect a dominant effect of thiol exchange on cell migration and invasion when ERp5 is expressed at high levels. These results not only show that cell surface disulfide exchange is critical for the activity of ERp5 on cell migration and invasion, they also suggest that inhibition of disulfide exchange may be beneficial in the treatment of tumor metastasis.
Activation of the ErbB2 Pathway Is Required for the Metastatic Activity of ERp5.
Because ERp5 is known to modulate cell surface receptors (38, 40), we next examined its effect on candidate genes known to play an important role in breast cancer development. ErbB2(HER2/NEU) is one such gene. It is a member of subclass I of the receptor tyrosine kinase superfamily (42), which when activated leads to the stimulation of many cell signaling pathways including PI3K-AKT pathways, the mitogen-activated protein kinase pathway, and the SRC tyrosine kinase pathway (43, 44). The expression of ErbB2 is altered in many epithelial tumors such as breast, ovarian, gastric, and non-small-cell lung cancers (45–48). ErbB2 expression has also been shown to be inversely correlated with the prognosis of breast cancer, indicating an important role in tumor metastasis (45, 49, 50).
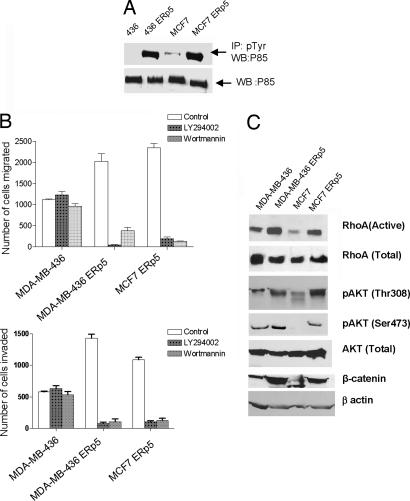
Overexpression of ERp5 did not affect ErbB2 expression levels in MCF7 and MDA-MB-436 cells as determined by immunoblot analysis (Fig. 3A). However, overexpression of ERp5 led to activation of ErbB2 as determined by immunoprecipitation and Western blot analysis of cell extracts from MCF7 and MDA-MB-436 cells. The level of ErbB2 phosphorylation is significantly higher in MCF7 and MDA-MB-436 cells expressing ERp5 than in cells with an empty vector (Fig. 3B). Similar results were obtained in 168FARN cells as well. ErbB2 triggers signal transduction by ligand-dependent heterodimerization or ligand-independent homodimerization (42, 51, 52). Consistent with this mechanism, ERp5 expression induces dimerization of ErbB2 in both MCF7 and MDA-MB-436 cells (Fig. 3C). To determine whether ErbB2 is required for the activity of ERp5 in tumor cell migration and invasion, a short hairpin RNA (shRNA) construct was used to knock down expression of ErbB2 in MCF7 and MDA-MB-436 cells that overexpress ERp5. Western blot analysis demonstrated that the shRNA is effective in suppressing ErbB2 when compared with a control shRNA (Fig. 3D). Migration and invasion assays were then performed to determine whether ERp5 activity depends on ErbB2 expression. The number of migrated MCF7 and MDA-MB-436 cells that express both ERp5 and the shRNA against ErbB2 is reduced by >90% and 85%, respectively, in comparison to cells expressing ERp5 and a control shRNA (Fig. 3D). Similar results were obtained in the invasion assay: invasive MCF7 and MDA-MB-436 cells that express both ERp5 and the shRNA against ErbB2 are reduced by >95% compared with cells that express ERp5 and a control shRNA (Fig. 3D). Interestingly, the effect of ErbB2 knockdown on migration and invasion in MCF7 and MDA-MB-436 cells overexpressing ERp5 is more prominent than in cells containing the control vector (Fig. 3 C and D). This may again be due to a dominant effect of ERp5 on migration and invasion. These results demonstrate that activation of the ErbB2 pathway is required for the effects of ERp5 on tumor migration and invasion.
Fig. 3.
ERp5 activates ErbB2 pathway. (A) The expression level of total ErbB2 does not change after ERp5 overexpression in human breast cancer MDA-MB-436 and MCF7 cells. (B) Immunoprecipitation of phosphorylated ErbB2 demonstrates ErbB2 is activated by ERp5 overexpression in MDA-MB-436 and MCF7 cells. (C) Immunoblots under nonreducing condition show that ERP5 promotes dimerization of ErbB2 in MDA-MB-436 and MCF7 cells. (D) Knockdown of ErbB2 by a specific shRNA reduces the effect of ERp5 on migration and invasion in MDA-MB-436 and MCF7 cells.
ERp5 Activates PI3K and Its Downstream Signaling Molecules RhoA and β-Catenin.
Activation of ErbB signaling results in a number of downstream events, including activation of the PI3K signaling cascade. The effects of ERp5 on PI3K activity were therefore examined by measuring the phosphorylation status of the p85 subunit of PI3K, which correlates with PI3K kinase activity in vivo (53, 54). p85 phosphorylation was determined by immunoprecipitation using a phosphotyrosine antibody followed by Western blot analysis by using an antibody specific for p85. The level of active PI3K is significantly higher in MDA-MB-436 cells expressing ERp5 than in those cells containing a control vector, whereas the total amount of PI3K is similar in these samples, demonstrating that PI3K is activated by ERp5 (Fig. 4A). The PI3K inhibitors LY294002 and wortmannin were then used to examine whether PI3K is required for the effects of ERp5 on migration and invasion. As shown in Fig. 4B, the addition of 10 μM LY294002 reduces migration of MCF7 and MDA-MB-436 cells that stably express ERp5 by >90%; likewise, treatment with 0.1 μM wortmannin reduces migration by >85% in these cells (Fig. 4B) (these PI3K inhibitors were not tested on MCF7 control cells because these cells have minimal migration and invasion capacity in the absence of ERp5 overexpression). Both compounds have no effect on cell growth at the concentrations that were used in the experiments (data not shown). LY294002 also reduced invasion of MCF7 and MDA-MB-436 cells that stably express ERp5 by >85% and 95%, respectively; wortmannin had similar effects (Fig. 4B). Taken together, these results strongly suggest that the PI3K pathway is activated by ERp5 and is required for the induction of cell migration and invasion by ERp5.
Fig. 4.
ERp5 activates PI3K pathway. (A) Immunoprecipitation of phosphorylated PI3K in MDA-MB-436 and MCF7 cells demonstrates that ERp5 overexpression activates PI3K, whereas it has no effect on the expression level of total PI3K. (B) PI3K inhibitors Ly294002 (10 μM) and wortmannin (0.1 μM) reverse the effect of ERp5 in the migration and invasion assay in MDA-MB-436 and MCF7 cells. (C) Stable ERp5 overexpression in MDA-MB-436 and MCF7 cells leads to the activation of RhoA and Akt but has no effect on the total protein expression level of these two proteins. The activation of Akt subsequently up-regulates β-catenin.
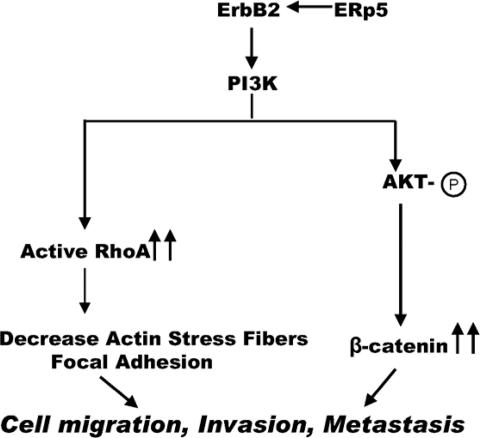
The activation of downstream signaling molecules in the PI3K pathway by ERp5 was examined next. RhoA is one of the downstream targets of PI3K and activation of RhoA is known to promote cell migration (55). Affinity precipitation of active RhoA by Rhotekin binding domain beads in the cell extracts of MCF7 and MDA-MB-436 cells overexpressing ERp5 revealed higher expression levels of the active form of RhoA when compared with cells containing a control vector; the total RhoA levels are similar in the presence or absence of ERp5 (Fig. 4C). Another major downstream target of activated PI3K is the serine-threonine kinase Akt, which is activated when it is phosphorylated at Thr308 and Ser473 (56, 57). As shown in Fig. 4C, the total protein levels of Akt are similar in cells stably expressing either ERp5 or a control vector. However, the phosphorylated forms (active forms) of Akt at both amino acids 308 and 473 are significantly increased in cells expressing ERp5 (Fig. 4C). To further explore the downstream consequences of PI3K/Akt activation, we examined the activation of β-catenin by Akt. Western blot analysis confirmed that the expression levels of β-catenin are significantly increased in MCF7 and MDA-MB-436 cells stably expressing ERp5 (Fig. 4C). These results suggest that activation of the PI3K pathway is responsible for tumor cell migration and invasion induced by ERp5 (Scheme 1).
Scheme 1.
The molecular mechanisms of ERp5 in promoting tumor invasion and metastasis.
ERp5 Is Up-Regulated in Invasive Clinical Breast Cancer Samples.
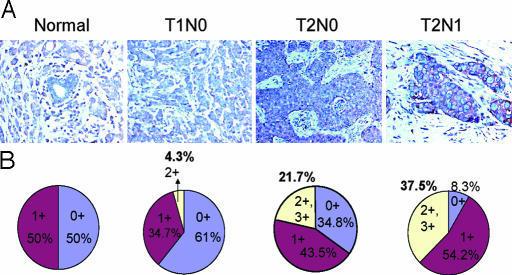
Finally, tissue microarrays were used to examine the expression of ERp5 in clinical breast cancer samples at various stages. Immunostaining of ERp5 showed that normal breast ducts as well as early stages of breast cancer (including ductal carcinoma in situ and T1N0 samples) exhibited little or no ERp5 expression. However, ERp5 expression was increased in more advanced stages of invasive ductal carcinoma (T2N0 and T2N1) (Fig. 5A): 60–100% of carcinoma cells were stained exclusively in the cytoplasm. None of the normal breast samples and only 4.3% of the T1N0 samples received 2+ scores, whereas 21.7% of the T2N0 samples and 37.5% of the T2N1 samples scored 2+ or higher (Fig. 5B), demonstrating that ERP5 is up-regulated in more invasive breast cancer samples. The P value of Fisher's exact test is 0.0034, indicating that there is indeed an association between the staining results and disease progression. These results further support the role of ERp5 in tumor progression. Interestingly, macrophages were strongly stained and endothelial cells were also moderately stained with ERp5 antibody, which may correlate with their enhanced mobility relative to normal epithelial cells.
Fig. 5.
ERp5 expression is up-regulated in the clinical tumor samples of invasive ductal carcinoma. (A) Immunostaining with an ERp5 antibody of the tumor tissue array containing normal breast tissues and breast ductal carcinoma at various stages was scored on a scale of 0 to 3: 0 represents no staining, and 3+ represents the strongest staining. ERp5 expression is elevated in T2N0 and T2N1 compared with normal breast duct and T1N0 tumors. (B) The percentage of tumor samples on the array with different scores was tabulated. The percentage of tumor samples with 2+ scores is higher in invasive breast cancer: 21.7 and 37.5% of T2N0 and T2N1 stage, respectively, compared with 0% in normal breast ductal structure and 4.3% in T1N0 tumors.
The immunostaining results with clinical breast cancers suggest that tumors with no ErbB2 amplification can still be aggressive due to the activation of ErbB2 by mechanisms other than gene amplification. Therefore, the activation of the ErbB2 pathway, rather than the amplification or overexpression of ErbB2, may be another indication of the need for chemotherapies targeting ErbB2. The finding that tumor cells overexpressing ERp5 are sensitive to ErbB2 and PI3K inhibition in migration and invasion assays (Figs. 3D and 4B) suggests that chemotherapy targeting ErbB2 and PI3K may be effective in patients in which ERp5 expression is up-regulated.
Conclusion
In summary, we have applied a novel gain-of-function in vivo selection system to identify the thiol isomerase ERp5 as an activator in the early steps of metastasis. ERp5 promotes tumor cell migration and invasion by activating the ErbB2/PI3K signaling pathway (Scheme 1). To our knowledge, this is the first report demonstrating the amplification of ErbB2/PI3K signaling by a disulfide isomerase. The activation of ErbB2 by ERp5 leads to the activation of PI3K and, subsequently, RhoA and β-catenin, which have been shown to play important roles in tumor invasion and metastasis (Scheme 1). Together with the finding that β-catenin was also identified in this forward genetic screen, these results demonstrate that the activation of ErbB2/PI3K pathway is critical in breast cancer metastasis, especially in the early stages of the process. Besides its location in the endoplasmic reticulum, ERp5 also exists on the cell surface based on a previous publication (39) and our own observation (unpublished data). Because the majority of cell surface proteins contains at least one disulfide bond, these proteins may require disulfide isomerases for their maturation. Alternatively, cell surface disulfide exchange may play an important role in the activation and regulation of cell signaling pathways (58). In either case, the selective inhibition of cell surface thiols may serve as a potential therapeutic approach to the suppression of tumor progression and metastasis.
Materials and Methods
Plasmids and Reagents.
Human and mouse ERp5 genes were cloned into the pBabe-puromycin plasmid and expressed by the LTR promoter. shRNA against ErbB2 and control shRNA were purchased from Open Biosystems (Huntsville, AL). Anti-ERp5 antibody was prepared as previously described (39). Anti-human Akt, phospho-Akt-(Ser-473), phospho-Akt-(Thr-308), and PI3K inhibitor Ly294002 were purchased from Cell Signaling Technology (Beverly, MA); β-catenin was purchased from BD Transduction Laboratories (San Jose, CA); and ErbB2, phosphorylated tyrosine, clone 4G10, PI3-kinase p85, PI3K inhibitor wortmannin, and the RhoA activation assay kit were purchased from Upstate Cell Signaling Solutions (Lake Placid, NY).
Cell Culture.
The mouse breast cancer cell line 168FARN and human breast cancer cell line MCF7 and MDA-MB-436 were cultured in DMEM with 10% FBS. All cells were incubated at 37°C with 5% CO2.
Recombinant Retroviruses and Viral Infection.
Virus production and infection were performed as previously described (21).
Immunoblotting and Immunochemistry.
Immunoblotting and immunoprecipitation were performed as previously described (21). Rho activity was measured by affinity precipitation of GTP-Rho with Rhotekin-agarose beads (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer's instructions.
Tissue microarrays of formalin-fixed, paraffin-embedded samples of human breast epithelium representing normal as well as different stages of tumor progression were prepared at Fox Chase Cancer Center. A total of 16 normal breast areas, 23 T1N0, 23 T2N0, and 24 T2N1 breast cancers cases, two cores for each sample, represented by a total of 172 cores were used in the tissue microarrays. Immunostaining was performed as previously described (59). ERp5 antibody (1:100) was used. Cytoplasmic staining was scored according to the stain intensity from 0 (no stain), 1 (marginal to moderate stain), 2 (moderate to intense stain), and 3 (strong intense stain).
Invasion and Migration Assays.
Matrigel invasion assays were performed as described (60) using transwell chambers (8-μM pore size; Costar). In some experiments, cells were preincubated with the PI3-kinase inhibitors LY294002 (100 μM), wortmannin (100 nM), DTNB (1 mM), or control medium for 30 min. After detachment with trypsin, cells were washed with PBS and resuspended in serum-free medium, and 250-μl cell suspension (2 × 105 cells/ml) was added to the upper chamber. DTNB (1 mM) was added to the cell suspension. Images of three different ×10 fields were captured from each membrane, and the number of invading cells was counted. The mean of triplicate assays for each experimental condition was used. For migration assays, the same procedure was performed except that transwell chambers were not coated with matrigel.
Functional Screen in an Orthotopic Animal Model.
To establish luciferase positive cancer cells, the luciferase gene from pGL3 (Promega, San Luis Obispo, CA) was cloned into pBabe-puromycin. 168FARN cells were infected with retroviruses containing luciferase to establish 168FARN-Luc cells that stably express luciferase. To establish the animal model for the forward genetic screen, 1 × 106 168FARN-Luc cells were orthotopically transplanted into the mammary fat pads of 10-week-old BALB/cJ mice or injected intravenously by tail vein. Mice bearing luciferase positive tumors were imaged 7–8 weeks after transplantation on the Xenogen IVIS system (Xenogen Corporation, Hopkinton, MA). Mice injected by tail vein were imaged 4–5 weeks after transplantation to monitor tumor growth in the lung. To perform the forward genetic screen, retroviruses containing a cDNA library were generated as described (23). 168FARN-Luc cells were infected with the viruses containing the library at 1 multiplicity of infection. 168FARN-Luc cells (1 × 106) containing the library were transplanted into the mammary fat pad of each mouse. Lung metastasis nodules were isolated 6–7 weeks after transplantation. Genomic DNA of metastatic cells was isolated with the Genomic DNA Purification kit according to supplier's instruction (Qiagen, Valencia, CA). PCRs were performed with the Expand High Fidelity kit (Roche Diagnostics, Indianapolis, IN) with primers as described (23). PCR products were cloned with a TA cloning kit (Invitrogen) and sequenced. To validate the hits from the screen, 168FARN-Luc cells were infected with retroviruses containing the full-length mouse ERp5 behind the LTR promoter; 1 × 106 cells were transplanted into the mammary fat pads of mice and imaged 6–8 weeks after transplantation.
Acknowledgments
We thank Dr. Fred Miller for 168FARN cells and Dr. George Daley for the cDNA library. Q.H. is supported by the Breast Cancer Alliance, the Pardee Foundation, and the Commonwealth Universal Research Enhancement Program (Pennsylvania Department of Health). We also acknowledge support for this work by the Novartis Research Foundation.
Abbreviations
- PI3K
phosphoinositide 3-kinase
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid).
Footnotes
The authors declare no conflict of interest.
References
- 1.Fidler IJ. Nat Rev Cancer. 2003;3:1–6. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Gupta GP, Massague J. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Steeg P. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Welch DR, Steeg PS, Rinker-Schaeffer CW. Breast Cancer Res. 2000;2:408–416. doi: 10.1186/bcr87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers AF, Naumov GN, Vantyghem SA, Tuck AB. Breast Cancer Res. 2000;2:400–407. doi: 10.1186/bcr86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA. Proc Natl Acad Sci USA. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 9.Clark EA, Golub TR, Lander ES, Hynes RO. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 10.Brinckerhoff CE, Matrisian LM. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 11.Yu Q, Stamenkovic I. Clin Exp Metastasis. 2004;21:235–242. doi: 10.1023/b:clin.0000037705.25256.d3. [DOI] [PubMed] [Google Scholar]
- 12.Cairns RA, Khokha R, Hill RP. Curr Mol Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- 13.Cavallaro U, Christofori G. Ann NY Acad Sci. 2004;1014:58–66. doi: 10.1196/annals.1294.006. [DOI] [PubMed] [Google Scholar]
- 14.Grimm S. Nat Rev Genet. 2004;5:179–189. doi: 10.1038/nrg1291. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta R, Kaykas A, Moon RT, Perrimon N. Science. 2005;308:826–832. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- 16.Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- 17.Friedman A, Perrimon N. Curr Opin Genet Dev. 2004;14:470–476. doi: 10.1016/j.gde.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Paddison PJ, Silva JM, Conklin DS, Schlabach M, Li M, Aruleba S, Balija V, O'Shaughnessy A, Gnoj L, Scobie K, et al. Nature. 2004;428:427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- 19.Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, et al. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 20.Kolfschoten IG, van Leeuwen B, Berns K, Mullenders J, Beijersbergen RL, Bernards R, Voorhoeve PM, Agami RA. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q, Raya A, DeJesus P, Chao S, Quon KC, Caldwell JS, Chanda SK, Izpisua-Belmonte JC, Schultz PG. Proc Natl Acad Sci USA. 2004;101:3456–3461. doi: 10.1073/pnas.0308562100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aslakson CJ, Miller FR. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 23.Koh EY, Chen T, Daley GQ. Nucleic Acids Res. 2002;30:e142p1–e142p7. doi: 10.1093/nar/gnf142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 25.Bienz M, Clevers H. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 26.Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Laure L. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 28.Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Cancer Res. 2001;61:3465–3471. [PubMed] [Google Scholar]
- 29.Naishiro Y, Yamada T, Takaoka AS, Hayashi R, Hasegawa F, Imai K, Hirohashi S. Cancer Res. 2001;61:2751–2758. [PubMed] [Google Scholar]
- 30.Brabletz T, Hermann K, Jung A, Faller G, Kirchner T. Am J Pathol. 2000;156:865–870. doi: 10.1016/s0002-9440(10)64955-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suarez A, Armas A. Am J Pathol. 1999;155:527–536. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Z, Pan J, Chu B, Wong Y, Cheung AL, Tsao S. Human Pathol. 1999;30:458–466. doi: 10.1016/s0046-8177(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 33.Ellgaard L, Ruddock LW. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari DM, Soling H. Biochem J. 1999;339:1–10. [PMC free article] [PubMed] [Google Scholar]
- 35.Sevier CS, Kaiser CA. Nat Rev Mol Cell Biol. 2002;3:836–846. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 36.Woycechowsky KJ, Raines RT. Curr Opin Chem Biol. 2000;4:533–539. doi: 10.1016/s1367-5931(00)00128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulleid NJ. Curr Biol. 2003;13:R380. doi: 10.1016/s0960-9822(03)00314-2. [DOI] [PubMed] [Google Scholar]
- 38.Turano C, Coppari S, Altieri F, Ferraro A. J Cell Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 39.Jordan PA, Stevens JM, Hubbard GP, Barrett NE, Sage T, Authi KS, Gibbins JM. Blood. 2005;105:1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 40.Ellerman DA, Myles DG, Primakoff P. Dev Cell. 2006;10(6):831–837. doi: 10.1016/j.devcel.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Markovic I, Stantchev TS, Fields KH, Tiffany LJ, Tomic M, Weiss CD, Broder CC, Strebel K, Clouse KA. Blood. 2004;103:1586–1594. doi: 10.1182/blood-2003-05-1390. [DOI] [PubMed] [Google Scholar]
- 42.Hynes NE, Lane HA. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 43.Schlessinger J. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 44.Yarden Y, Silwkowski MX. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 45.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 46.Falck VG, Gullick WJ. J Pathol. 1989;159:107–111. doi: 10.1002/path.1711590204. [DOI] [PubMed] [Google Scholar]
- 47.Tateishi M, Ishida T, Mitsudomi T, Kaneko S, Sugimachi K. Eur J Cancer. 1991;27:1372–1375. doi: 10.1016/0277-5379(91)90012-3. [DOI] [PubMed] [Google Scholar]
- 48.Stenman G, Sandros J, Nordkvist A, Mark J, Sahlin P. Genes Chromosomes Cancer. 1991;3:128–135. doi: 10.1002/gcc.2870030208. [DOI] [PubMed] [Google Scholar]
- 49.Yu D, Wang SS, Dulski KM, Tsai CM, Nicolson GL, Hung MC. Cancer Res. 1994;54:3260–3266. [PubMed] [Google Scholar]
- 50.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, et al. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Weiner DB, Liu J, Cohen JA, Williams WV, Greene MI. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- 52.Schlessinger J. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 53.Fruman DA, Meyers RE, Cantley LC. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 54.Pleiman CM, Hertz WM, Cambier JC. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- 55.Raftopoulou M, Hall A. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Rane MJ, Coxon PY, Powell DW, Webster R, Klein JB, Pierce W, Ping P, McLeish KR. J Biol Chem. 2001;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 57.Testa JR, Bellacosa A. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jordan P, Gibbins JM. Antioxid Redox Signal. 2006;8:312–324. doi: 10.1089/ars.2006.8.312. [DOI] [PubMed] [Google Scholar]
- 59.López de Cicco R, Bassi DE, Zucker S, Seidah NG, Klein-Szanto AJ. Cancer Res. 2005;65:4162–4171. doi: 10.1158/0008-5472.CAN-04-2820. [DOI] [PubMed] [Google Scholar]
- 60.Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]