Abstract
Four species in the ELEGANS group of subgenus the Caenorhabditis are distinguished by two very different mating systems: androdioecy in C. elegans and Caenorhabditis briggsae with males and self-fertilizing hermaphrodites and dioecy in Caenorhabditis remanei and Caenorhabditis sp. strain CB5161 with males and females. Using chemotaxis assays, we demonstrate that females secrete a potent sex pheromone that attracts males from a distance, whereas hermaphrodites do not. The female sex pheromone is not species-specific, with males of all four species attracted to both the C. remanei and Caenorhabditis sp. female sex pheromones. The pheromone is, however, sex-specific, with only females secreting the pheromone and attracting only males. Furthermore, the sex pheromone is stage-specific, with female secretion and male detection of the pheromone beginning near adulthood. Females lose their attractiveness immediately after mating but regain it several hours after mating ceases. Finally, the female somatic gonad is required for sex-pheromone production, and the male-specific cephalic neurons (CEM) are required for male response.
Keywords: chemotaxis, cephalic neuron, sex attractant, nematode mating
Direct observations show that the mating efficiency of the androdioecious species Caenorhabditis elegans is poor compared with the related dioecious species Caenorhabditis remanei and that both C. remanei and C. elegans males find C. remanei females more attractive than C. elegans hermaphrodites (1). Perhaps during evolution C. elegans hermaphrodites lost the ability to secrete a sex pheromone that is still secreted by C. remanei females and this pheromone still attracts C. elegans males. The focus of the present study is to definitively determine the presence of a sex pheromone in dioecious Caenorhabditis that is absent in related hermaphroditic species.
Although C. elegans is a well-studied organism, it is yet debatable whether there is a true sex pheromone in this species, although apparently males can sense a hermaphrodite-derived chemical (2, 3). If hermaphrodites do indeed secrete a true sex pheromone, it is surprising that males do not rapidly and consistently chemotax to hermaphrodites, because C. elegans is capable of chemotaxing to both water-soluble and volatile chemicals (4). In fact, detection of sex pheromones secreted by other nematode species has been fairly straightforward (5), with the secreting female commonly establishing a gradient of attractant readily followed by conspecific males.
Females, in contrast to self-fertilizing hermaphrodites, must mate with males to reproduce, and natural selection should favor those females that do so rapidly and consistently after reaching adulthood. Here, we demonstrate that females from the dioecious species C. remanei and Caenorhabditis sp. strain CB5161 attract conspecific males from a distance, whereas hermaphrodites from the androdioecious species C. elegans and Caenorhabditis briggsae do not. We then obtain, by soaking females, a supernatant solution that can attract males from a distance. Using this solution and standard chemotaxis assays (4), we explore the species, sex, and stage specificity of the Caenorhabditis sex pheromone.
Results
Females Attract Conspecific Males.
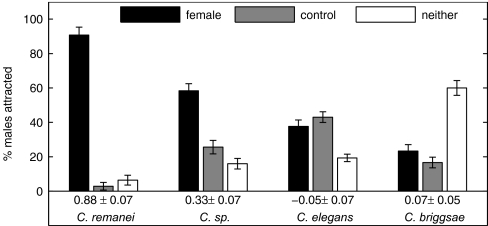
Individual virgin females and hermaphrodites were assayed for their ability to attract conspecific males. Results are summarized in Fig. 1, for which the chemotaxis index (CI) is defined as the difference between the number of worms scored on the test and the control spots, divided by the total number of animals tested. Evidently, females from the dioecious species C. remanei and Caenorhabditis sp. attract conspecific males from a distance, whereas no evidence for a similar potent attraction is observed for the hermaphroditic species (C. elegans and C. briggsae).
Fig. 1.
Only females attract conspecific males. Shown is the percentage of males attracted to females (a bacterial spot on which there is a conspecific virgin female or hermaphrodite), control (a bacterial spot with no worm), or neither (neither of the spots). The CI is shown below the grouped bars. One hundred twenty to 300 males were assayed from each species. Only the conspecific male attraction to the C. remanei and Caenorhabditis sp. female is significant at P < 0.0001.
Only Females Produce Attractant, and Only Males Respond.
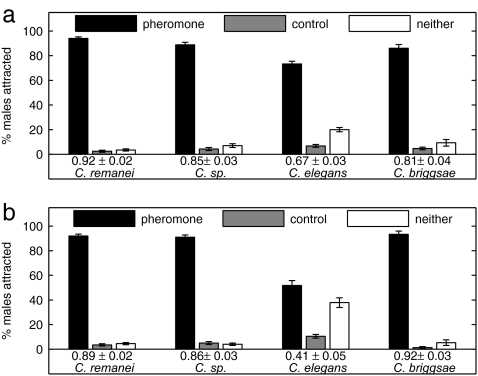
Supernatant solutions obtained from soaking of virgin C. remanei (Fig. 2a) and Caenorhabditis sp. (Fig. 2b) females were assayed for pheromone activity. Males of all four species are attracted to both the C. remanei and Caenorhabditis sp. pheromones. Experiments in which the C. remanei pheromone was placed on the Petri dish cover instead of the agar surface still attracted C. remanei males (200 males tested, CI = 0.87 ± 0.02), indicating that the pheromone is volatile and can be sensed through the air.
Fig. 2.
Female pheromone attracts males of all four species. Shown is the percentage of males scored on the pheromone spot, control spot, or neither spot. The CI is shown below the grouped bars. Three hundred to 400 males were assayed from each species. All attractions are significant at P < 0.0001. (a) C. remanei pheromone; (b) Caenorhabditis sp. strain CB5161 pheromone.
To determine whether production of, and response to, the attractant is sex-specific, supernatant solutions were obtained from overnight soaking of both sexes of C. elegans and C. remanei to obtain four different supernatant solutions. The results are presented succinctly by using the CI ± SEM in Table 1. The supernatant solutions obtained from C. elegans males, C. elegans hermaphrodites, and C. remanei males have only weak or no attraction. In contrast, the supernatant from C. remanei females strongly attracts C. remanei and C. elegans males.
Table 1.
Only females secrete pheromone, and only males are attracted
| Supernatant | Animals assayed for attraction to supernatant |
|||
|---|---|---|---|---|
|
C. elegans |
C. remanei |
|||
| Male | Hermaphrodite | Male | Female | |
| C. elegans male | 0.02 ± 0.07 | 0.02 ± 0.07 | 0.09 ± 0.02 | 0.03 ± 0.07 |
| C. elegans hermaphrodite | 0.11 ± 0.04 | 0.02 ± 0.05 | 0.09 ± 0.03 | −0.04 ± 0.06 |
| C. remanei male | 0.06 ± 0.05 | 0.14 ± 0.02 | 0.13 ± 0.02 | 0.21 ± 0.05 |
| C. remanei female | 0.75 ± 0.03* | 0.09 ± 0.05 | 0.94 ± 0.01* | 0.13 ± 0.02 |
Supernatant solutions from C. elegans or C. remanei males or hermaphrodites/females are used to attract C. elegans or C. remanei males or hermaphrodites/females. The tabulated results are CI ± SEM. Two hundred worms were assayed for each test.
*The strongest attraction observed is of C. elegans and C. remanei males to the C. remanei female supernatant (P < 0.0001).
To test further for the possibility that C. elegans hermaphrodites secrete both attractive and repulsive signals, we have mixed supernatant solutions obtained from C. remanei females and C. elegans hermaphrodites in a 1:1 ratio. Both C. remanei and C. elegans males were still attracted to the mixed-supernatant solution (C. remanei males had a CI of 0.96 ± 0.02 versus 0.97 ± 0.02 for pheromone mixed with M9; C. elegans males had an identical CI of 0.73 ± 0.04 in test and control experiments), indicating the absence of any C. elegans hermaphrodite secretions that can significantly degrade the attractiveness of the Caenorhabditis sex pheromone or repel males.
Plausibly, hermaphrodites secrete pheromone only under very specific environmental or physical conditions. We have tested C. elegans hermaphrodite supernatant obtained under the following conditions and from the following strains and have found no significant attraction: hermaphrodites starved for 1 or 2 days; hermaphrodites incubated at the high temperature of 30°C for 3 h; sperm-depleted hermaphrodites; hermaphrodites exiting from the dauer stage (using daf-4 temperature-sensitive dauer mutants); CB4108:fog-2(q71)V, which is a mutation that blocks hermaphrodites from producing self-sperm, essentially converting hermaphrodites into females; and the Hawaiian wild-type race CB4856, distinguished by its ability to maintain a large number of males in a population (M. Hammarlund and E. M. Jorgensen, personal communication).
Attractant Production and Response Begin at Sexual Maturity.
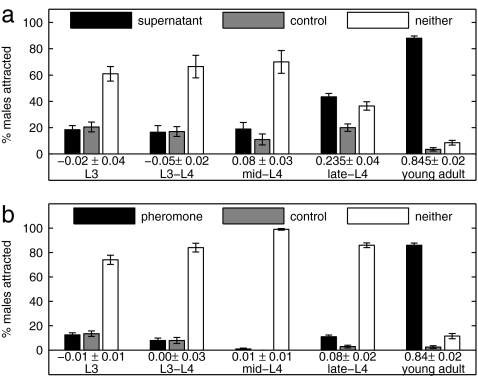
C. remanei worms were synchronized at the L1 larval stage and tested at various time points after release from L1 arrest to determine the life stages at which females produce and males are attracted to the sex pheromone. The data for female secretion (Fig. 3a) and male detection (Fig. 3b) of the pheromone demonstrate that secretion and detection are stage-specific, with maximum production and attraction occurring at adulthood, when the worms are ready to mate.
Fig. 3.
Attractant production and response begin at sexual maturity. C. remanei male and virgin female worms were harvested 39, 42, 45, 48, and 51 h after an L1 synchronization, corresponding to L3, L3–L4, mid-L4, late-L4, and young-adult stages, respectively. Shown is the percentage of C. remanei males scored on the supernatant or pheromone spot, control spot, or neither spot. The CI is shown below the grouped bars. Two hundred males were assayed for each tested stage. Only young-adult attraction is significant at P < 0.0001. (a) Staged females provide supernatant solution, and adult males are tested for their attraction. (b) Staged males are tested for their attraction to the standard pheromone solution.
Mated Females Lose Their Attractiveness.
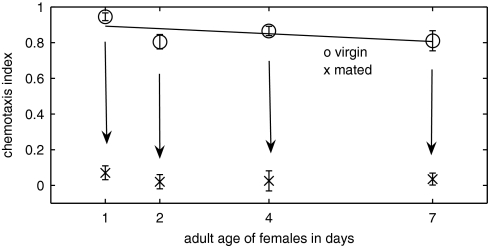
We tested young-adult C. remanei males for their attraction to supernatant solution obtained from either (i) 20 virgin adult C. remanei females on their first, second, fourth, and seventh days of adulthood or (ii) 20 virgin adult C. remanei females mated with 40 C. remanei males for 3 h on the same days. In Fig. 4, the circles and crosses indicate the CI of the supernatants obtained from the virgin and mated females, respectively. We observe only a slight decline in attractiveness as the females aged. The arrows drawn on the figure emphasizes our main result: although virgin females of adult age up to 7 days old secrete sex pheromone, mating with males at any age immediately results in females losing their attractiveness to males.
Fig. 4.
Mated females lose their attractiveness. Pheromone solution is obtained from 20 C. remanei females of adult age (1, 2, 4, and 7 days old), and C. remanei males are tested for their attraction to the female supernatants. The CI is plotted versus the adult age of the harvested females. Two hundred males were assayed for each data point. The circles correspond to the data from virgin females, and the crosses correspond to data from females that were mated for 3 h immediately before harvesting. The virgin-female data are fit by a least-squares line. The arrows highlight that virgin females lose their attractiveness soon after mating.
Mated Females Regain Attractiveness After Cessation of Mating.
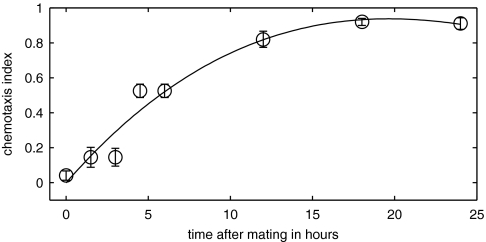
Twenty virgin adult C. remanei females were mated with 40 C. remanei males for 3 h. Starting from between 0 and 24 h after mating, a supernatant solution was obtained by soaking the mated females for 6 h. In Fig. 5, we observe that females begin to regain their attractiveness several hours after the cessation of mating, with the supernatant solution regaining maximum attractiveness 12–18 h postmating. Twelve hours postmating, females still have male sperm in their spermatheca and continue to lay fertilized eggs.
Fig. 5.
Mated females regain attractiveness after cessation of mating. C. remanei males are tested for their attraction to a supernatant solution obtained from mated C. remanei females from 0 to 24 h after mating. Two hundred males were assayed for each data point. The circles indicate the CI of the supernatants obtained from the mated females. The data are fit by a least-squares cubic polynomial.
Female Somatic Gonad Is Required for Pheromone Production.
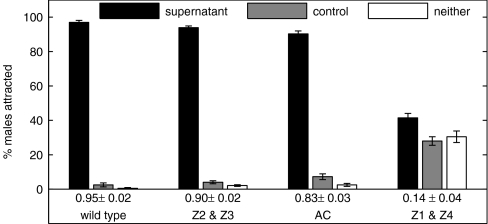
To ascertain which female organ is required for production of the sex pheromone, C. remanei females were subject to laser ablation and, as virgin young adults, individually soaked in 20 μl of M9 at 25°C for 6 h so that the resulting supernatant could be tested for its ability to attract C. remanei males. We ablated the Z1 and Z4 cells (required for development of somatic gonad, germ line, and vulva), the anchor cell (required for vulval induction but not gonad or germ-line development), or the Z2 and Z3 cells (germ-line precursors) (6, 7).
In Fig. 6, we observe that females with ablated Z1 and Z4 cells were significantly less attractive than the wild type (P < 0.0001, two-sample t test), implicating the gonad, germ line, or vulva as the female-specific cells required for sex-pheromone production. Females with ablated Z2 and Z3 cells or ablated anchor cell are still strongly attractive to males, ruling out the germ line and vulva as being required for female attractiveness. Females with ablated Z1 cells lack the anterior gonad arm, whereas females with ablated Z4 cells lack the posterior gonad arm. To further confirm the somatic gonad as the origin of pheromone production, additional experiments individually ablating the Z1 or Z4 cells were performed and resulted in females that were still attractive (data not shown), suggesting that the pheromone can be produced by somatic tissue of either gonad arm. Interestingly, females with a laser-ablated anchor cell are not significantly less attractive than the wild type, suggesting that the release of pheromone does not require the presence of a vulval channel.
Fig. 6.
Laser ablation implicates female somatic gonads in pheromone production. Supernatant solutions are obtained from individual C. remanei females and C. remanei females with the specified cells ablated. Shown is the percentage of C. remanei males scored on the supernatant spot, control spot, or neither spot. The CI is shown below the grouped bars. Four hundred to 800 males were assayed for each ablation type. Supernatant obtained from females with cells Z1 and Z4 ablated are significantly less attractive at P < 0.0001 than that obtained from wild type.
Male Cephalic Neurons (CEMs) Are Required for Pheromone Detection.
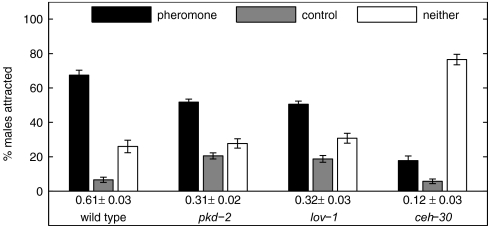
We assayed males with mutations that affect the male-specific CEMs. Fig. 7 shows the results from attraction assays for three C. elegans male mutant strains: pkd-2, lov-1, and ceh-30, together with C. elegans wild-type males. The proteins PKD-2 and LOV-1 are known to be expressed in the male CEMs (8), and the ceh-30 males used here lack all four CEMs without any other obvious mutant phenotype (H. Schwartz and H. R. Horvitz, personal communication). All three mutant strains are significantly less attracted to the pheromone than wild type (P < 0.0001, two-sample t test). Furthermore, the ceh-30 mutants that lack all four CEMs, as confirmed by loss of pkd-2::gfp expression in the head, are less attracted to the pheromone than are the male mutants pkd-2 and lov-1 males (P < 0.0001, two-sample t test) that have intact CEMs. The CI value of 0.12 ± 0.03 for ceh-30 is comparable to the value obtained for C. remanei males to C. remanei male supernatant (see Table 1) and may be attributed to background.
Fig. 7.
Sensory mutants implicate male CEMs in pheromone detection. C. elegans wild-type, pkd-2, lov-1, and ceh-30 mutant males are tested for their attraction to the C. remanei pheromone. Shown is the percentage of males scored on the pheromone spot, control spot, or neither spot. The CI is shown below the grouped bars. Four hundred males of each mutation were assayed. Mutant responses differ from wild type at P < 0.0001.
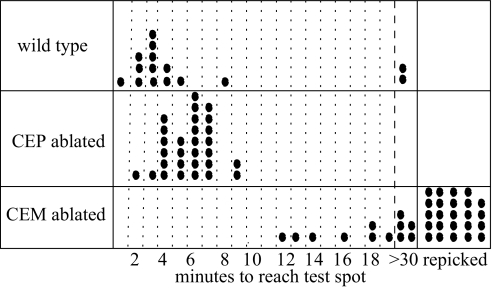
To obtain definitive physical evidence that male-specific CEMs are required for attraction to the sex pheromone, we laser-ablated all four CEMs from 12 C. elegans males and tested them in a single-worm attraction assay. As a control, we also laser-ablated all four cephalic neurons (CEPs) from 15 C. elegans males, leaving the CEMs intact. The CEPs and CEMs are components of the head cephalic sensilla; hermaphrodites only have a single dendrite (CEP), whereas males have two dendrites (CEP and CEM) (9). Each laser-ablated male was assayed one to four times. The results from these assays are presented in Fig. 8, showing that both wild-type and CEP laser-ablated control males were able to chemotax to the pheromone spot with a median time of ≈3 min for wild type and 5 min for control. By comparison, the median time for CEM laser-ablated males that found the pheromone spot was 16 min. Furthermore, a large number of CEM-ablated males would have crawled off the edge of the plate if they were not repicked to the start spot.
Fig. 8.
Laser ablation implicates male CEMs in pheromone detection. Wild-type C. elegans males and C. elegans males with their CEPs or CEMs ablated are scored by the time required to reach a test spot containing C. remanei sex pheromone. Each single-worm attraction assay is indicated by a circle, and the arrival times are rounded to the nearest minute. Males that could not reach the spot within 30 min are scored as “>30”; males that were about to crawl off the slide were repicked to the start position and scored as “repicked.”
Discussion
We began this research by demonstrating that females from the dioecious Caenorhabditis species C. remanei and Caenorhabditis sp. strain CB5161 placed overnight on an agar plate attracted conspecific males from a distance, whereas hermaphrodites from the androdioecious species C. elegans and C. briggsae did not. By soaking females in buffer, we recovered a biologically active supernatant solution that attracted males but did not attract females or hermaphrodites. Surprisingly, the Caenorhabditis pheromone is not species-specific, with both C. remanei and Caenorhabditis sp. strain CB5161 females secreting a pheromone that attracts males of all four Caenorhabditis species. Although there are no apparent premating reproductive barriers between these four species, most hybrid crosses arrest during embryogenesis (10). Postcopulatory, prefertilization interactions between sperm and oocyte, however, seem to be conserved to varying degrees (11).
The Caenorhabditis pheromone is, however, sex-specific, with only females secreting the pheromone and the pheromone attracting only males but not females or hermaphrodites.
Furthermore, stage specificity exists in both female secretion and male detection of the female sex pheromone. Pheromone secretion by females and male chemotaxis toward females requires energy from both females and males, and natural selection should favor female secretion and male detection of sex pheromone only for those worms that are ready to mate. We showed that secretion and detection of the pheromone clearly peaks in adulthood, as expected for a sex pheromone that facilitates immediate mating.
We also showed that virgin adult females continue to secrete pheromone as they age but are no longer attractive immediately after mating. Females, however, regain attractiveness soon after mating ceases. Indeed, it is well known that female behavior and physiology in many insect species change dramatically immediately after mating, and that secretions produced by the accessory reproductive glands of male insects, when transferred to females, trigger these changes (12). In a process called pheromonostasis, females in a number of moth species cease production of a sex pheromone after mating with males. In the corn earworm moth, Helicoverpa zea, pheromonostatic action has been attributed to a 57-aa pheromone-suppression peptide (13) that is present in the male seminal fluid. Indeed, the fruit fly, Drosophila melanogaster, has emerged as an important model for understanding the nature and specific function of a large number of male accessory-gland protein genes (Acps) that are required for this pheromonostatic control (12).
We have further demonstrated that C. remanei females that lack somatic gonads have significantly reduced attraction to males, implicating the gonad in the production of the female sex pheromone. Females missing only a vulva are still attractive to males, suggesting that a pheromone of small molecular size could be released by diffusion through the hypodermis or from the intestinal tract through excretion.
Previous behavioral analysis of worms in which head amphid neurons were laser-ablated showed that these neurons are necessary for chemotaxis behavior, with specific neurons detecting either water-soluble or volatile odorants (4). C. elegans males, compared with hermaphrodites, have 79 additional neurons, of which there is one male-specific sensory neuron in each of the four cephalic sensilla in the head (14). These four male-specific CEMs are similar in structure to the amphid sensory neurons, and their processes extend into the cephalic openings and possess ciliated endings (4). It was previously speculated that these CEMs might be used to sense a sex pheromone released by the C. elegans hermaphrodite (15), although no evidence for a sex pheromone yet existed. Our experiments with male-specific mutants and males without CEMs demonstrate that these neurons indeed are required for males to respond to a volatile sex pheromone of a related Caenorhabditis species. It is not clear whether the four CEMs in C. elegans males are used for any other function, but it would be surprising if they are only used to detect a sex pheromone that is not secreted by conspecific hermaphrodites. Of course, the dioecious ancestor of C. elegans may have secreted a female sex pheromone, and its continued detection by C. elegans males may be an evolutionary relic. Although C. elegans diverged from C. remanei ≈100 million years ago (16), which perhaps is a long time to maintain functional CEMs in the absence of purifying selection, C. elegans hermaphrodites may have only lost the ability to secrete a sex pheromone in the more recent past.
It was previously demonstrated (2, 3) that C. elegans males can detect a hermaphrodite-derived chemical. Simon and Sternberg (2) observed that males reverse direction of movement more frequently in regions of agar conditioned with hermaphrodites and tend to stay longer in these regions. Furthermore, they determined that males introduced at a distance found hermaphrodites somewhat more readily when these hermaphrodites were placed on previously hermaphrodite-conditioned regions. The hermaphrodite vulva is not required for male response. Lipton et al. (3) demonstrated that C. elegans exhibits a leaving behavior that is both sex- and stage-specific. Whereas juveniles and hermaphrodites rarely wander away from food, adult males leave food to wander unless adult hermaphrodites are present.
It is important, however, to distinguish between a hermaphrodite-derived chemical sensed by males and a female-derived chemical that is actively secreted to attract males from a distance. To further clarify the qualitative difference between these chemicals, we have repeated the “attraction assay” of Simon and Sternberg (2) (see their materials and methods section for further details). Ten C. remanei or C. elegans males were individually tested for their ability to find C. remanei females or C. elegans hermaphrodites placed on regions that were previously conditioned for 24 h by uncoordinated mutant females or hermaphrodites. All 10 C. remanei and C. elegans males were able to find the C. remanei female within 100 min, with a median finding time of 5 min for C. remanei males and 30 min for C. elegans males. In contrast, only 5 of 10 C. elegans males and 7 of 10 C. remanei males were able to find the C. elegans hermaphrodite within 100 min. Among those males that did find the hermaphrodite, the median finding time was 43 min for C. elegans males and 53 min for C. remanei males. The consistency and rapidity with which C. remanei males find their conspecific females clearly distinguishes the C. remanei female sex pheromone studied here from the previously studied Simon and Sternberg (2) “mate-finding cue” of C. elegans.
The secretion of a sex pheromone by females is only the first step in the mating process that enables females to attract males from a distance. Our results suggest that male–hermaphrodite couplings lack this first step: certainly, hermaphrodites no longer secrete a sex pheromone of potency comparable to that secreted by females. Still present in the androdioecious species, however, are the subsequent steps in the male–hermaphrodite mating process involving both mechanical and chemical sensory inputs at short range, including the characteristic male behaviors of searching, turning, and location of vulva.
We now speculate about the evolutionary trajectory on which hermaphrodites lost the ability to secrete a sex pheromone. The C. elegans and C. briggsae hermaphrodites are thought to be descendant from a common dioecious ancestor shared with C. remanei and Caenorhabditis sp. strain CB5161 females (17). The most fundamental step in the evolutionary transition from female to hermaphrodite must have been a mutation in which females gained the ability to produce internal sperm. Self-fertilized offspring from females of a previous obligate outcrossing species, however, most likely suffered high inbreeding depression, and natural selection would presumably still favor females that outcrossed in the presence of males. Yet, the ability of females to self-fertilize in the absence of males certainly has a selective advantage to animals colonizing new habitats. Presumably this advantage must have outweighed the disadvantage of accidental inbreeding, and the initial mutation, transforming females into protohermaphrodites, must have been fixed. Perhaps additional mutations that followed fine-tuned the physiology and mechanisms associated with sperm production in these protohermaphrodites. One can imagine that at some point in the evolutionary history of C. elegans and C. briggsae there existed hermaphroditic nematodes that still secreted a sex pheromone to attract males yet were able to self-fertilize when males were absent. Outcrossing may still predominate over selfing, but there may be a critical level of inbreeding depression at which the advantage of a selfing population, namely the elimination of the 2-fold cost of males (18), balances the disadvantage of inbreeding. When inbreeding depression falls below this critical level, perhaps by the purging of recessive deleterious mutations over many generations, any new mutation resulting in the loss of sex-pheromone production by hermaphrodites could become fixed. The ultimate loss of sex-pheromone production transformed C. elegans and C. briggsae into essentially self-fertilizing hermaphroditic species, with relatively low outcrossing rates (19, 20). Whether the continuing presence of males in these species is adaptive (21) or males are evolutionary relics (1) is debatable and directly relevant to the long-standing and important problem as to why sexual reproduction and outcrossing is so widespread in nature (22).
Materials and Methods
Worm Strains.
For wild-type C. elegans, we have used the N2 CB4088:him-5(e1490)V strain with a high incidence of male progeny. We have also tested the mutants EM401:daf-4(m63)III; him-5(e1490)V, which enters dauer stage at 25°C and exits at 20°C; CB4108:fog-2(q71)V, which blocks hermaphrodites from producing self-sperm; and the Hawaiian wild-type race CB4856. The strain EM464 was used for C. remanei; CB5161 was used for Caenorhabditis sp.; and AF16 was used for C. briggsae. Males from AF16 were induced by heat shock and were maintained as a mating stock. In tests of C. elegans CEM mutants, we have made use of PS3151:lov-1(sy552)II; him-5(e1490)V; PT8:pkd-2(sy606)IV; him-5(e1490)V; MT13261:nIs133I; him-5(e1467ts)V; and ceh-30(n3714,n4111)X. To aid in laser ablation of the CEMs and CEPs, KC818:unc-119(e2498)III; him-5(e1490)V; wxEx64[ppkd-2 pkd-2::gfp + M142XbaIselfL]; and BZ555:egIs1 [Pdat-1::GFP] were used.
Single Female or Hermaphrodite Attraction Assay.
To assay the attractiveness of a single virgin female or hermaphrodite, two rubber O-rings of 1-cm diameter are placed on opposite sides of a 5-cm agar plate and 5 μl of OP-50 bacterial solution dropped inside the O-rings. A virgin young female (or hermaphrodite) is trapped inside one of the O-rings on the bacterial spot and stored overnight with attraction assays to be run the following day. For the assay, the female and both O-rings are removed, and a 2-μl drop of 1 M sodium-azide solution is added to both bacterial spots to paralyze males that reach these spots. Ten previously picked males, which were isolated on single plates for several hours, are then placed on the midline between the two bacterial spots, and the plate is covered and stored for ≈30 min. The males are then scored for their residence. Males not found on either spot because they have crawled off the plate or are still wandering are recorded as “neither spot.”
Pheromone-Attraction Assay.
To simplify the attraction assay further, five young virgin C. remanei or Caenorhabditis sp. females were put in 100 μl of M9 buffer for 6 h at 25°C. The supernatant solutions were then removed and assayed for pheromone activity. The assay was performed on a microscope slide with a layer of 1.5% agar placed on top of an empty plate. Two 2-μl drops of 1 M sodium-azide solution were separated by 3 cm and allowed to dry. Then, a drop of 2 μl of supernatant and 2 μl of M9 buffer were separately added on top of the sodium-azide spots. Twenty young-adult males from the species to be tested were placed on the midline between the spots. After 30 min, the paralyzed male worms were scored on the basis of their location. The remaining worms were scored as “neither spot.” To test the volatility of the pheromone, the attraction assay was repeated by placing the 2-μl pheromone-solution drop on the Petri dish cover directly above the sodium-azide spot, preventing direct contact of males with the pheromone solution. Also, in a series of control experiments, we have collected supernatant solutions from C. remanei or C. elegans males, females, or hermaphrodites and tested whether these supernatants can attract C. remanei or C. elegans males, females, or hermaphrodites.
Pheromone-Attraction Assay (Single Worm).
Adult males (possibly laser-ablated) were assayed one at a time for their attraction to a pheromone spot. The assay was performed on a microscope slide as described above, and the movement of the male was observed under a microscope with very low light intensity to reduce excessive heating. A single male was picked to a spot 1.5 cm from a 2-μl pheromone drop, and a stopwatch was started. If the male reached the edges of the slide, it was scored as “repicked,” and the male was placed back on the start spot and the stopwatch was restarted. If the male reached the pheromone spot, the time was recorded. If the male could not reach the pheromone spot within 30 min, it was scored as “>30.” Males completing the assay were picked to a bacteria-seeded worm plate for recovery for 1 h. The males were again assayed for diacetyl response by using the same regime. If a male could not reach the diacetyl spot within 30 min, the preceding attraction datum for this male was discarded.
Laser Ablation of Female Z1–Z4 Cells, Anchor Cells, and Male CEMs and CEPs.
A C. remanei population was synchronized at 20°C. Ablation of gonadal progenitor cells was performed with synchronized L1 animals (for Z1–Z4 ablation) or L3 females (for anchor-cell ablation). Worms were immobilized on agar pads for laser microsurgery. Successfully ablated females were recovered by adding a drop of M9 buffer and were transferred to a seeded worm plate for 2 additional days before the supernatant solution was prepared. Ablation of four CEMs or four CEPs was performed on 1-day-old adult C. elegans males with their CEMs marked by pkd-2::gfp or their CEPs marked by dat-1::gfp. Successful ablation was confirmed by the elimination of the gfp signals. Ablated males were transferred to a seeded worm plate for 3 h of recovery and then used in single-worm pheromone-attraction assays.
Calculation of CI.
Much of our data from the chemotaxis assays are presented by showing the percentage of worms reaching the test spot, control spot, or neither spot, as well as the SE in the percentages. The three numbers (test, control, and neither) may be distilled further by defining a CI as the difference between the numbers of worms scored on the test and the control spots, divided by the total number of tested animals (4). A CI of unity indicates that all worms are attracted to the pheromone spot, whereas a CI near zero indicates no evident attraction. We further compute the SEM in the average CI value obtained from multiple trials for each experiment. All hypothesis testing is performed by using the CI data, and two CI series from multiple trials in an experiment may be compared against each other by using a two-sample t test or compared against the null-hypothesis CI = 0 by using a one-sample t test.
Acknowledgments
We thank colleagues of the K.L.C. laboratory for help. We thank Kent Liu for improving the design of the phenomone attraction assay. Worm strains were kindly provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. We also thank Hillel Schwartz of the Horvitz lab for the ceh-30 mutant. This study was supported by a grant from the Research Grants Council (Hong Kong).
Abbreviations
- CI
chemotaxis index
- CEM
male-specific cephalic neuron
- CEP
cephalic neuron.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Chasnov JR, Chow KL. Genetics. 2002;160:983–994. doi: 10.1093/genetics/160.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon JM, Sternberg PW. Proc Natl Acad Sci USA. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. J Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargmann CI, Mori I. In: C. elegans II: Monograph 33. Riddle DL, Blumenthal T, Meyers BJ, Priess JR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 717–737. [Google Scholar]
- 5.Green CD. Helminthology Abstracts Series A. 1980;49:327–339. [Google Scholar]
- 6.Schedl T. In: C. elegans II: Monograph 33. Riddle DL, Blumenthal T, Meyers BJ, Priess JR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 241–269. [Google Scholar]
- 7.Greenwald I. In: C. elegans II: Monograph 33. Riddle DL, Blumenthal T, Meyers BJ, Priess JR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 519–542. [Google Scholar]
- 8.Barr MM, Sternberg PW. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 9.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 10.Baird SE, Yen WC. Evol Dev. 2000;2:9–15. doi: 10.1046/j.1525-142x.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 11.Hill KL, L'Hernault SW. Dev Biol. 2001;232:105–114. doi: 10.1006/dbio.2000.0136. [DOI] [PubMed] [Google Scholar]
- 12.Gillott C. Annu Rev Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 13.Kingan TG, Bodnar WM, Raina AK, Shabanowitz J, Hunt DF. Proc Natl Acad Sci USA. 1995;92:5082–5086. doi: 10.1073/pnas.92.11.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmons SW, Sternberg PW. In: C. elegans II: Monograph 33. Riddle DL, Blumenthal T, Meyers BJ, Priess JR, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 295–334. [Google Scholar]
- 15.Ward S, Thomson N, White JG, Brenner S. J Comp Neurol. 1975;160:313–338. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 16.Coghlan A, Wolfe KH. Genome Res. 2002;12:857–867. doi: 10.1101/gr.172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiontke K, Gavin NP, Raynes Y, Roehrig C, Piano F, Fitch DH. Proc Natl Acad Sci USA. 2004;101:9003–9008. doi: 10.1073/pnas.0403094101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JM. The Evolution of Sex. Cambridge, UK: Cambridge Univ Press; 1978. [Google Scholar]
- 19.Barrière A, Félix MA. Curr Biol. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Cutter AD. Genetics. 2006;172:171–184. doi: 10.1534/genetics.105.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutter AD, Avilés L, Ward S. Genet Res. 2003;81:91–102. doi: 10.1017/s001667230300613x. [DOI] [PubMed] [Google Scholar]
- 22.Otto SP, Lenormand T. Nat Rev Genet. 2002;3:252–261. doi: 10.1038/nrg761. [DOI] [PubMed] [Google Scholar]