Abstract
The prion protein (PrP) level in muscle has been reported to be elevated in patients with inclusion-body myositis, polymyositis, dermatomyositis, and neurogenic muscle atrophy, but it is not clear whether the elevated PrP accumulation in the muscles is sufficient to cause muscle diseases. We have generated transgenic mice with muscle-specific expression of PrP under extremely tight regulation by doxycycline, and we have demonstrated that doxycycline-induced overexpression of PrP strictly limited to muscles leads to a myopathy characterized by increased variation of myofiber size, centrally located nuclei, and endomysial fibrosis, in the absence of intracytoplasmic inclusions, rimmed vacuoles, or any evidence of a neurogenic disorder. The PrP-induced myopathy correlates with accumulation of an N-terminal truncated PrP fragment in the muscle, and the muscular PrP displayed consistent mild resistance to protease digestion. Our findings indicate that overexpression of wild-type PrP in skeletal muscles is sufficient to cause a primary myopathy with no signs of peripheral neuropathy, possibly due to accumulation of a cytotoxic truncated form of PrP and/or PrP aggregation.
Keywords: doxycycline, inducible transgenic mice, muscle disease
Diseases affecting the skeletal muscles are fairly common. Many muscle disorders are secondary to pathological conditions affecting the nervous system. Others are related to genetic mutations, immune dysfunction, or exogenous toxins and drugs (1). However, in many primary myopathies, the etiology and pathogenic mechanisms remain unclear (1).
The normal or cellular prion protein (PrPC) is a ubiquitous small glycoprotein attached to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor. Although PrP is well established as the central protein in prion diseases or transmissible spongiform encephalopathies (2), its cellular function remains elusive.
PrPC has been reported to be up-regulated in the skeletal muscles of patients with sporadic and hereditary inclusion body myositis (3–4), as well as in patients with polymyositis, dermatomyositis, and neurogenic muscle atrophy (5). Furthermore, transgenic (Tg) mice overexpressing PrPC also developed necrotizing myopathy associated with demyelination of peripheral nerves and vacuolization of the CNS at advanced ages (6). It is not clear whether the elevated level of PrPC in skeletal muscle is a cause or result of myopathy in the affected patients. Although PrPC overexpression is the cause of the muscle disorders in the above-mentioned experiments (6), body-wide PrPC overexpression and the presence of pathology in central and peripheral nervous tissues raise questions about the role of muscular PrPC in the pathogenic process in the muscles.
The present study reports that strictly muscle-specific overexpression of wild-type human PrPC under tight regulation by doxycycline in Tg mice leads to a primary myopathy. These data argue that overexpression of wild-type PrP in the skeletal muscle is in itself sufficient to cause a myopathy. In addition, our data show that the myopathy correlates with preferential accumulation in the skeletal muscles of an N-terminal truncated PrPC fragment, which corresponds to the previously reported C1 fragment (7–11), suggesting that the C1 fragment is involved in the pathogenesis.
Results
Transgenic Mice Exhibiting Doxycycline-Inducible and Skeletal Muscle-Specific Expression of PrPC.
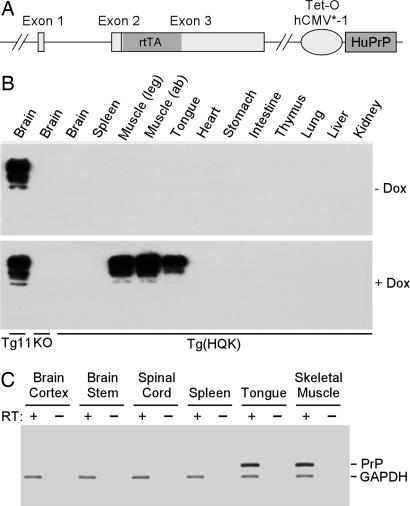
Doxycycline-inducible Tg mice were generated by using the HQK transgene construct with the ORF of two genes: the reverse tetracycline responsive transcription activator (rtTA) under the control of the mouse PrP promoter from the half-genomic vector (12) and human PrPC regulated by the tetracycline-responsive promoter (tetO-hCMV*-1) from the core plasmid (13) (Fig. 1A). The two genes were in tandem and separated by a spacer sequence. The HQK transgene was introduced into the wild-type FVB mice, and Tg(HQK)/Prnp0/0 mice were obtained through breeding with the Zurich I PrP-null mice (14) in FVB background. Four founders were obtained; line Tg(HQK)18 [referred to as Tg(HQK) for simplicity] was selected for further study.
Fig. 1.
Dox-induced, muscle-specific expression of PrP in the Tg(HQK) mice. (A) The HQK transgene construct. The murine PrP ORF in pHGPRP was replaced with that of rtTA, and human PrP (HuPrP) ORF under the control of tetO-hCMV*-1 promoter was placed downstream of exon 3 in pHGPRP after a spacer sequence. Exons 1–3 denote the 3 Prnp exons in pHGPRP; the rtTA ORF and HuPrP ORF are shaded. (B) Western blot analysis. The Tg(HQK) mice were given 6 g of Dox per kg of food pellets for 10 days. Brain, skeletal muscles from the hind leg (quadriceps) and abdomen (ab), as well as other indicated organs/tissues were collected from Dox-treated (+) and untreated (−) Tg(HQK) mice and subjected to Western blot analysis with the mAb 3F4. Twenty micrograms of total protein was loaded for each sample. Tg11, a Tg line with constitutive expression of human PrP in the brain at a level that is ≈6-fold that of PrP in the brain of wild-type FVB mice (Q.K., unpublished data); KO, FVB/Prnp0/0 mice. (C) RT-PCR analysis. Brain (cortex), brainstem, spinal cord, spleen, tongue, and skeletal muscles (quadriceps) were collected from Tg(HQK) mice treated the same way as in B and subjected to RT-PCR analysis for PrP mRNA and GAPDH mRNA.
Skeletal muscles from hind legs (quadriceps), abdominal wall, tongue, brain, spleen, heart, stomach, intestine, thymus, lung, liver, and kidney were collected from Tg(HQK) mice that had been fed food pellets either lacking or containing doxycycline (Dox) for 10 days to induce PrPC expression. No PrP was detectable on Western blots from skeletal muscle and the various organs when Dox was not administered (Fig. 1B Upper). After Dox treatment, large amounts of PrP accumulated in the skeletal muscles from hind legs, abdominal wall, and tongue, but not in any other organ (Fig. 1B Lower) except for the heart, where a small amount of PrP was detected after prolonged exposure (data not shown). The muscle-specific, Dox-inducible PrP expression in Tg(HQK) mice was confirmed by RT-PCR analysis, demonstrating the presence of PrP mRNA only in skeletal muscles (Fig. 1C). The reason for the strictly skeletal muscle-specific PrP expression in the Tg(HQK) mice after induction with Dox is unclear. It appears to be the intrinsic property of the HQK transgene construct because the muscle specificity is observed in all Tg(HQK) lines (data not shown).
PrP expression in the muscles of Tg(HQK) mice was dependent on the dosage of Dox (Fig. 2). After 7 days of treatment with 20, 50, 200, 1,000, or 6,000 mg of Dox per kg of food, muscle PrPC was first detected at a dose of 50 mg of Dox per kg of food, and the PrPC levels increased with higher Dox dosages. Near peak level of induction was achieved with 1 g of Dox per kg of food, but the dosage of 6 g of Dox per kg of food was used to maximize the consistency of induced PrP expression.
Fig. 2.
Dose response of Dox-induced HuPrP expression in the skeletal muscle of the Tg(HQK) mice. (A) Western blot analysis. (B) Plot of the dose-dependent induction of PrP. The Tg(HQK) mice were treated for 7 days with indicated doses of Dox. Skeletal muscles from an uninduced Tg(HQK) mouse (0 Dox) and brain tissues from a Tg11 mouse and a PrP KO mouse (KO FVB) served as controls. Tg11 is a transgenic mouse line expressing human PrP in the brain at approximately six times that of wild-type FVB mice (Q.K., unpublished data). Twenty micrograms of total protein was loaded for each sample. The blot was probed with mAb 3F4. The Tg(HQK) mice were all in the PrP-null FVB background.
Time Courses of PrP Induction and Clearance.
After induction with 6 g of Dox per kg of food, PrPC was first detected in the skeletal muscles at 2 days postinduction (data not shown). The amount of PrP increased with time and reached a near maximum stable level at 14 days postinduction (Fig. 3A). At the highest level of expression, the PrPC level in the muscles of Tg(HQK) mice was ≈40-fold that of wild-type FVB mice (Fig. 3A Lower), exemplifying the high inducibility of PrP expression by oral administration of Dox in the Tg(HQK) mice.
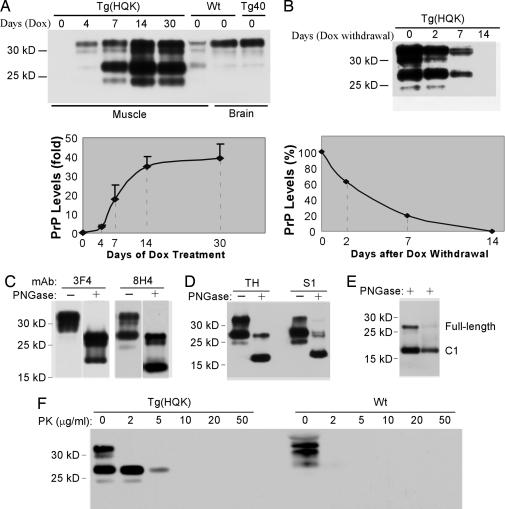
Fig. 3.
PrP in the skeletal muscles of the Tg(HQK) mice: induction, clearance, and characterization. (A) Time course of induction. The Tg(HQK) mice were treated with 6 g of Dox per kg of food for 0–30 days before being killed. Skeletal muscle from a wild-type (Wt) FVB mouse served as a control, as did brain tissues from the same Wt FVB mouse and a Tg40 mouse. Ten micrograms of total protein was loaded for each sample. (Upper) Representative Western blot probed with 8H4. (Lower) Plot of the PrP levels (expressed in fold of the PrP level in the skeletal muscle of Wt FVB mice) against the duration of Dox treatment, and the error bars were based on three duplicate blots. (B) Time course of clearance. The Tg(HQK) mice were treated with 6 g of Dox per kg of food for 7 days before the drug was withdrawn. The mice were killed at 0, 2, 7, and 14 days after withdrawal of Dox. (Upper) Western blot. (Lower) Plot of the PrP levels (expressed as percentages of the PrP level before Dox withdrawal). Ten micrograms of total protein was loaded for each sample. The blots were probed with mAb 8H4. The Tg(HQK) and Tg40 mice are all in the Prnp0/0 background. (C) PrP species in the muscles. Homogenate of skeletal muscle from a Tg(HQK) mouse treated with 6 g of Dox per kg of food for 30 days was Western blotted and probed with biotinylated 3F4 followed by 8H4. Two micrograms of total protein was loaded for each sample. The four lanes were assembled from the same blot. (D) The 27-kDa PrP was glycosylated. Total muscle homogenate (TH) (left two lanes) and the supernatant (S1) after immunoprecipitation with mAb 8B4 (binding to PrP37–44) (right two lanes) were subjected to Western blot analysis with 8H4. (E) The deglycosylated 18-kDa PrP comigrated with the C1 fragment. Total muscle homogenate from Tg(HQK) mice (left lane) and tissue homogenate from the autolysed brain of a Tg40 mouse (right lane) were probed with 8H4. “−” and “+” indicate with or without PNGase treatment to remove glycans from PrP, respectively. (F) PrP in the Tg(HQK) mice was mildly resistant to protease digestion. Total muscle homogenates from Tg(HQK) mice (1 μg of total protein) or Wt mice (40 μg of total protein) were subjected to proteinase K (PK) digestion at the indicated PK concentrations followed by Western blot analysis with 8H4.
The rate of PrPC clearance was assessed by feeding 6 g of Dox per kg of food to Tg(HQK) mice for 7 days followed by administration of regular food lacking Dox, and the PrPC levels in skeletal muscles (quadriceps) collected at 0, 2, 7, and 14 days after Dox withdrawal were examined by Western blot analysis with mAb 8H4 (binding to mouse PrP175–185 and human PrP176–186). PrP expression was found to decrease progressively with time, becoming undetectable at 14 days (Fig. 3B). Similar results were obtained with muscles from the abdominal body wall (data not shown).
Characterization of PrP in Tg(HQK) Mice.
The PrP in muscles from Tg(HQK) mice treated with 6 g of Dox per kg of food for 7 days or more distributed mostly into a very prominent band of 32 kDa and a much weaker band of 30 kDa on Western blots probed with biotinylated 3F4 (binding to human PrP109–112) (Fig. 3C). Treatment with PNGase that cleaves off the glycans revealed a strong 27-kDa band and weaker 24- and 20-kDa bands, indicating that the 32- and 30-kDa bands were glycosylated (Fig. 3C). After reprobing the blot with the mAb 8H4, besides a moderate 32-kDa band and a much weaker 30-kDa band, an additional very strong 27-kDa band and a very weak 24-kDa band were observed (Fig. 3C); the same band patterns were confirmed when probed directly with 8H4 (Fig. 3A). After deglycosylation, these bands migrated to 27, 24, and 18 kDa (Fig. 3C), confirming that most of the PrP expressed in the muscles of Tg(HQK) mice were glycosylated. Similar 32-, 30-, and 27-kDa PrP bands were detected with 8H4 in the skeletal muscles from wild-type FVB mice, but the 32- and 27-kDa bands were of comparable intensity (Fig. 3A). In the brains of wild-type FVB mice and the Tg40 transgenic mouse line expressing human PrP at wild-type level (15), the 32-, 30-, and 27-kDa PrP bands were also detected with 8H4, but a vast majority was the 32-kDa form (Fig. 3A).
The prominent 27-kDa PrP fragment in the muscles of Tg(HQK) mice, which shifted to 18 kDa after glycan removal (Fig. 3C), appears similar to the previously reported C1 fragment (7–11). The C1 fragment is an N-terminus-truncated, glycosylated 27-kDa PrPC fragment with N terminus at residues 111/112 generated during normal PrP metabolism, which also shifts to 18 kDa after glycan removal (9). To evaluate whether the 27-kDa PrP fragment in the muscles of Dox-induced Tg(HQK) mice is actually the same as the C1 fragment, full-length PrP was removed by immunoprecipitation with the mAb 8B4 (against human PrP37–44) (Fig. 3D). The supernatant contained mostly the 27-kDa PrP fragment and a much smaller amount of 24-kDa PrP fragment; both bands shifted to 18 kDa after PNGase treatment (Fig. 3D), indicating that they both were glycosylated. Muscle homogenates from Tg(HQK) mice were then compared by Western blot with tissue homogenate from autolysed Tg40 mouse brains. Autolysis of human brain tissue has been shown to convert most full-length PrP to the C1 form (16). Fig. 3E shows that the deglycosylated 18-kDa PrP fragment from the muscle of Tg(HQK) mice migrated at the same position as the C1 fragment from the autolysed brain of the Tg40 mice. In addition, the 27-kDa fragment and the deglycosylated 18-kDa fragment were not detected by mAb 3F4 (Fig. 3C), which recognizes human PrP residues 109–112, indicating that these PrP fragments have an N terminus downstream of residue 109. Together these data confirm that the 27-kDa PrP fragment in the muscles of Tg(HQK) mice is indeed the C1 fragment.
To evaluate the protease resistance of PrP in the Tg(HQK) mice, skeletal muscle tissue homogenates from Tg(HQK) or wild-type mice treated with 6 g of Dox per kg of food for 7 days were subjected to digestion with an increasing amount of proteinase K (PK). Fig. 3F shows that PrP in the Tg(HQK) mice is partially resistant to 5 μg/ml of PK, whereas PrP in the wild-type mice is totally digested at 2 μg/ml of PK. Similar results were obtained for Tg(HQK) and wild-type mice treated with Dox for 2, 4, 14, 30, and 60 days (data not shown). These data indicate that, in the Tg(HQK) mice, muscular PrP is moderately more resistant to PK digestion than that of wild-type mice, and the PK resistance remained constant throughout the course of Dox induction.
Progressive Primary Myopathy in Tg(HQK) Mice with Dox-Induced PrP Expression.
The skeletal muscles from Tg(HQK) mice after increasing duration of Dox treatment were subjected to histological examinations. For Tg(HQK) mice, a few scattered cells with central nuclei in skeletal muscles were visible after as early as 7 days of Dox treatment. After 4–5 weeks of Dox induction, ≤15% of the fibers in muscle fascicles exhibited one or more centrally located nuclei; some showed rare necrotic fibers and regenerating fibers. These changes were accompanied by a mild decrease in fiber diameters affecting both slow- and fast-twitch fibers when compared with those in similarly treated wild-type FVB mice. There was no evidence of mitochondrial proliferation, fiber splitting, or denervation atrophy (e.g., target fibers or fiber type grouping). Acid phosphatase staining revealed a prominent increase of lysosomes within muscle fibers. Minimal endomysial fibrosis was identified (data not shown). These morphological abnormalities became increasingly more pronounced in later stages of the disease (7–29 weeks after induction), with prominent atrophy, degeneration, and regeneration of myofibers accompanied by central nuclei in over one-third of the muscle fibers. In addition, hypertrophic fibers, sparse split fibers (partially or completely split into two by a thin septum extending from the endomysium), and excessive lysosomal activity (acid phosphatase) were seen in virtually all muscle fibers at this stage (Fig. 4D). Mild mitochondrial proliferation occurred in some of the muscle fibers and in some instances even approximated “ragged-red” fiber formation. Neither inclusions nor amyloid-like materials were detected by H&E (Fig. 4B) or Congo red stains (data not shown). To examine the effect of prolonged Dox treatment, wild-type FVB mice and PrP-null mice (FVB/Prnp0/0) were also subjected to the same Dox treatment and examination, but little pathological change was observed in these mice after treatment with Dox for 200 days (Fig. 4) or >1 year (data not shown). In addition, immunohistochemical staining with 3F4 shows that PrP was distributed throughout the muscle cells, whereas a small number of muscle fibers contained many intensely stained dots (Fig. 4F), consistent with the presence of PrP aggregates.
Fig. 4.
Myopathy in the Tg(HQK) mice treated with Dox. Wild-type FVB mice (A and C) and Tg(HQK) mice (B, D, and F) were treated with 6 g of Dox per kg of food for 200 days (A and B), 30 days (C and D), or 14 days (F). The skeletal muscles (quadriceps) from the hind legs were cryosectioned and subjected to H&E staining (A and B) or acid phosphatase staining (C and D) with standard procedures. Similar muscle tissues from a PrP-null mouse (E) or a Tg(HQK) mouse (F) were fixed, embedded, sectioned, and stained with 3F4.
Correlation of the Myopathy in Tg(HQK) Mice with Accumulation in Muscles of an N-Terminal-Truncated PrP Fragment.
The amount of the C1 fragment appears to be much more than that of the full-length PrP in the muscle of Tg(HQK) mice (Fig. 3), which is unusual because the level of the C1 fragment was reported to be 30–50% of the full-length PrP in the autopsied brains of normal humans (9). The time course of Dox-induced expression of the full-length PrP and the C1 fragment in the muscle of Tg(HQK) mice was examined more closely after deglycosylation by Western blot with 8H4 and compared with that of similarly treated wild-type FVB mice (Fig. 5). The data show that, in Tg(HQK) mice, both full-length PrP and the C1 fragment increased with time and reached near peak levels at 14 days (Fig. 5A). The C1 fragment was in submolar amount relative to the full-length PrP up to 4 days, but the C1 fragment was detected at approximately three times the level of the full-length PrP from 7 days and beyond (Fig. 5 A and C). In contrast, the levels of the C1 fragment and the full-length PrP and their ratios remained relatively constant throughout the time course in the Dox-treated wild-type FVB mice, with the C1/full-length ratio fluctuating between 0.98 and 1.25 (Fig. 5 B and C). The difference in the ratios of C1/full-length PrP between the Tg(HQK) and wild-type FVB mice is highly significant (P < 0.001, Student's unpaired t test). This experiment was repeated three times with similar results. These findings indicate that the myopathy is correlated with the high ratio of C1/full-length PrP in the skeletal muscles of Tg(HQK) mice.
Fig. 5.
Preferential accumulation of a truncated PrP fragment in the muscles of Tg(HQK) mice induced with Dox. (A) Muscles of Tg(HQK) mice. (B) Muscles of wild-type FVB mice. The Tg(HQK) and wild-type FVB mice were treated with 6 g of Dox per kg of food for 0 (lanes 1–3), 4 (lanes 4–6), 7 (lanes 7–9), 14 (lanes 10–12), and 30 (lanes 13–15) days, respectively, and skeletal muscles (quadriceps) were collected. Muscle homogenates from each mouse [5 and 25 μg of total proteins for Tg(HQK) mice and wild-type FVB mice, respectively] were treated with PNGase F, loaded onto 10–20% Tris-Tricine gels, and subjected to Western blotting analysis with 8H4. (C) Plot of relative ratios of the C1/full-length PrP during the course of Dox treatment. The error bars were based on the three samples at each time point. The experiment was repeated three times with similar results.
Discussion
We have generated four lines of Tg(HQK) mice where expression of PrP is highly regulated by Dox and stringently limited to the skeletal muscles. After sustained PrP overexpression, these Tg(HQK) mice develop a rapidly progressive primary myopathy. Furthermore, an N-terminus-truncated PrP fragment, which matches the previously described C1 fragment (7–11), is present at three times the level of full-length PrP in the muscle of Dox-induced Tg(HQK) mice.
Transgenic mice overexpressing wild-type PrP in a body-wide fashion have been reported to exhibit myopathy in the advanced age (6), but these mice also overexpress PrP in the nervous system and developed peripheral neuropathy, making it unclear whether the myopathy is primarily due to the accumulation of PrP in the skeletal muscles or it represents neurogenic atrophy secondary to the impairment of the CNS. Furthermore, transgenic mice (named PG14) with whole-body expression of a mutant PrP with nine extra octapeptide repeats were reported to develop a progressive primary myopathy (17). The PG14 mice also developed spontaneous neurological illness, and the mutant PrP in the brain and peripheral tissues (including skeletal muscles) showed mild resistance to protease digestion and other biochemical features reminiscent of the pathogenic form of PrP (PrPSc) found in prion-affected mammals (17–18). In addition, hamsters subjected to daily i.p. injections of chloroquine for 60 days also developed a myopathy that was accompanied by accumulation of PrP in the muscle fibers (19).
Our data demonstrate that overexpression of wild-type PrP in the skeletal muscles alone is sufficient to cause myopathy. These findings strongly suggest that the elevated levels of PrP found in the skeletal muscles of human primary myopathies, such as inclusion body myositis (3–5) and inflammatory myopathies including polymyositis and dermatomyositis (5), may play an important role in the pathogenesis.
The pathogenic mechanism of the PrP-mediated myopathy remains to be determined. The preferential accumulation of the N-terminal-truncated C1 fragment pointed to the C1 fragment. Tg mice expressing N-terminus-truncated forms of PrP following deletion of residues 23–121 or 23–134 develop loss of neurons in the cerebellum (20). Similarly, Doppel (Dpl), a protein with limited sequence homology to the C-terminal part of PrP, is also toxic when expressed in neurons in the absence of wild-type PrP (21), suggesting that Dpl and some C-terminal fragments of PrP are neurotoxic and the toxicity can be neutralized by the presence of sufficient full-length PrP. The toxicity of the experimentally generated N-terminus-truncated PrP has been tentatively explained by the significant inhibition of PrP internalization (22–23), delayed translocation to the cell surface, and prolonged half-life of the truncated PrPC (23).
In contrast to these experimentally generated N-terminus-truncated fragments, the N-terminal-truncated PrP fragment observed in large amounts in the skeletal muscles of Tg(HQK) mice corresponds to the C1 fragment reported to result from normal PrPC processing (7–11). The C1 fragment was also observed at much lower levels in the skeletal muscles of wild-type mice, where the C1/full-length PrP molar ratio was close to 1 (Fig. 5). After synthesis in the endoplasmic reticulum, most of the PrPC is transported to the cell surface and attached by a GPI anchor to the caveolae, specialized plasma membrane microdomains that mediate key processes such as signal transduction and transcytosis (24). Surface PrPC undergoes constant internalization (25), and it is cleaved by the disintegrin family of metalloproteases (26), likely in an endocytic compartment (25), to generate the C1 fragment. The cellular function of the C1 fragment is not known.
In the Dox-induced Tg(HQK) mice, the high levels of the C1 fragment in skeletal muscles imply that production of this fragment was increased and/or its turnover was slowed down in the Tg(HQK) mice. The prominent increase of lysosomal activity in the muscle fibers starting from the early stages of Dox treatment (Fig. 4D) would provide a mechanism for enhanced production of the C1 fragment.
In Tg(HQK) mice treated with 6 g of Dox per kg of food, the ratio of C1/full-length PrP reached 3.0 after 7 days and it remained relatively constant afterward; initial signs of myopathy also became visible after 7 days, and the disorder progressed rapidly. The correlation of the unusually high C1/full-length PrP ratio with myopathy in the Tg(HQK) mice suggests that the observed muscle cell toxicity is likely mediated by the C1 fragment through a mechanism similar to the neurotoxicity mediated by the experimental N-terminus-truncated PrP forms (20). The C1 fragment observed in normal cultured cells and brain tissues may be tolerated because of its low concentration and coexistence of roughly equal or higher molar amounts of full-length PrPC.
Muscular PrP in the Tg(HQK) mice is moderately more resistant to proteinase K than that of wild-type mice (Fig. 3F), suggesting low levels of PrP aggregation in the Tg(HQK) mice. However, the significance of this finding is unclear because the PrP PK resistance was constant throughout the course of Dox treatment, whereas signs of myopathy were not detectable until after 7 days of Dox treatment and increased thereafter. Immunohistochemical staining revealed PrP-positive dots and small deposits in at least some muscle cells (Fig. 4F), arguing for the presence of PrP aggregates. Because Congo red staining is negative, the PrP aggregates in the Tg(HQK) mice are different from the Congo red-positive amyloid inclusions containing PrP, amyloid-β, and its precursor protein found in inclusion body myositis (3). Nevertheless, PrP aggregation might still play a role in the pathogenic process in the Tg(HQK) mice.
The muscle-specific PrP expression in the Tg(HQK) mice appears to be an intrinsic property of the HQK construct because all independent Tg(HQK) lines showed the same muscle specificity (data not shown). Therefore, the HQK construct has the potential to restrict the Dox-dependent expression of any gene to skeletal muscles when the ORF of this gene is used to replace the PrP ORF in HQK. Consequently, the HQK construct may be very valuable for generation of other regulated Tg mouse models of muscle diseases. It can also potentially be used for gene therapy through regulated expression of a specific protein lacking in muscle cells due to a gene defect.
Materials and Methods
The HQK Transgenic Mice.
The HQK transgene was created by replacing the mouse PrP ORF in the half genomic PrP (HGPRP) clone (12) with that of rtTA and placed upstream of tetO-hCMV*-human PrP ORF of the core plasmid (13) after a spacer sequence. The HQK transgene construct was microinjected into fertilized FVB/NJ eggs at Albert Einstein College of Medicine. Founder pups were screened for human PrP ORF by PCR as previously described (15). Human PrP ORF-positive founder mice were further screened by PCR for the rtTA ORF with primers rtTA-1 (CTTGGTGTAGAGCAGCCTAC) and rtTA2r (CCGCATATGATCAATTCAAGG). Selected founder mice were bred with FVB/Prnp0/0 mice to obtain Tg(HQK) mice that were HQK+/0/Prnp0/0. The FVB/Prnp0/0 mice were obtained from the Prusiner laboratory, where the Zurich I Prnp0 allele was from the Weissmann laboratory (14). The transgene expression in the brain and other tissues was examined by immunoblotting analysis by using 3F4 or 8H4.
Doxycycline Treatment.
Treatment of mice with Dox was done with rodent food from Bio-Serv (Frenchtown, NJ) containing indicated amounts of Dox (20 mg, 50 mg, 200 mg, 1 g, or 6 g per kg of food), and the food was changed three times per week. The dose of 6 g of Dox per kg of food was used except for the dose-response experiment.
Western Blot Analysis.
Tissues were homogenized and immunoblotted onto PVDF membranes after separation on 10–20% gradient Tris-Tricine SDS/PAGE gels (15). Total protein concentrations of the tissue homogenates were measured with the BCA Protein Assay Reagents (Pierce Chemical, Rockford, IL). Deglycosylation where indicated was performed with PNGase F (New England Biolabs, Ipswich, MA) following the manufacturer's instructions except for overnight incubation at 37°C. Immunoprecipitation of full-length PrP with mAb 8B4 was performed as described (27). The blots were probed with mAb 8H4 (for human PrP176–186 and mouse PrP175–185) or 3F4 (for human PrP109–112; Signet Laboratories, Dedham, MA) in conjunction with HRP-conjugated goat anti-mouse IgG Fc antibody (Chemicon International, Temecula, CA) and developed with BM chemiluminescence blotting substrate (Roche Diagnostics, Indianapolis, IN).
RT-PCR.
Total RNA was extracted from indicated tissues by using the RNeasy mini kit (QIAGEN, Valencia, CA). RT-PCR was performed with standard procedures by using primer pairs ACTTTGGCATTGTGGAAGGGCT and ACCCTGTTGCTGTAGCCGTATTC for GAPDH mRNA and GGATCAGCAGACCGATTCTG and GGTGACCGTGTGCTGCTTG for the transgene PrP mRNA. These primers are complementary to different exons of the respective genes.
Generation of the C1 Fragment.
The C1 fragment was generated by autolysis of brain tissue of the Tg40 mice expressing human PrP (15) as described (16), except that the brain tissue was autolysed at room temperature for 72 h.
Histological, Enzyme, and Immunohistochemical Analysis.
Skeletal muscles (quadriceps) were cryosectioned and subjected to H&E staining and enzyme immunohistochemical analysis by standard methods (28) for NADH-dehydrogenase, succinate dehydrogenase, and acid phosphatase. The sections were also stained by Congo red as described (29) and examined by using rhodamine optics to amplify the signal for congophilia. PrP immunostaining with 3F4 on paraffin-embedded, fixed-muscle sections was done as described (15).
Acknowledgments
We thank Dr. Stanley Prusiner (University of California, San Francisco, CA) for providing the FVB/Prnp0/0 mice derived from the Zurich I PrP-null mice and Dr. Wen-Hwa Lee (University of California, Irvine, CA) for providing the core plasmid. The study was funded by National Institutes of Health Grants AG14359 and NS052319.
Abbreviations
- Dox
doxycycline
- PrP
prion protein
- rtTA
reverse tetracycline responsive transcription activator
- Tg
transgenic.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Karpati G, Sinnreich M. J Neuropathol Exp Neurol. 2003;62:1203–1210. doi: 10.1093/jnen/62.12.1203. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askanas V, Bilak M, Engel WK, Alvarez RB, Tome F, Leclerc A. Neuroreport. 1993;5:25–28. doi: 10.1097/00001756-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sarkozi E, Askanas V, Engel WK. Am J Pathol. 1994;145:1280–1284. [PMC free article] [PubMed] [Google Scholar]
- 5.Zanusso G, Vattemi G, Ferrari S, Tabaton M, Pecini E, Cavallaro T, Tomelleri G, Filosto M, Tonin P, Nardelli E, et al. Brain Pathol. 2001;11:182–189. doi: 10.1111/j.1750-3639.2001.tb00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westaway D, DeArmond SJ, Cayetano-Canlas J, Groth D, Foster D, Yang SL, Torchia M, Carlson GA, Prusiner SB. Cell. 1994;76:117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 7.Caughey B, Race RE, Ernst D, Buchmeier MJ, Chesebro B. J Virol. 1989;63:175–181. doi: 10.1128/jvi.63.1.175-181.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris DA, Huber MT, van Dijken P, Shyng SL, Chait BT, Wang R. Biochemistry. 1993;32:1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- 9.Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. J Biol Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- 10.Taraboulos A, Scott M, Semenov A, Avrahami D, Laszlo L, Prusiner SB. J Cell Biol. 1995;129:121–132. doi: 10.1083/jcb.129.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent B, Paitel E, Frobert Y, Lehmann S, Grassi J, Checler F. J Biol Chem. 2000;275:35612–35616. doi: 10.1074/jbc.M004628200. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, Rulicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 13.Utomo AR, Nikitin AY, Lee WH. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- 14.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 15.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, et al. J Neurosci. 2005;25:7944–7949. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Huete A, Lievens PM, Vidal R, Piccardo P, Ghetti B, Tagliavini F, Frangione B, Prelli F. Am J Pathol. 1998;153:1561–1572. doi: 10.1016/S0002-9440(10)65744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiesa R, Pestronk A, Schmidt RE, Tourtellotte WG, Ghetti B, Piccardo P, Harris DA. Neurobiol Dis. 2001;8:279–288. doi: 10.1006/nbdi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 18.Chiesa R, Piccardo P, Ghetti B, Harris DA. Neuron. 1998;21:1339–1351. doi: 10.1016/s0896-6273(00)80653-4. [DOI] [PubMed] [Google Scholar]
- 19.Furukawa H, Doh-ura K, Sasaki K, Iwaki T. Lab Invest. 2004;84:828–835. doi: 10.1038/labinvest.3700111. [DOI] [PubMed] [Google Scholar]
- 20.Shmerling D, Hegyi I, Fischer M, Blattler T, Brandner S, Gotz J, Rulicke T, Flechsig E, Cozzio A, von Mering C, et al. Cell. 1998;93:203–214. doi: 10.1016/s0092-8674(00)81572-x. [DOI] [PubMed] [Google Scholar]
- 21.Moore RC, Mastrangelo P, Bouzamondo E, Heinrich C, Legname G, Prusiner SB, Hood L, Westaway D, DeArmond SJ, Tremblay P. Proc Natl Acad Sci USA. 2001;98:15288–15293. doi: 10.1073/pnas.251550798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KS, Magalhaes AC, Zanata SM, Brentani RR, Martins VR, Prado MA. J Neurochem. 2001;79:79–87. doi: 10.1046/j.1471-4159.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- 23.Nunziante M, Gilch S, Schatzl HM. J Biol Chem. 2003;278:3726–3734. doi: 10.1074/jbc.M206313200. [DOI] [PubMed] [Google Scholar]
- 24.Harris DA, Peters PJ, Taraboulos A, Lingappa V, DeArmond SJ, Prusiner SB. In: Prion Biology and Diseases. 2nd Ed. Prusiner SB, editor. Plainview, NY: Cold Spring Harbor Lab Press; 2004. pp. 492–543. [Google Scholar]
- 25.Shyng SL, Huber MT, Harris DA. J Biol Chem. 1993;268:15922–15928. [PubMed] [Google Scholar]
- 26.Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, Grassi J, Lopez-Perez E, Checler F. J Biol Chem. 2001;276:37743–37746. doi: 10.1074/jbc.M105677200. [DOI] [PubMed] [Google Scholar]
- 27.Pan T, Li R, Wong BS, Liu T, Gambetti P, Sy MS. J Neurochem. 2002;81:1092–1101. doi: 10.1046/j.1471-4159.2002.00909.x. [DOI] [PubMed] [Google Scholar]
- 28.Dubowitz V. In: Muscle Biopsy: A Practical Approach. Dubowitz V, editor. Philadelphia: Baillière Tindall; 1985. [Google Scholar]
- 29.Mendell JR, Sahenk Z, Gales T, Paul L. Arch Neurol. 1991;48:1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]