Abstract
Cocaine is a psychostimulant and a drug widely abused by humans. Cocaine elicits its effects primarily by blocking the activity of the dopamine (DA) transporter, leading to elevated levels of extracellular DA in areas receiving dopaminergic innervation, with the consequent activation of DA receptors. Cocaine, however, also elevates other neurotransmitter levels, leading to a general activation of interconnected brain circuitries. Studies aimed at unraveling the molecular mechanisms underlying the effects of cocaine have shown a leading role of DA D1 receptors in the cascade of cellular events elicited by this drug. In this study, we have analyzed the acute response to cocaine in animals deleted for the expression of DA D2 receptors (D2R), an inhibitor of DA signaling. Importantly, we show that although D1 receptor-mediated functions are preserved and even enhanced in D2R−/− mutants, the behavioral response to acute cocaine administration is severely altered. In addition, c-fos response to acute cocaine administration, in contrast to wild-type mice, is absent in D2R−/− mutants. Our findings show that the absence of D2R, very likely through a presynaptic mechanism, uncovers an inhibitory signaling pathway normally masked by the activity of this receptor on brain circuitries engaged by abused drugs.
Keywords: behavior, c-fos, dopamine D2 receptor-deficient mice, drug abuse
Drugs of abuse share the common property of generating an outstanding elevation of dopamine (DA) in brain areas of the motor and limbic systems (1), which has been related to their addictive properties (2–5). Thereby, activation of the dopaminergic (DAergic) system is a leading step in the mechanisms underlying behavioral, cellular, and molecular effects of drugs in the CNS.
The psychostimulant cocaine, a highly abused substance, primarily acts by blocking the activity of the DA transporter, elevating extracellular DA levels in the nucleus accumbens (Acb), dorsal striatum, and frontal cortex (1, 6), leading to activation of DA receptors in these areas. Among DA receptors, D1 and D2 receptors (D1- and D2R) have a leading role in the behavioral, cellular, and molecular response to DA signaling (7). With respect to cocaine, pharmacological blockade of D1R (8, 9), as well as genetically induced ablation of D1R-mediated signaling, has been shown to strongly impair the behavioral and cellular response to this drug (10, 11). Further studies have assessed the key role of D1R in stimulating the cellular response to drugs by modulating downstream kinases and phosphatases (12).
The expression of immediate early genes (IEG) has been used as a final readout of the cellular response to illicit drugs in the brain. It is now well established that activation of c-fos and other IEGs in the striatum (13–15) upon acute cocaine, as well as other drug, treatments is dependent on activation of the DAergic and glutamatergic pathways (16, 17). Indeed, c-fos induction is lost in D1R−/− mice challenged with cocaine (14, 18), and similarly, blockade of glutamatergic signaling results in a blunted c-fos cellular response (17, 19). Nonetheless, the role of inhibitory signaling pathways in the modulation of the response to drugs certainly participates in the behavioral and cellular outcome of drug intake. In this study, we analyzed the behavioral and cellular effects of the psychomotor stimulant cocaine in animals carrying the deletion of the DA D2R.
Loss of D2R in D2R−/− mice leads to a blunted response to the rewarding (20) and reinforcing properties of morphine (21) and alcohol (22). This phenotype is observed despite no changes in the affinity or number of D1R in these mice (23). Similarly, D2R−/− mice also present impaired responses to a cocaine discrimination task (24) and an abnormal response to cocaine self-administration (25). D2 but not D1R is crucial for the induction of morphological changes in DAergic neurons by cocaine (26). Loss of D2R-mediated signaling affects both presynaptic and postsynaptic DAergic responses (27, 28). These are differentially mediated by the two D2R isoforms, D2L and D2S. D2L has major postsynaptic effects, whereas D2S has presynaptic autoreceptor functions (28). Upon cocaine treatments, a massive DA release is observed in the striatum of D2R−/− mice (27), and D2Rs modulate glutamate and GABA release (29, 30) in GABAergic neurons.
Here we show that direct activation of D1R-mediated signaling by D1 agonists in D2R−/− mice results in a dose-dependent stimulation of locomotion and c-fos expression in the striatum and cortex as it does in WT littermates. The cellular response in D2R−/− mice to a D1 agonist is even increased with respect to WT animals, as it would be expected by an unopposed stimulation of D1R by D2-mediated signaling. On the contrary, cocaine treatment of D2R−/− mice uncovered disruption of the expected D1R-mediated effects both at the molecular and behavioral levels. These results appear dependent on the loss of presynaptic D2S-mediated effects, as shown by the analysis of D2L−/− mice in which cocaine psychomotor properties are similar to WT mice. These results strongly point to a key modulator role of D2R on striatal neurons activity in the full integration of the molecular and behavioral responses to drugs of abuse.
Results
Induction of Forward Locomotion and Enhanced Activation of Striatal and Cortical c-fos mRNA in D2R−/− Mice in Response to a D1R Agonist.
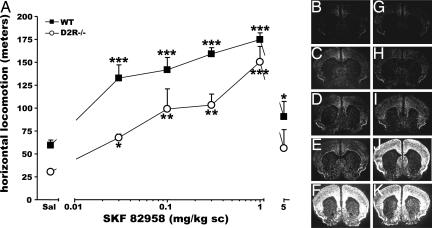
Striatal homogenates from D2R−/− mice present unaltered number and pharmacological properties of D1R sites in comparison to WT animals (23). However, because striatal DA receptors sensitivity is not correlated to their density (31), we tested the behavioral and cellular response of D2R−/− mutants to stimulation by D1 agonists. Systemic administration of increasing concentrations of the D1R-specific agonist SKF 82958 (0.03, 0.1, 0.3, 1, and 5 mg/kg s.c.) resulted in a dose-dependent increase of forward locomotion in D2R−/− mice as in WT littermates (Fig. 1A). Statistical analyses showed no [genotype × treatment] interaction (F(5, 46) = 0.864, P = 0.512). In particular, the highest dose of SKF 82958 used (5 mg/kg) resulted into a drop in forward locomotion in D2R−/− mice very similar to that of WT animals because of the development of stereotypies, and in particular of grooming behavior (32). These results were supported by previous studies using SKF 81297 (28).
Fig. 1.
Behavioral and cellular effects of D1R activation in D2R−/− mice. (A) Increase of motor activity by the D1 agonist SKF 82958 in D2R−/− (■) and WT littermates (○). A significant treatment effect for both WT and D2R−/− mice was observed over 1 h. Induction of c-fos mRNA expression 1 h after saline (B and G) and 0.01 mg/kg (C and H), 0.1 mg/kg (D and I), 0.3 mg/kg (E and J), and 1 mg/kg (F and K) SKF 82958 injections in WT (B–F) and D2R−/− mice (G–K) is shown. Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P < 0.0001.
The comparable behavioral responses induced by SKF 82958 between genotypes were mirrored by the molecular and cellular events produced downstream of the D1R signaling. The induction of IEGs, in particular c-fos, has been extensively used as a readout of neuronal second messenger activation (33). In situ hybridization experiments showed a comparable pattern of D1R-induced c-fos expression in the striatum and cortex in both genotypes (Fig. 1 B–K). Quantifications of c-fos mRNA expression showed a 2.2-fold stronger increase of this gene in the Acb and caudate-putamen (CP) of D2R−/− mice at doses of 0.3 and 1 mg/kg in comparison to WT littermates.
Altered Motor Response to Acute Cocaine Challenge in D2R−/− Mice.
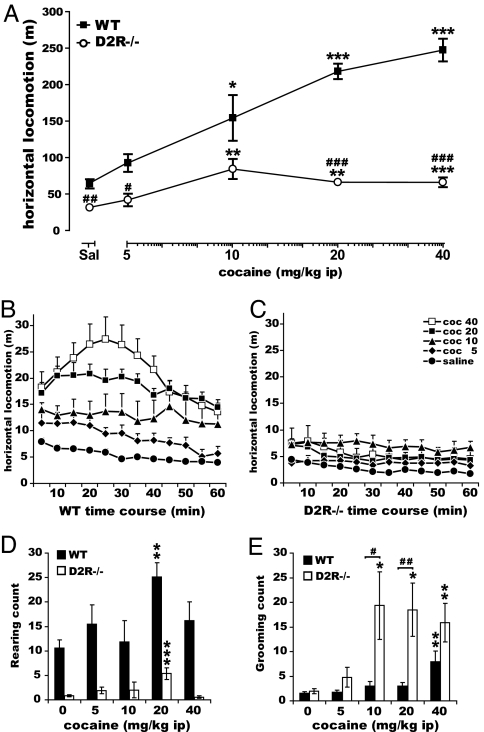
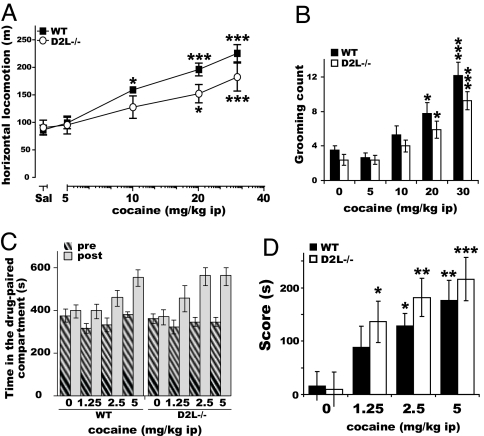
Next, we analyzed the acute response of D2R−/− mice to a wide range of cocaine doses starting from 2.5 to 40 mg/kg. The lowest cocaine doses were used to assess whether the higher sensitivity of D2R−/− animals revealed by direct D1R stimulation might be obscured by restricting the analyses only to high concentrations of the drug. The effect of cocaine on motor behavior was assessed during the first hour after the injection. Behavioral observations, followed by statistical analyses, indicated that although WT animals increase their forward locomotion in a cocaine dose-dependent manner, the motor stimulant effects of cocaine were dramatically reduced in the absence of D2R at all doses tested (Fig. 2A). Two-way ANOVA analyses, using genotype and treatment as main factors, pointed to a significant [genotype × treatment] interaction (F(4, 114) = 19.34, P < 0.0001). The response to cocaine in D2R−/− reached a plateau at 10 mg/kg and did not increase further at 20 and 40 mg/kg. The time-course profile of forward locomotion in animals of both genotypes showed that the reduced forward locomotion in D2R−/− mice (Fig. 2A) was due to a severe quantitative and qualitative alteration of the response to cocaine effects (Fig. 2 B and C) rather than a simple attenuation of the amplitude of this response over time. A three-way ANOVA with repeated measures over time confirmed this observation, indicating a significant [time × treatment × genotype] interaction (F(44, 331) = 2.39, P < 0.0001).
Fig. 2.
Altered motor response to acute cocaine challenge in D2R−/− mice. (A) Horizontal locomotion over 1 h after cocaine injection in WT (■) and D2R−/− (○). Statistics showed significant genotype and treatment effects and [genotype × treatment] interaction. (B and C) Time course of horizontal locomotion (5 min bins) after cocaine injections in WT (B) and D2R−/− (C). Post hoc analyses revealed absent [time × treatment] interaction (F(44, 147) = 0.99, P = 0.4926) only in D2R−/− mice. (D) Rearing behavior upon cocaine injections in WT (filled bars) and D2R−/− (open bars). Statistics showed significant genotype and treatment effects and a trend for a [genotype × treatment] interaction [F(4, 106) = 2.17, P = 0.0773]. (E) Grooming behavior upon cocaine in WT (filled bars) and D2R−/− (open bars) mice. Results showed significant genotype and treatment effects and [genotype × treatment] interaction. Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P < 0.0001. Genotype difference for the same treatment: #, P < 0.05; ##, P < 0.001; ###, P < 0.0001.
Analysis of stereotyped behaviors (34) showed a downward trend in cocaine-induced rearing behavior (Fig. 2D, [genotype × treatment] interaction: F(4, 106) = 2.17, P = 0.0773) in D2R mutants while indicating a highly exacerbated cocaine-induced grooming frequency in D2R−/− mice compared with WT (Fig. 2E, significant [genotype × treatment] interaction: F(4, 131) = 3.37, P = 0.0116). In D2R−/− mice, high cocaine doses also induced characteristic intense grooming episodes which very likely traded off forward locomotion. This might participate in the dramatic reduction in the cocaine-induced horizontal activity in these mutants. These results also suggest that although D1R-dependent signaling appears highly stimulated in the absence of D2R (Fig. 1), stimulation by cocaine cannot be converted into a normal motor output in the absence of D2R-mediated events.
Reduced Sensitivity to Cocaine-Induced Conditioned Place Preference (CPP) in D2R−/− Mice.
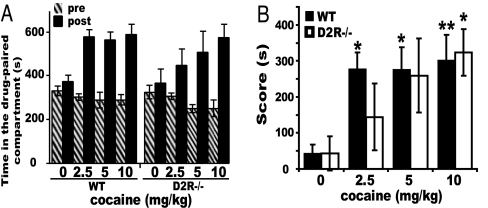
We then extended our analysis to the rewarding properties of cocaine in these mutants. For this, D2R−/− and WT littermates were tested in the CPP paradigm (35). Experiments were performed by using three different doses of cocaine (2.5, 5, and 10 mg/kg). Cocaine induced CPP in animals of both genotypes, as shown by the increase in the time spent in the drug-associated compartment during the postconditioning test (Fig. 3A). However, statistical analyses using the score (defined as the difference in the time spent during post- versus preconditioning phases in the drug-paired compartment) showed a difference in the dose-response effect in D2R−/− versus WT mice (Fig. 3B). Post hoc comparison in D2R−/− mice showed a decreased CPP to cocaine, reaching statistical significance (P < 0.05) compared with the saline group only at the highest dose tested (10 mg/kg). On the contrary, WT littermates showed a robust CPP response at all doses, statistically significant already at 2.5 mg/kg (Fig. 3B). These results show decreased sensitivity to the rewarding properties of cocaine in D2R−/− mice. Significant CPP response, only at the highest cocaine dose, in D2R−/− mice might suggest the involvement of other neuromodulator systems in cocaine addiction (36).
Fig. 3.
Cocaine-induced CPP in D2R−/− mice. (A) Time spent in the cocaine-paired compartment during pre- (hatched bars) and postconditioning (black bars) phases in WT and D2R−/− littermates. (B) CPP score [difference in time spent (s) in post- vs. preconditioning phases in the drug-paired compartment]. Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001.
Absence of D2R Signaling Uncovers a Divergent Postsynaptic Activation of IEGs.
The mRNA of c-fos and zif268 (also named Egr-1/NGFI-A/Krox-24/TIS8/ZENK) is strongly induced by acute cocaine treatments (37) through a D1R-mediated mechanism (13, 14, 18, 31).
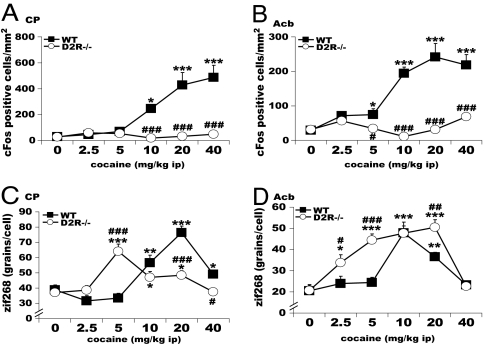
Dose-response in situ hybridization analyses were performed to evaluate the c-fos mRNA induction pattern 1 h after acute cocaine treatment (2.5, 5, 10, 20, and 40 mg/kg) in the home cage (Fig. 5 A and B). As previously reported (14, 37), a dose-dependent increase in c-fos positive cells was observed in the frontal cortex, CP, and Acb. Strikingly, induction of c-fos expression by cocaine in ventral and dorsal striatum, as well as in all of the major cortico-limbic targets of DAergic projections, was completely abolished in D2R−/− animals (Fig. 5 A and B). Loss of c-fos induction in D2R−/− mice was observed also in the orbito-frontal cortex, the most sensitive brain region in WT siblings. A thorough quantification of the number of c-fos positive cells in D2R mutants vs. WT littermates confirmed the absence of any cocaine-induced c-fos-positive cells in the mutants (F(1, 84) = 0.26, P = 0.6129). These results were further confirmed at the protein level by Western blot and gel shift analyses comparing striatal extracts from WT and D2R−/− mice (data not shown).
Fig. 5.
Cocaine induced c-fos and zif268 expression in D2R−/− mice. c-fos induction in the CP (A) and Acb (B) in WT (■) but not D2R−/− (○), whereas zif268 induction is present in both WT and D2R−/− after acute cocaine. zif268 quantifications indicate a significant [genotype × treatment] interaction in the CP (C) and also in Acb (D). Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P < 0.0001. Genotype difference for the same treatment: #, P < 0.05; ##, P < 0.001; ###, P < 0.0001.
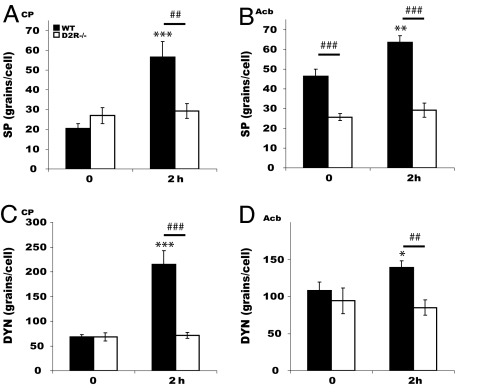
Similarly, analyses of the pattern of induction of substance P (SP) (Fig. 4 A and B) and dynorphin (Dyn) (Fig. 4 C and D) 2 h after cocaine treatment showed that these genes failed to be induced in D2R−/− striata either at 10 or 20 mg/kg cocaine. However, in WT striata, both genes were induced 2 h after cocaine injections (20 mg/kg).
Fig. 4.
SP and Dyn expression after acute cocaine. SP and DYN mRNAs induction 2 h after cocaine (20 mg/kg) in CP (A and C) and Acb (B and D) of WT (filled bars) but not of D2R−/− (open bars) mice. Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P < 0.0001. Genotype difference for the same treatment: ##, P < 0.001; ###, P < 0.0001.
c-fos expression in striatal neurons has been reported to require the simultaneous activation of D1R and NMDA receptors (3, 16). Our data show that the D1R pathway is greatly stimulated in D2R−/− mice, thus suggesting that activation of the NMDA glutamatergic pathway might be impaired in D2R−/− mice. To assess this possibility, we analyzed the behavioral effect of a glutamatergic antagonist in D2R−/− and WT littermates. Administration of phencyclidine induces hyperlocomotion through blockade of cortical NMDA receptors (30). Interestingly, we found that although both genotypes respond to phencyclidine with an increased motor behavior, the response of D2R−/− mice to 7.5 mg/kg phencyclidine is greatly attenuated [supporting information (SI) Fig. 7]. Thus, loss of c-fos induction by cocaine might only partially depend on a diminished glutamatergic signaling in D2R mutants, suggesting the implication of an inhibitory signal, unmasked in the absence of D2R, on the expression of this IEG.
In contrast, the expression of zif268, which has a similar pattern and induction properties as c-fos, was strongly stimulated by cocaine in D2R−/− mice (Fig. 5 C and D). We detected and quantified a higher sensitivity of D2R−/− mice in the induction level of this gene. The dose–response curve of zif268 expression was left-shifted, showing a statistical significant response at 2.5 and 5 mg/kg cocaine in the Acb of D2R−/− mice, whereas in WT animals there was no increase of zif268 mRNA at these doses (Fig. 5D). Also in the CP we observed a similar profile, although in this brain area a significant stimulation by cocaine in D2R−/− mice was observed at 5 mg/kg cocaine, whereas zif268 expression in WT littermates becomes evident only at 10 mg/kg (Fig. 5C). We also observed a stronger zif268 response in the Acb of D2R−/− vs. WT mice as compared with the CP, where the dose–response profile and maximal level of expression were comparable in the two genotypes (Fig. 5 C and D). These experiments reveal a dissociation in the cocaine-induced c-fos and zif268 expression in the absence of D2R-mediated signaling. zif268 induction might be less dependent on alteration of other neurotransmitters, such as glutamate, than c-fos. However, zif268 induction by cocaine was completely blocked by pretreatment of WT and D2R mutants with the NMDA antagonist MK801 (SI Fig. 8). These results suggest that absence of c-fos induction could not result from altered glutamate signaling in D2R mutants. c-fos and zif268 expression is partly regulated by different signaling molecules (38), possibly suggesting a lack of D2R-mediated activation of specific pathways, leading to c-fos induction in D2R mutants. Alternatively, these results might suggest the presence of an inhibitory signal, unmasked in the absence of D2R, which counteracts the activation by cocaine of specific signaling molecules.
Pre- Versus Postsynaptic D2R-Mediated Effects of Cocaine.
D2Rs exist in vivo in two isoforms, D2L and D2S. We have previously shown that D2S has major presynaptic autoreceptor functions, because in D2L−/− mice (28) regulation of DA release is maintained (27) despite loss of postsynaptic D2-mediated effects (12, 28). Thus, we assessed whether behavioral alterations to cocaine in D2R−/− mice are dependent on pre- or postsynaptic D2-mediated effects. Motor behavior of WT and D2L−/− littermates was analyzed in response to acute increasing doses of cocaine. Interestingly, these experiments showed that both genotypes similarly ([genotype × treatment] [F(4, 60) = 1.07, P = 0.3792]) increase their forward locomotion in a cocaine dose-dependent manner (Fig. 6A). Statistical analyses also revealed a genotype effect (F(4, 60) = 5.86, P = 0.0186) on motor behavior, which has been already observed in response to mixed DAergic agonists in D2L−/− mice (28).
Fig. 6.
Cocaine effects in D2L−/− mice. (A) Horizontal locomotion over 1 h after cocaine injection in WT (filled squares) and D2L−/− (open circles). Statistics showed significant genotype and treatment effects but no [genotype × treatment] interaction. (B) Grooming behavior upon cocaine injection in WT (filled bars) and D2L−/− mice (open bars). There were significant treatment and genotype effects but no [genotype × treatment] interaction. (C) Time spent in the cocaine-paired compartment during pre- (hatched bars) and postconditioning (gray bars) phases in WT and D2L−/− littermates. (D) Score (s) of WT (filled bars) and D2L−/− (open bars) mice after cocaine CPP test showing similar responses in WT and D2L−/− mice except at 1.25 mg/kg. Treatment effect compared with its control: ∗, P < 0.05; ∗∗, P < 0.001; ∗∗∗, P< 0.0001.
By difference with D2R−/− mice, grooming behavior after acute cocaine (Fig. 6B) similarly increased in WT and D2L−/− mice in a dose-dependent manner.
Similar results were also obtained in the CPP test. Cocaine conditioning (1.25, 2.5, and 5 mg/kg) produced robust CPP in both WT and D2L−/− mice (Fig. 6C). Two-way ANOVA using the score as the dependent variable showed significant treatment effect (F(3, 58) = 11.78, P < 0.0001) and no genotype effect (F(1, 58) = 1.84, P = 0.17) or [treatment × genotype] interaction (F(3, 58) = 0.35, P = 0.78). Post hoc comparison between saline and cocaine-treated mice revealed a significant effect of cocaine in CPP at 1.25 mg/kg in D2L−/− but not in WT mice (Fig. 6D). Comparison of results obtained in D2R−/− versus D2L−/− mice reveal an important role of presynaptic D2S-dependent signaling in the behavioral response to psychostimulants.
Discussion
Genetic and pharmacological studies on the molecular mechanisms underlying the response to drugs of abuse converge toward the conclusion that D1R-mediated signaling is an absolute requirement in the events that modulate the motor and limbic pathways (18, 39, 40). In addition, a clear involvement of the glutamatergic system has also been shown (3, 17). Blockade of either D1R or of the glutamate signaling strongly impairs the effect of cocaine and other drugs. Nevertheless, inhibitors of both the DAergic and glutamatergic signaling also have to be considered in the final physiological output generated by the use of illicit drugs.
Pharmacological studies have previously addressed the role of D2R in the motor effect of cocaine in mice (9, 39). D2R-specific antagonists were shown to dose-dependently block the psychomotor effects of cocaine. However, the interpretation of pharmacological results is confounded by the motor inhibitory effects of high doses of D2-antagonists on locomotion, even in the absence of cocaine. Furthermore, not all D2R antagonists were able to block cocaine effects (9). Our studies, thereby, adds to previous analyses by elucidating the role of D2R in genetically altered mice in which the pharmacological caveats can be eliminated. In support of pharmacological analyses, D2R−/− mice present a reduction of motor behavior under basal conditions (23) but are still able to move. Loss of D2R does not significantly alter the expression of other DA receptors (23, 41), including D1R. Indeed, D2R−/− mice respond to apomorphine, a mixed D1-D2 agonist, by an increase of motor activity (28). In addition, like the full D1 agonist SKF 81297 (28), SKF 82958 (42, 43) elicits a similar dose-response increase in forward locomotion in D2R−/− and WT littermates (Fig. 1).
In the absence of D2R, cocaine treatments result in an exaggerated striatal increase of extracellular DA levels, due to loss of D2R-mediated presynaptic autoreceptor functions (27). This might lead to D1R overstimulation in D2R−/− mice as indicated by the reduction of forward locomotion in favor of stereotypies in these mutants. This argument appears supported by the observations that the onset and intensity of stereotypies in D2R−/− mice are already at a maximal level at 10 mg/kg cocaine, whereas they become significant only at the dose of 40 mg/kg in WT littermates. Alternatively, the flat motor response to doses of cocaine higher than 10 mg/kg might suggest the involvement of additional inhibitory mechanisms revealed in the absence of D2R-mediated signaling.
The hedonic properties of cocaine are substantially preserved in D2R−/− mice, although attenuated, in agreement with previous reports (24). Similarly, in the CPP analysis, whereas WT mice show a clear CPP at 2.5 mg/kg cocaine, D2R−/− mice showed a statistical significant response only at 10 mg/kg. D2R−/− mice self-administer cocaine at higher rates than WT littermates (25), suggesting missing mechanisms, in these mutants, that normally limit rates of high-dose cocaine self-administration. This might also indicate the involvement of other neuromodulators in the induction of CPP and self-administration by cocaine in D2R mutants. Indeed, the rewarding (44) and reinforcing properties (36) of cocaine are preserved in mice lacking the DA transporter, a primary target of this drug.
At the gene expression level, as expected (13), D1R-mediated induction of IEGs such as c-fos by SKF 82958 is conserved in D2R−/− mice. SKF 82958 elicits a stronger c-fos induction in the cortex and striatum of D2R−/− as compared with WT mice, suggesting that D1R-mediated postsynaptic functions are not defective in D2R−/− mice after direct agonist challenge. The enhanced molecular response to D1 stimulation in D2R−/− is what would be expected by the removal of the D2R-mediated inhibitory signaling.
However, D1R activation by cocaine in D2R mutants is translated into a differential activation of zif268 and c-fos. zif268 expression, activated by cocaine through similar mechanisms than c-fos (15, 18), is normally induced in D2R−/− mice. Interestingly, we report an increased sensitivity of zif268 expression to very low cocaine doses in D2R−/− mice compared to WT siblings. The mechanism underlying the higher sensitivity of zif268 to cocaine in D2R−/− mice is presently unclear. It might correlate with the increased stimulation of D1R due to the massive DA release in D2R−/− mice (27). But it might also depend on the activation of this gene by different neuromodulators uncovered only in the absence of D2R.
On the contrary, by difference with WT, we report absence of induction of c-fos, Dyn, and SP in D2R−/− mice. It is classically assumed that D1R and D2R are segregated in different striatal neurons, which belong to the striato-nigral and striato-pallidal pathways, respectively (13). Striatal neurons are further organized in striosomes and matrix compartments (37). Our results are at odds with the reported linearity between stimulation of the D1R-mediated signaling and expression of zif268, c-fos, Dyn, and SP in striato-nigral neurons (10, 40) comprising the striosomes, which do not express D2R (13, 37). Because c-fos is not induced in any striatal neurons, and because we have not assessed whether zif268 induction is observed in striato-nigral or D2R-expressing striato-pallidal neurons, we cannot exclude the possibility that zif268 expression might be induced in different neurons than striato-nigral. In addition, although c-fos and zif268 have usually been reported to follow common induction pathways, recent studies have shown a similar differential activation of IEGs by cocaine in animals lacking the expression of the mitogen- and stress-activated protein kinase-1 (38) as in D2R−/− mice. This might suggest a possible link between D2R-mediated signaling and this kinase; however, in MSK1-KO mice, c-fos expression can be rescued at high cocaine concentrations, by difference with what we report in D2R−/− mice at any dose tested. Several hypotheses can be drawn to explain lack of c-fos induction in D2R−/− mice. First, D1R overstimulation might lead to increased c-fos induction, which then blocks c-fos expression by an autoregulatory mechanism (33, 45). However, the time course of c-fos autoregulation (≈2 h) does not match the blunted c-fos expression in D2R−/− mice observed 1 h after cocaine. Furthermore, results obtained by direct stimulation of D1R using D1-specific ligands (Fig. 1) in D2R mutants argue against this possibility. Second, glutamatergic stimulation of striatal medium spiny neurons in D2R mutants required for a full induction of this gene (16) is also altered. However, we show that the response to phencyclidine in D2R mutants is present although attenuated, thus possibly contributing but not explaining the cause of loss of cocaine effects on c-fos expression. In addition, zif268 expression, which also requires glutamate stimulation, is unaffected in D2R mutants and pretreatment of D2R−/− mice with MK801 before cocaine eliminates zif268 induction in WT and D2R mutants. This definitely rules out a major glutamatergic effect on loss of c-fos induction. Importantly, electrophysiological studies have shown that cocaine depresses striatal GABAergic synaptic transmission through D2R-mediated effects (29). In D2R mutants, a presynaptic D2-mediated modulation of the GABAergic response to cocaine is lost, resulting in a stronger inhibitory control of striatal neurons by this neurotransmitter. This might counteract the cellular and psychomotor response to cocaine in D2R−/− mice.
D2R is composed of two isoforms, D2L and D2S, yet with different functions in vivo. Behavioral and neurochemical analyses of mice lacking D2L have shown that in D2L−/− mice, the presynaptic D2-mediated responses are intact (15, 27, 28), identifying D2S as the isoform implicated in presynaptic functions (15, 27, 28). In line with a major contribution of loss of presynaptic D2S receptors, in the aberrant response to cocaine of D2R−/− mice, D2L−/− mutants respond to this drug quite similar to WT mice.
In conclusion, the analysis of D2R−/− mice in response to cocaine reveals the relevance of the inhibitory control exerted by presynaptic D2-mediated signaling, very likely D2S-dependent, on the molecular mechanisms activated by this drug. Absence of presynaptic D2-mediated effects impinges on DAergic as well as cortical and striatal neurons physiology. This, together with the wide range of cocaine effects in the brain, makes the identification of a possible inhibitory system unmasked by absence of D2Rs quite complex. Nonetheless, based on these and previous studies, we would like to speculate that the absence of presynaptic D2R-mediated functions by profoundly modifying GABAergic signaling lead to altered integrated responses to psychostimulants.
Materials and Methods
Animals.
Mice were housed under standard conditions (12 h light/dark cycle). D2R−/− (23), D2L−/− (28), and their WT littermates (25% 129/Sv and 75% C57BL/6J) were obtained from heterozygotes breeding; age-matched males were used for experiments.
Treatments.
SKF 82958 (Sigma-RBI) in saline at 0.03, 0.1, 0.3, 1, or 5 mg/kg or saline was administered s.c. (n = 5 WT and n = 5 D2R−/− mice per group).
For acute treatments, saline or cocaine (cocaine hydrochloride; Sigma, St. Louis, MO) in saline were injected i.p. at the following doses: 2.5, 5, 10, 20, or 40 mg/kg in WT and D2R−/− (n = 8–21 per dose) and 5, 10, 20, or 30 mg/kg in WT and D2L−/− (n = 7–8 per dose).
SKF82958 and cocaine were tested in habituated mice (i.e., individually placed in testing cages 2 h before treatment).
For CPP experiments, WT and D2R−/− were injected with saline (WT: n = 11; D2R−/−: n = 8) or cocaine at 2.5 mg/kg (n = 10 per genotype), 5 mg/kg (WT: n = 11; D2R−/−: n = 9), or 10 mg/kg (WT: n = 9; D2R−/−: n = 10). WT and D2L−/− were injected with saline (WT: n = 12; D2L−/−: n = 9) or cocaine at 1.25 mg/kg (WT: n = 8; D2L−/−: n = 7), 2.5 mg/kg (n = 8 per genotype), or 5 mg/kg (n = 7 per genotype).
Behavioral Analyses.
Motor activity was evaluated in the home cage (20 × 30 cm) for 1 h by using a VIDEOTRACK system (Viewpoint, Lyon, France). Cocaine-induced stereotypies were visually scored as described (46).
CPP apparatus consists of two squared compartments (height 30 × 15 × 15 cm) with circled or hatched visual motif, separated by a guillotine door. Each compartment contained visual and tactile cues (three identical cylinders, spaced by 0.5 cm, diameter of 2 cm, height 1 cm), positioned either horizontally or vertically. The time spent in each compartment during pre- and postconditioning tests (15 min) was measured (47).
In Situ mRNA Hybridization and Quantification.
Brain coronal cryostat sections (10 μm) were hybridized (23) with [35S]CTP sense or antisense riboprobes specific for murine c-fos (48), zif268 (49), proDyn, and SP (23). Autoradiographic films and photographic emulsions of in situ experiments were used to quantify gene expression (50) by using ImageJ software (National Institutes of Health, Bethesda, MD). (Observations used to quantify expression/gene/condition: c-fos, n = 8; zif268, n = 24; SP, n = 12; Dyn, n = 12–44.)
Statistical Analysis.
Results were analyzed by ANOVA followed by the appropriate post hoc comparisons (P < 0.05 was considered statistically significant).
Supplementary Material
Acknowledgments
We thank Valerie Heidt and Eric Erbs for assistance and Valeria DiDato for discussions. This work was supported by grants from the Mission Interministerielle de Lutte contre la Drogue et la Toxicomanie (MILDT) and European Community Contract LSHM-CT-2004-005166 (to E.B.). M.W. was the recipient of European Union Contract LSHM-CT-2004-005166 and Region Alsace fellowships.
Abbreviations
- DA
dopamine
- D2R
dopamine type 2 receptor
- DAergic
dopaminergic
- Acb
nucleus accumbens
- CP
caudate-putamen
- D1R
dopamine type 1 receptor
- IEG
immediate early gene
- CPP
conditioned place preference
- SP
substance P
- Dyn
dynorphin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610790104/DC1.
References
- 1.Di Chiara G, Imperato A. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Chiara G. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE, Malenka RC, Nestler EJ. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Iaccarino C, Saiardi A, Heidt V, Bozzi Y, Picetti R, Vitale C, Westphal H, Drago J, Borrelli E. Proc Natl Acad Sci USA. 2004;101:11465–11470. doi: 10.1073/pnas.0402028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett RL, Appel JB. Psychopharmacology (Berlin) 1989;99:13–16. doi: 10.1007/BF00634445. [DOI] [PubMed] [Google Scholar]
- 9.Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. Psychopharmacology (Berl) 1991;105:335–339. doi: 10.1007/BF02244427. [DOI] [PubMed] [Google Scholar]
- 10.Steiner H, Gerfen CR. Exp Brian Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. Proc Natl Acad Sci USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Moine C, Svenningsson P, Fredholm BB, Bloch B. J Neurosci. 1997;17:8038–8048. doi: 10.1523/JNEUROSCI.17-20-08038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JQ, McGinty JF. Neuroscience. 1996;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- 16.Konradi C, Leveque JC, Hyman SE. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drago J, Gerfen CR, Westphal H, Steiner H. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 19.Sorg BA, Guminski BJ, Hooks MS, Kalivas PW. Brain Res Mol Brain Res. 1995;29:381–386. doi: 10.1016/0169-328x(94)00281-i. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado R, Saiardi A, Valverde O, Samad TA, Roques BP, Borrelli E. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 21.Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. J Neurosci. 2002;22:RC224. doi: 10.1523/JNEUROSCI.22-10-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. Psychopharmacology (Berlin) 2000;152:343–350. doi: 10.1007/s002130000548. [DOI] [PubMed] [Google Scholar]
- 23.Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- 24.Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Psychopharmacology (Berlin) 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- 25.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. J Neurosci. 2002;22:2977–2988. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. J Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 29.Centonze D, Picconi B, Baunez C, Borrelli E, Pisani A, Bernardi G, Calabresi P. Neuropsychopharmacology. 2002;26:164–175. doi: 10.1016/S0893-133X(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 30.Takahata R, Moghaddam B. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- 31.LaHoste GJ, Yu J, Marshall JF. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cromwell HC, Berridge KC. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan JI, Curran T. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA, Bozarth MA. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 35.Tzschentke TM. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 36.Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 37.Graybiel AM, Moratalla R, Robertson HA. Proc Natl Acad Sci USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chausmer AL, Katz JL. Psychopharmacology (Berlin) 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]
- 41.Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, Borrelli E. Crit Rev Neurobiol. 1997;11:121–142. doi: 10.1615/critrevneurobiol.v11.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 42.Nisenbaum ES, Mermelstein PG, Wilson CJ, Surmeier DJ. Synapse. 1998;29:213–224. doi: 10.1002/(SICI)1098-2396(199807)29:3<213::AID-SYN3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Ruskin DN, Rawji SS, Walters JR. J Pharmacol Exp Ther. 1998;286:272–281. [PubMed] [Google Scholar]
- 44.Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sassone-Corsi P, Sisson JC, Verma IM. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- 46.Clifford JJ, Usiello A, Vallone D, Kinsella A, Borrelli E, Waddington JL. Neuropharmacology. 2000;39:382–390. doi: 10.1016/s0028-3908(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 47.Acquas E, Carboni E, Leone P, Di Chiara G. Psychopharmacology (Berlin) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- 48.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. Cell. 1988;55:917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 49.Christy BA, Lau LF, Nathans D. Proc Natl Acad Sci USA. 1988;85:7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerfen CR, Keefe KA, Gauda EB. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.