Abstract
Angiogenesis is necessary for tumor growth beyond a volume of approximately 2 mm3. This observation, along with the accessibility of tumor vessels to therapeutic targeting, has resulted in a research focus on inhibitors of angiogenesis. A number of endogenous inhibitors of angiogenesis are found in the body. Some of these are synthesized by specific cells in different organs, and others are created by extracellular proteolytic cleavage of plasma-derived or extracellular matrix-localized proteins. In this review, we focus on angiostatin, endostatin, PEX, pigment epithelial-derived factor, and thrombospondin (TSP)-1 and -2, either because these molecules are expressed in malignant glioma biopsies or because animal studies in malignant glioma models have suggested that their therapeutic administration could be efficacious. We review the known mechanisms of action, potential receptors, expression in glioma biopsy samples, and studies testing their potential therapeutic efficacy in animal models of malignant glioma. Two conclusions can be made regarding the mechanisms of action of these inhibitors: (1) Several of these inhibitors appear to mediate their antiangiogenic effect through multiple protein-protein interactions that inhibit the function of proangiogenic molecules rather than through a specific receptor-mediated signaling event, and (2) TSP-1 and TSP-2 appear to mediate their antiangiogenic effect, at least in part, through a specific receptor, CD36, which initiates the antiangiogenic signal. Although not proven in gliomas, evidence suggests that expression of specific endogenous inhibitors of angiogenesis in certain organs may be part of a host antitumor response. The studies reviewed here suggest that new antiangiogenic therapies for malignant gliomas offer exciting promise as nontoxic, growth-inhibitory agents.
Angiogenesis, the growth of a new vasculature from preexisting vessels, is a multistep process that occurs normally during a number of bodily functions, such as wound healing, embryogenesis, the female reproductive cycle, and the development of a collateral blood circulation following the occlusion of vessels (Liekens et al., 2001). It also occurs in pathologic processes, such as tumor invasion and metastasis, rheumatoid arthritis, and psoriasis (Liekens et al., 2001). Angiogenesis may also be promoted by stem cells that are recruited to a tumor bed and differentiate into endothelial cells or into a supportive cell (Allport et al., 2004; Annabi et al., 2004; Fears et al., 2004). The most frequently used quantitative measurement of angiogenesis is an assessment of microvessel density in a given tissue area based on immunohistochemical identification of microvessels with an antibody directed toward CD31 (PECAM-1) or CD34 (Liekens et al., 2001).
For angiogenesis to occur, the balance of proangiogenic and antiangiogenic factors must favor the proangiogenic factors, and this has been termed the “angiogenic switch” (Hanahan and Folkman, 1996). In tumor angiogenesis, proangiogenic growth factors, such as basic fibroblast growth factor (bFGF)3 and vascular endothelial cell growth factor (VEGF), are secreted by the tumor cells, as well as by platelets and potentially vascular mesenchymal cells (Hanahan and Folkman, 1996; Liekens et al., 2001). These factors bind specific receptors on endothelial cells, which leads to the activation of the endothelial cell. The activation of endothelial cells results in the upregulation of specific integrin receptors on the cell surface such as integrin αvβ3 and α5β1, cell proliferation, and protease secretion (Brooks, 1996; Gladson, 1996; Kim et al., 2000a). Proteases degrade the underlying basement membrane and provide a “route” for sprouting or migrating endothelial cells (Liekens et al., 2001). Tube or lumen formation occurs in the sprouted endothelial cells. However, the new microvessels formed in tumor angiogenesis are abnormal and remain leaky, as they lack a properly formed basement membrane and demonstrate other morphologic abnormalities (Hanahan and Folkman, 1996; Liekens et al., 2001).
For this review, we selected endogenous inhibitors of angiogenesis that either have been expressed in malignant glioma biopsies or have been suggested as an efficacious therapy by the results of animal studies in malignant glioma models. We focus on angiostatin, endostatin, PEX, pigment epithelial-derived factor (PEDF), and thrombospondin (TSP)-1 and -2, and we include a description of the known mechanisms of action, potential “receptors” (receptor-like molecules), expression in glioma biopsy samples, and studies testing their potential therapeutic efficacy in animal models of malignant glioma.
Proteolytic Fragments of Proteins as Inhibitors of Angiogenesis
Angiostatin
Mechanism of Action and Known Interactions with Potential Receptors or Other Proteins
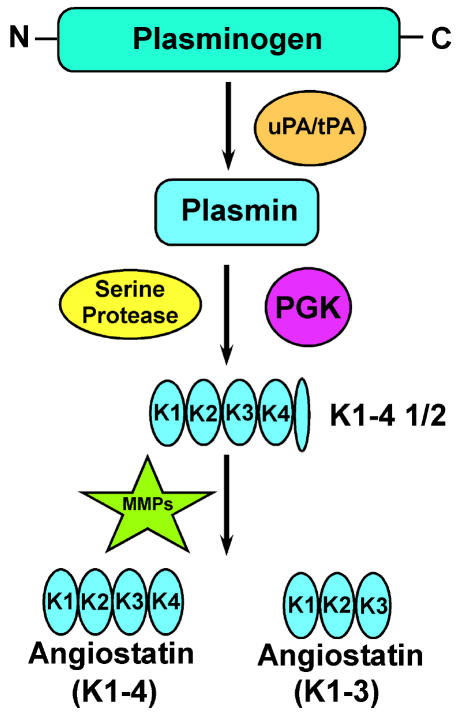
Angiostatin is an internal fragment of plasminogen that was first described by O’Reilly and colleagues (1994). This fragment contains the first three or four kringle (K) domains of plasminogen (K1–3 or K1–4). The process by which angiostatin is generated is thought to be as follows: Urinary-type plasminogen activator or tissue-type plasminogen activator proteolytically cleaves plasminogen to generate plasmin, followed by reduction of the disulfide bonds in the fifth K domain, potentially by phosphoglycerate kinase. Subsequent proteolysis of peptide bonds by an unknown serine protease results in the generation of the K fragment K1–4½, followed by proteolytic cleavage by matrix metalloproteinase (MMP) to yield angiostatin (K1–3 or K1–4) (Geiger and Cnudde, 2004) (Fig. 1).
Fig. 1.
Pathway for the generation of angiostatin from plasminogen (outlined by Geiger and Cnudde [2004]). Angiostatin is thought to be generated from plasminogen by the following steps: u-PA (urikinase plasminogen activator) or t-PA (tissue plasminogen activator) proteolytically cleaves plasminogen to generate plasmin, and a reduction of disulfide bonds (potentially by phosphoglycerate kinase [PGK]) allows a serine protease to proteolytically cleave plasmin, generating K1–4½, followed by proteolytic cleavage by MMPs to generate angiostatin (K1–4 or angiostatin K1–3).
Four potential “receptors” for angiostatin have been identified: integrin αvβ3, ATP synthase, angiomotin, and the NG2 chondroitin sulfate proteoglycan (CSPG) (Chekenya et al., 2002; Moser et al., 1999; Tarui et al., 2001; Troyanovsky et al., 2001).
Integrin αvβ3
Integrin αvβ3 mediates cell adhesion to a variety of extracellular matrix proteins including fibronectin, degraded collagen, and vitronectin (Janssen et al., 2002; Tarui et al., 2001). Integrin αvβ3 is upregulated on the surface of proliferating endothelial cells in angiogenic microvessels, including those in glioblastoma (grade IV malignant astrocytoma) tumors (Brooks, 1996; Gladson, 1996). Antagonists directed toward integrin αvβ3 have been shown to inhibit tumor neovascularization in several animal models of different cancers, which supports a proangiogenic role for integrin αvβ3 (Brooks et al., 1994; Janssen et al., 2002; Reinmuth et al., 2003). Integrin αvβ3 on the surface of bovine aortic endothelial cells mediates attachment to angiostatin (Tarui et al., 2001), and in Chinese hamster ovary cells expressing the β3 integrin, adherence to angiostatin results in decreased cell spreading and decreased stress fiber formation (Tarui et al., 2001). These data suggest that angiostatin may act as an antagonist of integrin αvβ3 by binding to this integrin and competitively inhibiting its interaction with matrix ligands, thereby reducing cell attachment and adhesion, which are necessary for endothelial cell survival and migration (see Fig. 2A).
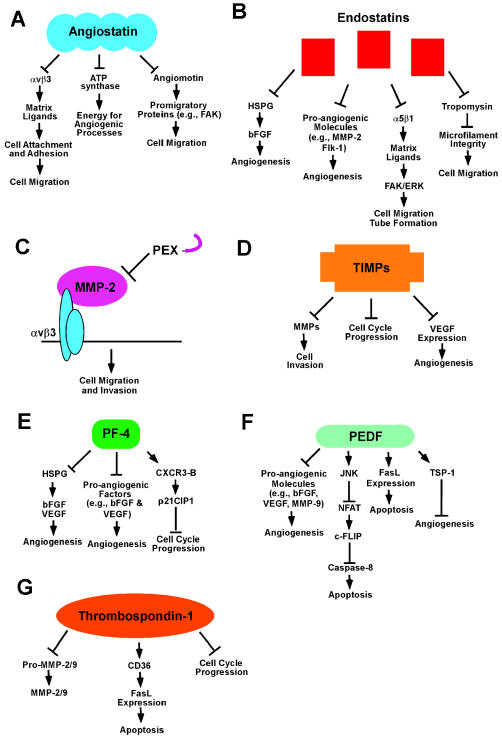
Fig. 2.
Mechanism of inhibition of angiogenesis by angiostatin, endostatin, PEX, TIMPs, PF-4, PEDF, and TSP-1. A. Potential “receptors” by which angiostatin mediates its antiangiogenic effect(s) include integrin αvβ3, ATP synthase, and angiomotin. B. Endostatin mediates its antiangiogenic effects via interactions with HSPG, proangiogenic molecules, α5β1, and tropomyosin. C. PEX inhibits angiogenesis by binding integrin αvβ3 and preventing the proangiogenic interaction of this integrin with MMP-2. D. TIMPs block angiogenesis by directly binding MMPs and inhibiting their activity, by blocking cell proliferation, and by downregulating VEGF expression. E. PF-4 can inhibit angiogenesis by competing with proangiogenic growth factors for binding to HSPG, by directly binding to proangiogenic growth factors, or by binding the chemokine receptor CXCR3-B and upregulating p21CIP1. F. PEDF mediates its antiangiogenic effect through the downregulation of proangiogenic molecules, such as bFGF, VEGF, and MMP-9; the induction of apoptosis; and the upregulation of antiangiogenic molecules such as TSP-1. G. TSP-1 can inhibit angiogenesis, at least in part, through an interaction of the type 1 repeat domain with the CD36 receptor and induce apoptosis. TSP-1 and -2 can potentially inhibit angiogenesis through an interaction with pro-MMP-2 and -9, with MMP-2 and -9, or through an induction of cell cycle arrest.
The expression of integrin αvβ3 is also upregulated on tumor cells in grade III and IV malignant astrocytoma biopsies (Gingras et al., 1995; Gladson and Cheresh, 1991; Gladson et al., 1995; Paulus et al., 1993). Furthermore, integrin αvβ3 has been shown to mediate the attachment of glioblastoma cells and their migration toward various ligands in vitro (Ding et al., 2002; Gladson et al., 1995). Investigators probing the function of integrin αvβ3 on these tumor cells have shown that the overexpression of the integrin β3 subunit in astrocytes that have been transformed with constitutively active Ras suppresses in vivo tumor growth when these cells are propagated intracranially in an immunocompromised rat and that this inhibitory effect is overcome by the expression of VEGF or constitutively active Akt (Kanamori et al., 2004). In grade IV malignant astrocytoma tumor biopsies (glioblastomas), the upregulation of VEGF and the constitutive activation of Akt are frequently observed (Chakravarti et al., 2004; Choe et al., 2003; Goldman et al., 1993; Wang et al., 2004a). These data suggest that in this model the functional effects of integrin αvβ3 expression may be dependent on, or regulated by, other genetic alterations commonly found in glioblastomas. Other interpretations of these data are possible, and one must bear in mind that the overexpression of an integrin receptor that is capable of organizing the cytoskeleton can promote a stationary as opposed to a migratory cell phenotype and that integrin receptor signaling optimally occurs in cooperation with a growth factor receptor (Giancotti and Ruoslahti, 1999; Hynes, 1992). As the proliferating endothelial cells in glioblastoma tumors likely do not contain the genetic alterations found in glioblastoma tumors, the mechanisms regulating the function of integrin αvβ3 are likely different. The tumors derived from the intracerebral propagation of the Ras-transformed astrocytes overexpressing αvβ3 were accompanied by a reduced number of pericytes in tumor vessels. This suggests there is a complex regulation of the proliferating endothelial cells in these tumors and a complex interaction of proliferating endothelial cells with the tumor cells.
ATP synthase
Angiostatin binds to the α/β -subunits of a second potential receptor, ATP synthase, which are found in plasma membrane extracts of human umbilical vein endothelial cells (HUVECs), and it binds to bovine F1 ATP synthase. This results in an inhibition of ATP synthase activity (Moser et al., 1999, 2001). Thus the binding of angiostatin may interfere with ATP synthase activity, inhibiting this source of cellular energy and thereby inhibiting proangiogenic processes (Moser et al., 2001).
Angiomotin
The third potential receptor for angiostatin, angiomotin, is a 72-kDa, cell-surface-associated protein that was initially identified as a receptor for angiostatin by a yeast two-hybrid screen (Troyanovsky et al., 2001). This protein belongs to the family of coiled-coil, PDZ-domain containing molecules and is expressed in a variety of human cell lines, including dermal microvascular endothelial cells (MvECs) and HUVECs (Troyanovsky et al., 2001). Angiomotin has been co-localized with focal adhesion kinase (a promigratory, nonreceptor cytoplasmic tyrosine kinase) to lamellipodia at the leading edge of migrating NIH3T3 cells, as well as to circular ruffles in spreading HUVECs, which suggests a role for angiomotin in promoting cell motility (Troyanovsky et al., 2001). Consistent with this proposed promigratory function, mouse aortic endothelial cells transfected with angiomotin demonstrate increased random migration (Troyanovsky et al., 2001), increased invasion on type 1 collagen (Levchenko et al., 2004), and stabilization of endothelial cell tube structures in in vitro assays (Levchenko et al., 2004). Treatment of angiomotin-expressing mouse aortic endothelial cells with angiostatin inhibits both endothelial cell migration and capillary tube formation on Matrigel (BD Biosciences, San Jose, Calif.); thus, investigators have proposed that angiostatin could act as an antagonist of angiomotin by blocking the ability of angiomotin to interact with other promigratory proteins (Troyanovsky et al., 2001) (see Fig. 2A).
CSPG
A physical interaction between angiostatin and the NG2 CSPG has been identified by co-immuno-precipitation and cell-binding assays (Chekenya et al., 2002). Below we discuss the results of a study in which the NG2 CSPG was overexpressed in glioblastoma cells, followed by the propagation of these cells intracerebrally in the nude rat.
Angiostatin Inhibition of Tumor Growth in Animal Models of Malignant Glioma
A potential mechanism whereby the antiangiogenic effect of angiostatin could be blocked in glioblastoma tumors is through an interaction with the NG2 CSPG (Chekenya et al., 2002). This proteoglycan is expressed on a subpopulation of glioblastoma cells that are localized adjacent to proliferating endothelial cells (Schrappe et al., 1991). Chekenya et al. (2002) showed that when glioblastoma tumor cells transfected with the NG2 CSPG were propagated intracranially in nude rats, an increased tumor volume and microvessel density were found, as compared with tumors derived from implanted glioblastoma cells expressing a control vector. Also, angiostatin was localized to the vasculature of tumors derived from the implantation of glioblastoma cells transfected with a control vector, whereas in tumors derived from glioblastoma cells transfected with the NG2 CSPG, angiostatin was bound to this proteoglycan on the surface of the tumor cells (Chekenya et al., 2002). This report suggests that the NG2 CSPG expressed on the surface of glioblastoma tumor cells may bind to angiostatin and sequester it away from the vasculature of the tumor, thereby inhibiting the antiangiogenic effects of angiostatin and promoting tumor neovascularization (Chekenya et al., 2002). As the NG2 CSPG is expressed on a specific population of tumor cells in glioblastoma tumors, its potential inhibitory effect on angiostatin therapy in patients with glioblastoma tumors remains to be determined.
Rat C6 glioma tumors propagated subcutaneously in the athymic nude mouse followed by injection with adenovirus expressing angiostatin exhibit decreased tumor volume, decreased tumor vascularity, and increased tumor cell apoptosis (Griscelli et al., 1998). Furthermore, intravenous delivery of angiostatin-adenovirus 24 h prior to C6 glioma cell implantation inhibited the establishment of glioma tumors in 80% of treated animals, whereas tumor formation was detected in all animals administered control adenovirus prior to tumor cell implantation (Griscelli et al., 1998). Similarly, when C6 glioma cells were propagated intracerebrally and injected with an adeno-associated-virus angiostatin vector, markedly smaller tumors with reduced neovascularization and higher apoptotic indices were found (Ma et al., 2002). Recombinant angiostatin has also been reported to inhibit growth and neovascularization of intracerebral glioma xenografts, as well as to increase tumor cell apoptosis (Joe et al., 1999).
The efficacy of angiostatin as a therapeutic agent may be increased if administered in conjunction with other gene therapies. For example, in C6 glioma tumors propagated in rats, intratumoral delivery of adeno-associated- virus angiostatin vector in combination with an adenovirally expressed suicidal thymidine kinase gene resulted in decreased tumor volume and prolonged animal survival (Ma et al., 2002). Furthermore, fragments of angiostatin containing K1–3 may also be clinically useful, because when such a fragment was expressed in the SHG44 human glioma cells followed by propagation of these cells subcutaneously in the flank of nude mice, a significantly reduced tumor growth and angiogenesis was found (Zhang et al., 2000). In the above studies angiostatin therapy did not result in observed toxicity, and this suggests it could be a highly efficacious new therapy for patients with malignant glioma.
Endostatin
Mechanism of Action and the Known Protein Interactions (Receptors)
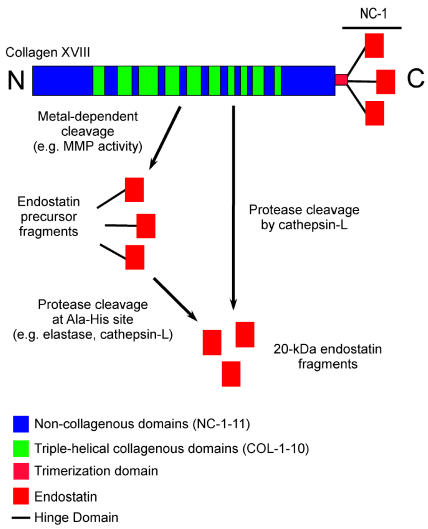
Endostatin is a 20-kDa carboxyl-terminal proteolytic cleavage fragment of collagen type XVIII (O’Reilly et al., 1997). It is thought to be generated through a two-step process, as follows: a metal-dependent early cleavage of collagen type XVIII, followed by cleavage at an Ala-His site (Wen et al., 1999) (Fig. 3). It is not entirely clear which protease(s) is responsible for endostatin generation from collagen type XVIII in vivo, as multiple proteases can cleave recombinant fragments of human collagen type XVIII to generate endostatin-like fragments (Ferreras et al., 2000). In a study testing approximately 12 different proteases, elastase and cathepsin-L were the only two proteases found to efficiently cleave fragments of recombinant human collagen type XVIII, generating endostatin-like fragments (Ferreras et al., 2000). In support of a role for cathepsin-L in the generation of endostatin, cathepsin-L can proteolytically generate endostatin from its precursor in murine hemangioendothelioma cells propagated in vitro (Felbor et al., 2000).
Fig. 3.
Schematic of collagen type XVIII and the protease cleavage to generate endostatin. Endostatin is derived from the NC-1 domain of collagen type XVIII. Endostatin can be directly cleaved from collagen type XVIII by cathepsin-L (Felbor et al., 2000). Alternatively, endostatin can be generated by a two-step process that is both metal dependent and elastase dependent (Wen et al., 1999).
Endostatin was originally reported to inhibit the proliferation of bovine capillary endothelial cells, but not the proliferation of cells of nonendothelial origin, and to also inhibit angiogenesis in the chick chorioallantoic membrane model (O’Reilly et al., 1997). The receptor or receptors mediating the biologic effects of endostatin are not known. However, endostatin can interact with several cell surface molecules, including heparan sulfate proteoglycans (HSPGs), such as glypican, integrin α5β1, tropomyosin, and the VEGF receptor KDR/Flk-1 (Ericksson et al., 2003; Karumanchi et al., 2001; MacDonald et al., 2001; Sasaki et al., 1999; Sudhakar et al., 2003).
Several reports have addressed the hypothesis that endostatin interacts with one or more HSPGs and thereby inhibits angiogenesis. As the proangiogenic growth factor bFGF requires an interaction with HSPG in order to optimally engage and activate its receptor, and endostatin binds HSPG, endostatin could theoretically displace bFGF from its interaction with HSPG and thereby inhibit bFGF signaling (Sasaki et al., 1999). Consistent with the above hypothesis, one report suggested that endostatin can inhibit bFGF-induced, but not VEGF-induced, angiogenesis (Sasaki et al., 1999). The interaction of endostatin with glypican is intriguing, as glypicans are HSPGs that can promote bFGF signaling through its receptor (FGFR1) in certain cellular contexts (Steinfeld et al., 1996). In addition, glypican-1 is upregulated on the proliferating MvECs in glioblastoma (grade IV malignant astrocytoma) tumors, and it promotes bFGF signaling in these cells (Qiao et al., 2003). Therefore, in glioblastoma tumors, endostatin interaction with the HSPG glypican-1 could potentially inhibit bFGF signaling (Fig. 2B).
An interaction of endostatin with integrin α5β1 has been reported (Sudhaker et al., 2003). Integrin α5β1 is thought to promote angiogenesis (Kim et al., 2000a), and its signaling requires engagement of its ligand fibronectin. Endostatin binding to integrin α5β1 appears to inhibit focal adhesion kinase and Erk signaling, but it does not affect signaling through the Akt/protein kinase B pathway (Eriksson et al., 2003; Sudhakar et al., 2003). Activation of focal adhesion kinase is one of the early signaling events that typically occurs with integrin binding to its ligand, and focal adhesion kinase activation can promote cell migration, proliferation, or survival, depending on the cellular context and the environment (reviewed in Hecker and Gladson [2003]). Furthermore, we have shown that focal adhesion kinase is necessary for brain MvEC tube formation and migration (Haskell et al., 2003). In a related potential proposed mechanism for the antiangiogenic effect of endostatin, the binding of endostatin to tropomyosin was reported to cause disruption of microfilament integrity and to inhibit cell motility (MacDonald et al., 2001). Last, endostatin has been reported to interact with several proangiogenic molecules and to block their function, such as the VEGF receptor KDR/Flk-1 and the activation of an MMP (Kim et al., 2000b, 2002) (Fig. 2B).
Expression in Malignant Glioma Biopsy Samples
Endostatin appears to be expressed by various cell types in glioma biopsy samples. One group analyzed endostatin expression in frozen tissue samples from 51 astrocytic tumors (13 grade II, 9 grade III, and 29 grade IV tumor biopsy samples) and reported that endostatin protein levels were increased in grade IV tumor biopsy samples, as compared with lower-grade tumor biopsy samples (Morimoto et al., 2002). The investigators in this latter study utilized both immunohistochemistry and immunoblotting in their analyses, and the highest level of expression of endostatin was detected at the hyperplastic vessels surrounding tumor cells. This study suggests the possibility that the generation of endostatin could be a host antiangiogenic antitumor response. Another group, examining endostatin expression in astrocytoma tumors using immunohistochemistry alone, detected endostatin in tumor cells, endothelial cells, macrophages, and lymphocytes in 41 biopsy samples (13 fibrillary astrocytomas, 3 protoplasmic grade II astrocytomas, 7 grade III astrocytomas, and 18 grade IV astrocytoma tumor samples) (Strik et al., 2001). These investigators concluded that endostatin expression was decreased in grade IV astrocytoma tumors as compared with lower grade tumors (Strik et al., 2001).
Inhibition of Tumor Growth in Animal Models of Malignant Glioma
Several reports have suggested that endostatin is effective in inhibiting tumor growth in animal models of malignant glioma. Two studies reported that endostatin encapsulated within alginate beads was efficacious in treating U87 MG and BT4C glioma tumors propagated in rodents (Joki et al., 2001; Read et al., 2001). Rat C6 glioma cells stably expressing murine endostatin and implanted subcutaneously into nude mice resulted in a decrease in the tumor growth rate but not a complete inhibition of tumor growth, as compared with the implantation of control C6 glioma cells (Peroulis et al., 2002). In BT4Cn gliosarcoma cells propagated both subcutaneously and intracranially in rats, treatment with recombinant endostatin resulted in decreased tumor size and a longer survival (Sorensen et al., 2002). Furthermore, in a xenograft model in which U87 MG human glioblastoma cells were propagated in the nude mouse brain, direct intracerebral microinfusion of endostatin was more effective in decreasing tumor volume and increasing tumor cell apoptosis, as compared with systemic administration of endostatin (Schmidt et al., 2004). No toxicity was detected with endostatin therapy in these animal models, which suggests recombinant endostatin could be a highly useful new therapeutic tool for patients with malignant glioma.
PEX
Mechanism of Action and Receptor
PEX is a 210 amino acid fragment of MMP-2, and it corresponds to the hemopexin domain of MMP-2 (Brooks et al., 1998). PEX binds to integrin αvβ3 and is thought to competitively inhibit the binding of MMP-2 to integrin αvβ3 (Brooks et al., 1998) (Fig. 2C). The interaction of MMP-2 and integrin αvβ3 through the hemopexin domain promotes the activity of MMP-2. Furthermore, the interaction of MMP-2 and integrin αvβ3 is probably proangiogenic, as MMP-2 bound to integrin αvβ3 is catalytically active and promotes endothelial cell invasion (Brooks et al., 1996). PEX binding to integrin αvβ3 inhibits the binding of MMP-2 and inhibits MMP-2 collagenolytic activity in the chick chorioallantoic membrane model of angiogenesis (Brooks et al., 1998). A purified human PEX fragment inhibits tube formation of endothelial cells isolated from multiple sites and plated on Matrigel (BD Biosciences), and it decreases endothelial cell proliferation, as well as the proliferation of several malignant glioma cell lines (U87 MG, U373, and U118) (Bello et al., 2001a).
Expression in Malignant Glioma Biopsy Samples and Testing as a Therapeutic Agent in Animal Models of Malignant Glioma
PEX has been detected in glioma biopsy specimens and in the conditioned media of several malignant glioma cell lines (U87 MG, U373, and U118), which suggests that PEX generation is a physiologic event (Bello et al., 2001a). PEX has been shown to inhibit tumor growth and angiogenesis in both subcutaneous and intracerebral xenograft models of malignant glioma (U87 MG and U373 cell lines) (Bello et al., 2001a, b). PEX in combination with carboplatin and etoposide has also been shown to prolong animal survival in an intracranial xenograft model of glioma (Bello et al., 2001b).
Tissue Inhibitors of Matrix Metalloprotease and Platelet Factor-4
TIMPs: Mechanisms of Action and Expression in Glioma Biopsies
Tissue inhibitors of matrix metalloprotease (TIMP-1, -2, -3, and -4) are another group of endogenous inhibitors. TIMPs are reported to bind directly to MMPs and inhibit their activity, as well as to inhibit cell proliferation and downregulate VEGF expression (Hajitou et al., 2001; reviewed in Jiang et al. [2002]; Murphy et al., 1993) (Fig. 2D). However, the literature suggests that TIMPs may also have proangiogenic effects. For example, by blocking MMP activity, TIMPs block MMP-mediated generation of angiostatin and endostatin, TIMPs have antiapoptotic effects, and TIMPs can bind one of the membrane-type MMPs (MT1-MMP) and assist MT1-MMP in the activation of pro-MMP-2 (a proangiogenic molecule) (Jiang et al., 2002).
The upregulation of specific TIMPs in tumors may explain in part the paradoxical effects of TIMPs on angiogenesis. Several groups have reported an upregulation of TIMP-1 in high-grade astrocytomas, neurinomas, and meningiomas (Groft et al., 2001; Lampert et al., 1998; Nakagawa et al., 1994). One group analyzed TIMP expression in four normal brains, eight low-grade gliomas, five malignant oligodendrogliomas, seven anaplastic astrocytomas, and 19 glioblastomas and reported that TIMP-1 mRNA expression increased with increasing tumor grade, but there was no detectable change in TIMP-2 or -3 expression (Groft et al., 2001). In addition, TIMP-4 expression was found to decrease with increasing tumor grade in glioma tumors (Groft et al., 2001). Expression of TIMP-1 was localized to tumor cells and the surrounding vasculature by in situ hybridization, whereas TIMP-4 expression was localized mostly to tumor cells with minor expression in the vasculature (Groft et al., 2001). When TIMP-4 was overexpressed in the U87 MG human glioblastoma cells, reduced invasion in an in vitro assay was found, along with a decrease in MT1-MMP-mediated activation of MMP-2 (Groft et al., 2001). Transfection of SF-188 human malignant astrocytoma cells with TIMP-2 also decreased invasion across Matrigel filters (BD Biosciences) (Matsuzawa et al., 1996), and co-transduction of U87 MG cells with TIMP-2 and the phosphatase PTEN resulted in a further increased inhibition of invasiveness in vitro, as compared with either transfection of TIMP-2 or PTEN alone (Lu et al., 2004). The data regarding the effects of TIMPs in animal models of tumors is conflicting (Jiang et al., 2002), and to our knowledge there are no reports examining the effects of TIMP overexpression in in vivo glioma models. It will be important to determine whether the antiangiogenic or proangiogenic effects of specific TIMPs predominate in in vivo models of malignant glioma.
PF-4: Mechanism of Action and Animal Glioma Models Expressing or Administered PF-4
Both the full-length platelet factor-4 (PF-4) protein and its carboxyl-terminal fragment have been reported to promote antiangiogenic and antitumorigenic effects (reviewed in Bikfalvi [2004]). Three potential mechanisms of action have been described: (1) competitive inhibition of bFGF and VEGF binding to HSPG and consequent inhibition of growth factor receptor signaling; (2) direct binding to angiogenic growth factors, such as bFGF and VEGF, which prevents the binding of these growth factors to their cognate growth factor receptor; and (3) binding to the chemokine receptor CXCR3-B, causing an increase in cAMP and p21CIP1, thereby blocking cell cycle progression (Bikfalvi, 2004) (Fig. 2E).
In an intracranial mouse model of malignant glioma created by the injection of human glioma cells virally transduced with the full-length PF-4, prolonged animal survival, slower-growing tumors, and decreased tumor blood vessel density were found (Tanaka et al., 1997). Furthermore, a 24-mer peptide derived from the carboxyl-terminus of PF-4 also inhibited angiogenesis and tumor growth in an intracranial xenograft model of glioma (Hagedorn et al., 2002). The effects of this PF-4 peptide appeared to be increased through domain swapping of the DLQ motif (Hagedorn et al., 2002) or by combining this peptide with anti-invasive therapies, such as PEX (Bello et al., 2004).
Pigment Epithelial-Derived Factor
Mechanism of Action
Pigment epithelial-derived factor is a 50-kDa glycoprotein that is a member of the serpin family (Bouck, 2002). PEDF promotes neuronal differentiation and survival, as well as inhibiting angiogenesis. A specific PEDF receptor promoting the antiangiogenic effect of PEDF has not been identified. However, PEDF has distinct collagen-binding and heparin-binding regions that could mediate its interactions with other proteins and thereby promote an antiangiogenic effect (Meyer et al., 2002; Yasui et al., 2003).
PEDF appears to inhibit neovascularization through a regulation of the Fas death receptor pathway (Bouck, 2002; Dawson et al., 1999; Stellmach et al., 2001) (Fig. 2F). PEDF treatment of human dermal MvEC results in increased cell surface expression of Fas ligand (FasL) (Volpert et al., 2002a). As proangiogenic growth factors, such as bFGF and VEGF, are present at high concentrations in the tumor microenvironment and these proangiogenic factors promote the upregulation of Fas expression on the cell surface of dermal MvEC (Volpert et al., 2002a), newly forming tumor vessels could be selectively receptive to PEDF-stimulated FasL expression and the induction of apoptosis. The proposed induction of the extrinsic pathway of apoptosis by PEDF appears to be due to PEDF induction of more than one proapoptotic protein. In addition to increasing FasL expression, PEDF stimulation results in c-jun N-terminal protein kinase activation, which prevents the signaling of the transcription factor NFAT (nuclear factor of activated T cells) (Zaichuk et al., 2004). One target of NFAT is an endogenous inhibitor of caspase-8 activity termed cellular Fas-associated death domain-like interleukin 1β-converting enzyme inhibitory protein (c-FLIP) (Zaichuk et al., 2004). c-FLIP is similar in structure to caspase-8, but it lacks enzymatic activity; therefore, it acts as a competitive inhibitor of caspase-8. PEDF may also mediate its antiangiogenic effect in part through a modulation of the expression of proangiogenic or other anti-angiogenic molecules. In U251 MG human glioblastoma cells propagated in vitro, the overexpression of exogenous PEDF resulted in the upregulation of TSP-1 and the downregulation of bFGF, VEGF, and MMP-9. In the U251 MG glioblastoma cells expressing exogenous PEDF, there was also an increase in cell death, as compared with control U251 MG cells (Guan et al., 2004).
Expression in Normal Brain and Malignant Glioma Tumor Biopsy Samples
Pigment epithelial-derived factor is expressed in many adult and fetal tissues, including most areas of the brain (Tombran-Tink et al., 1996). In one study evaluating PEDF mRNA and protein expression in five normal brain biopsy samples, five grade I astrocytomas, seven grade II astrocytomas, 10 grade III astrocytomas, and 10 grade IV astrocytoma biopsies, PEDF expression was found to be inversely correlated with tumor histologic grade (Guan et al., 2003). In the latter study PEDF mRNA and protein expression were analyzed by RT-PCR and immunohistochemistry. In a glioma xenograft model of U251 MG malignant astrocytoma (glioblastoma) in which these tumor cells were modified to express exogenous PEDF and then injected and propagated subcutaneously, tumor size was significantly reduced (Guan et al., 2004). The role of PEDF in neuroblastoma progression has also been studied; PEDF is produced by Schwann cells and differentiated neuroblastoma cells but not by primitive neuroblastoma tumor cells (Crawford et al., 2001). Host Schwann cells are thought to infiltrate neuroblastoma tumors and secrete antiangiogenic molecules, such as PEDF, as well as molecules that promote neuronal differentiation, consistent with a host antitumor response (Crawford et al., 2001).
Thrombospondin-1 and -2
Thrombospondin-1 and -2 are highly related and belong to the larger family of TSP proteins. Both TSP-1 and -2 contain three type 1 repeat domains, referred to by others as TSR domains (Adams and Lawler, 2004). Many molecules contain a TSP type 1 repeat domain; however, only a small number of these molecules have been shown to have antiangiogenic activity. We limit our discussion of proteins with a type 1 repeat domain to TSP-1 and -2, while acknowledging that other molecules with this domain are expressed in the brain or gliomas. For example, brain angiogenesis inhibitor-1 (BAI-1) contains a TSP-1 type 1 repeat domain, and it is expressed in glioma tumors (Kaur et al., 2003). In models of non-glioma tumors, exogenous expression of BAI-1 has been shown to inhibit angiogenesis (Nishimori et al., 1997).
Mechanism of Action and Receptor(s)
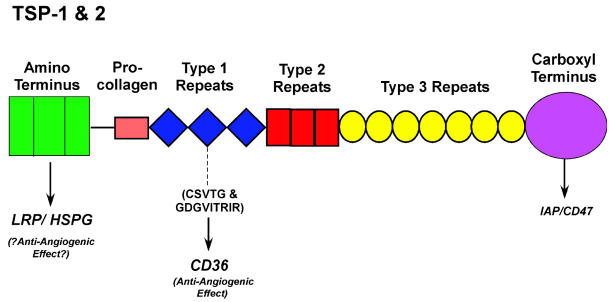
Thrombospondin-1 and -2 contain an amino-terminal heparin-binding domain, a pro-collagen-like domain, three type 1 repeats, three type 2 or EGF-like repeats, seven type 3 or calcium-binding repeats, and a globular carboxyl-terminal domain (Adams and Lawler, 2004) (Fig. 4). TSP-1 and -2 are the only members of the TSP family that contain a type 1 repeat domain (Adams and Lawler, 2004). The antiangiogenic effect of intact TSP-1 has been mapped, at least in part, to the type 1 repeat domains (Iruela-Arispe et al., 1999; Tolsma et al., 1993). Other domains of TSP-1 and -2 may also contribute to the antiangiogenic effect. Peptide sequences within the type 1 repeat, CSVTCG and GDGVITRIR, along with flanking residues, bind to the CD36 receptor expressed on the surface of MvEC (Dawson et al., 1997). This peptide sequence in the type 1 repeat of TSP-1 and -2 is highly conserved. Both TSP-1 and -2 have been reported to inhibit mouse or rat corneal neovascularization in a CD36 receptor-dependent manner, as well as to inhibit endothelial cell migration (Good et al., 1990; Rastinejad et al., 1989; Volpert et al., 1995).
Fig. 4.
Schematic of TSP-1 and -2 domain structure. Low-density lipoprotein receptor-related protein (LRP) recognizes the amino-terminal heparin-binding domain. The CD36 receptor recognizes the CSVTCG and the GDGVITRIR sequences along with flanking residues in the type 1 repeats. IAP/CD47 recognizes a sequence in the carboxyl-terminal domain (reviewed in Adams and Lawler [2004]).
One mechanism by which TSP-1 and -2 promote their antiangiogenic effect is through CD36 receptor signaling that results in apoptosis of dermal MvEC (Guo et al., 1997; Jimenez et al., 2000; Nor et al., 2000; Volpert et al., 2002a). A recent abstract suggests TSP-1 can also induce apoptosis of primary human brain MvEC (Rege et al., 2004). Using human dermal MvEC, investigators have shown that TSP-1 induces apoptosis through upregulation of FasL expression, resulting in the activation of the extrinsic pathway of apoptosis (Volpert et al., 2002a) (Fig. 2G). The importance of the Fas/FasL death receptor pathway in TSP-1-induced apoptosis is supported by studies in the Fas- and FasL-deficient mice; in these mice TSP-1 was unable to inhibit corneal neovascularization (Volpert et al., 2002a). Also, investigators have shown that TSP-1 treatment of dermal MvEC results in the activation of c-jun N-terminal protein kinase and p38 mitogen-activated protein kinase (Jimenez et al., 2000, 2001). Activation of other death receptor pathways was not examined in these studies.
A second potential mechanism for the antiangiogenic effect of TSP-1 and -2 is an inhibition of MvEC proliferation. In two studies, again using dermal MvEC, TSP-1 and -2 were reported to inhibit proliferation and to cause cell cycle arrest at the G0/G1 phase by a caspase-independent mechanism (Armstrong et al., 2002; Tomii et al., 2002) (Fig. 2G). The receptor involved is not clear. A third potential mechanism for the antiangiogenic effect of TSP-1 and -2 could be through an interaction with MMP-2. The type 1 repeats of TSP-1 and -2 interact with the zymogens of MMP-2 and -9 and have been reported to prevent their activation (Bein and Simons, 2000; Rodriguez-Manzaneque et al., 2001) (Fig. 2G). Also, TSP-1 and -2 bind to the low-density lipoprotein receptor-related protein (LRP), or potentially other LDL receptor family members, and are internalized by LRP, as well as targeted for degradation in the lysosome (Chen et al., 1996a; Godyna et al., 1995; Mikhailenko et al., 1995). LRP recognizes the amino-terminal heparin-binding domain of TSP-1 and -2 and cooperates with HSPG in the internalization and the subsequent degradation of TSP-1 and -2 (Chen et al., 1996a, b; Wang et al., 2004b). LRP is a scavenger receptor that internalizes several proteins and protease-inhibitor complexes. TSP-1 or -2 complexed with MMP-2 similarly binds to LRP and is internalized and degraded in the lysosome (Emonard et al., 2004; Yang et al., 2001). Thus, TSP-1 and -2 could theoretically reduce the level of MMP-2 in the cellular environment of a tumor through LRP internalization of a complex of TSP-1 or -2 and MMP-2. As MMP-2 promotes endothelial cell invasion, a reduction in the level of MMP-2 expression would likely inhibit angiogenesis.
Expression in Malignant Glioma Biopsy Samples
Thrombospondin-1 and -2 are expressed in some malignant tumors. Our lab has reported an increase in TSP-1 expression in glioblastoma (grade IV astrocytoma) biopsy samples, as compared with lower grade astrocytoma tumors and the normal brain, using immunohistochemistry of formalin-fixed and paraffin-embedded tissue samples (Pijuan-Thompson et al., 1999). We found TSP-1 expression to be localized predominantly to vascular mesenchymal cells with minimal tumor cell expression, suggesting a host antiangiogenic antitumor response (Pijuan-Thompson et al., 1999). This is supported by studies of TSP-2 expression in skin cancer. When skin carcinomas were induced with a chemical carcinogen, TSP-2 expression was upregulated in the mesenchymal stromal cells, which suggests a host antitumor response (Hawighorst et al., 2001). This is also supported by work in other tumors showing TSP-1 expression is altered as compared with the normal tissue counterpart. For example, TSP-1 expression is upregulated in the stroma of breast and cholangiocarcinoma cancer tissue (Bertin et al., 1997; Kawahara et al., 1998). Another group examining TSP-1 expression in glioma tumor cells as compared with normal brain cells reported TSP-1 expression was decreased in the tumor cells of grade IV astrocytoma biopsies (13 samples) as compared with its expression in normal brain and lower grade tumor cells (9 samples), again using immunohistochemical analysis (Hsu et al., 1996). Also, consistent with the study of Pijuan-Thompson et al. (1999), endothelial cells in the high-grade tumors retained TSP-1 expression (Hsu et al., 1996). In a third study, an increase in TSP-1 expression was detected in nine of 11 glioblastoma biopsy samples as compared with lower grade tumors (6 anaplastic astrocytomas, 8 low-grade astrocytomas, and 4 normal brain tissue samples) (Kawataki et al., 2000). The latter study found TSP-1 was localized to some vascular endothelial cells surrounding the tumor as well as to tumor cells; this study also utilized immunohistochemistry of paraffin-embedded sections (Kawataki et al., 2000). In a study examining TSP-1 and -2 message levels in 37 glioma biopsies (1 pilocytic astrocytoma, 4 fibrillary astrocytomas, 1 protoplasmic astrocytoma, 1 mixed oligoastrocytoma, 13 anaplastic astrocytomas, and 17 glioblastomas) and no normal brain samples, lower levels of TSP-2 mRNA expression appeared to correlate with a higher histologic grade, and TSP-1 mRNA expression levels appeared to have no prognostic value (Kazuno et al., 1999). As angiogenesis is found in most malignant glioma biopsies, the antiangiogenic effect of TSP-1 protein found in malignant glioma tumor biopsies is likely overcome by a higher level of expression of pro-angiogenic molecules.
TSP-1 Regulation
Expression of TSP-1 may be regulated by tumor suppressor genes and oncogenes. The tumor suppressors PTEN and p53 are reported to increase TSP-1 expression in certain cells (Dameron et al., 1994; Good et al., 1990), and the re-expression of wild-type p53 in U251 MG human glioblastoma cells propagated in vitro was reported to result in increased levels of TSP-1 mRNA (Harada et al., 2003). However, Tenan et al. (2000) reported that TSP-1 expression in glioblastoma cells propagated in vitro is not regulated by p53, but it is downregulated by anoxia. In various cell lines the oncogenes Ras, c-myc, v-src, c-jun, and Id1 have all been reported to repress TSP-1 expression (Volpert et al., 2002b), and Watnick et al. (2003) recently reported a pathway of TSP-1 repression involving phosphatidylinositol-3 kinase, Rho A, ROCK, myc, and Ras activation.
TSP-1 Inhibits Tumor Growth in Animal Models of Malignant Glioma
In the nude mouse injected subcutaneously with the LN-229 glioblastoma cell line, stable expression of intact TSP-1 resulted in a reduction in mean tumor size and a decrease in mean tumor microvessel number (Tenan et al., 2000). Furthermore, Kragh et al. (2002) also over-expressed intact TSP-1 in the LN-229 human glioblastoma cell line and, when propagating it in a xenograft model, found decreased tumor growth and decreased vascular density but no effect on perfusion. These data suggest that in glioblastoma tumors TSP-1 promotes a growth-limiting phenotype via an inhibition of angiogenesis (Kragh et al., 2002). Consistent with the above findings, a report by another group described nude mice that had been injected subcutaneously or intracerebrally with C6 rat glioma cells expressing a fragment of TSP-1 that contained the pro-collagen domain and the three type 1 repeats (de Fraipont et al., 2004). The injected mice produced tumors with decreased vascularity. However, tumors produced from the implanted C6 glioma cells expressing the TSP-1 fragment were also more aggressive, and the animals had a decreased survival (de Fraipont et al., 2004). These results suggest the possibility that fragments of TSP-1 may promote an anti-angiogenic effect but that protumorigenic effects may be unmasked (de Fraipont et al., 2004). Resistance to antiangiogenic TSP-1 therapy could potentially occur, as reported in another study. When C6 rat glioma cells were injected subcutaneously along with stromal fibroblasts expressing TSP-1, the tumor became insensitive to the effects of TSP-1, demonstrating prolonged growth, likely due to an increase in the production of proangiogenic growth factors (Filleur et al., 2001). In an earlier study, Bogdanov et al. (1999) used rats injected with C6 glioma cells or 9L gliosarcoma cells to test the effects of a peptide derived from the type 1 repeats of TSP-1 that did not contain the CD36 receptor binding sequence but contained the TGF-β activating sequence and the tryptophan-rich sequence. These investigators found that the tumors were significantly smaller in rats treated with this specific peptide, but no change in the vascular volume fraction, mean vascular area, or microvessel density was found (Bogdanov et al., 1999). The activation of TGF-β and its signaling of an antiproliferative effect could theoretically explain these results, as this peptide cannot bind the CD36 receptor to promote an antiangiogenic effect.
In nonglioma models of malignant tumors, TSP-1 and -2 have also been shown to inhibit angiogenesis and tumor growth. In a model of malignant melanoma, nude mice injected systemically with TSP-1 showed an increase in the percentage of apoptotic endothelial cells, particularly at the periphery of the tumor, which was detected by co-labeling with anti-CD34 and annexin V (Jimenez et al., 2000). In a syngeneic model of malignant melanoma and in an orthotopic model of human bladder carcinoma, treatment with a type 1 repeat peptide from TSP-1 that contained the CD36 receptor binding sequence decreased tumor growth and microvessel density (Reiher et al., 2002). In addition, TSP-1 treatment in combination with radiotherapy enhanced the antitumor effects of radiation therapy (Rofstad et al., 2003). TSP-2 has also been reported to have a potent antitumor effect. When A431 human squamous carcinoma cells transfected with TSP-2 were injected subcutaneously into the nude mouse, decreased tumor growth and decreased microvessel density were found, as compared with mice injected with control A431 cells (Streit et al., 1999). In support of an antiangiogenic role for TSP-2, TSP-2 null mice were found to be more susceptible to the development of chemical-carcinogen-induced skin cancer, and less tumor cell apoptosis was detected in the tumors induced in the TSP-2 null mice as compared with those induced in the control mice (Hawighorst et al., 2001). Recombinant fragments of TSP-2 have also been reported to inhibit angiogenesis and tumor growth of nonglioma tumors. Adenoviral-mediated or intraperitoneal injections of a fragment of recombinant TSP-2 containing the type 1 repeats, pro-collagen domain, and a portion of the amino-terminal domain also inhibited tumor growth and microvessel density in A431 human squamous cell carcinoma xenografts (Hahn et al., 2004; Noh et al., 2003).
Conclusions
Endogenous inhibitors of angiogenesis exert their effects through multiple mechanisms that include the induction of MvEC apoptosis, the inhibition of MvEC proliferation, the inhibition of the function of proangiogenic molecules, or the altered regulation of proangiogenic and antiangiogenic molecules. Two conclusions can be made regarding the mechanism of action of these inhibitors: (1) Several of these inhibitors appear to exert their effect by competing with or blocking the binding of natural ligands/binding partners for proangiogenic receptors/ molecules, and (2) TSP-1 and -2 can exert their anti-angiogenic effect, at least in part, by binding to a specific receptor, CD36. Expression of certain endogenous inhibitors of angiogenesis, such as TSP-1 and -2, could be part of a host antitumor response. The endogenous inhibitors of angiogenesis reviewed here provide promising new treatment options for patients diagnosed with malignant glioma tumors, as they appear unlikely to cause a host immune response (Boehm et al., 1997) and toxicity appears to be absent. Several antiangiogenic therapies are currently in phase 1/2 human clinical trials in patients with malignant gliomas, and these clinical trials include 2-methoxyestradiol, the αv antagonist EMD 121974, and the MMP-2 and -9 inhibitor COL-3 (reviewed in Jansen et al. [2004]). Questions regarding the development of resistance to these inhibitors do remain; therefore, combining new antiangiogenic therapy with established antiproliferative therapy should be considered. Also, as radiation of glioma cells may potentiate the expression of proangiogenic molecules (Parthymou et al., 2004), combining radiotherapy with antiangiogenic therapy may also prove to be clinically useful and effective.
Questions Remaining To Be Answered
Is the expression of some endogenous inhibitors of angiogenesis, such as TSP-1 and -2, a host antiangiogenic antitumor response? Our data regarding TSP-1 expression in malignant astrocytoma tumor biopsies suggest TSP-1 is expressed as an antiangiogenic antitumor response (Pijuan-Thompson et al., 1999). Studies of chemical-carcinogen-induced skin tumors suggest TSP-2 expression in the dermis is part of a host antitumor response (Hawighorst et al., 2001). Also, PEDF expression by Schwann cells in neuro-blastoma tumors is likely part of a host antiangiogenesis antitumor response (Crawford et al., 2001). Studies delineating whether endogenous inhibitors of angiogenesis are synthesized by host stromal cells (reactive astrocytes, microglia, endothelial cells, pericytes, and smooth muscle cells) in malignant glioma tumor biopsies need to be performed, including in situ hybridization studies to detect the message. This could be supported by studies propagating malignant glioma cells in specific knockout mice. Focal or transient tumor cell synthesis of an endogenous inhibitor of angiogenesis, such as TSP-1 or -2, does not detract from or disprove the possibility that endogenous inhibitors of angiogenesis can be expressed as part of a host antitumor response. Tumor cell synthesis of certain endogenous inhibitors of angiogenesis, such as TSP-1, could transiently promote disadhesion and migration of a tumor cell and thereby serve a tumor cell function.
Do endogenous inhibitors of angiogenesis downregulate a set of proangiogenic genes or upregulate a set of proapoptotic/pro-death genes? In a recent study by Abdollahi et al. (2004), genome-wide expression profiling of dermal MvEC following endostatin treatment showed that multiple antiangiogenic molecules were upregulated. Therefore, determining whether other endogenous inhibitors of angiogenesis also modify the regulation of sets of proangiogenic or antiangiogenic genes will assist us in determining their complete mechanism of action.
Acknowledgments
We thank Brian Dranka for reading this review and Jo Self for assistance in preparing this manuscript. The authors acknowledge the many studies that were not cited because of the need to limit the number of references.
Footnotes
This work was supported by predoctoral fellowship NS49674 from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (T.A.R.), predoctoral training grant T32 HL07918-06 (C.Y.F.), and grants CA97110 and CA097247 (Project 5) from the National Institutes of Health, National Cancer Institute (C.L.G.).
Abbreviations used are as follows: bFGF, basic fibroblast growth factor; c-FLIP, cellular Fas-associated death domain–like interleukin 1β-converting enzyme inhibitory protein; CSPG, chondroitin sulfate proteoglycan; FasL, Fas ligand; HSPG, heparan sulfate proteoglycan; HUVEC, human umbilical vein endothelial cell; K, kringle domain; LRP, low-density-lipoprotein receptor-related protein; MMP, matrix metalloprotease; MT, membrane type; MvEC, microvascular endothelial cells; PEDF, pigment epithelial-derived factor; PF-4, platelet factor-4; TIMP, tissue inhibitor of matrix metalloprotease; TSP-1 and -2, thrombospondin-1 and -2; VEGF, vascular endothelial cell growth factor.
References
- Abdollahi A, Hahnfeldt P, Maercker C, Grone HJ, Debus J, Ansorge W, Folkman J, Hlatky L, Huber PE. Endostatin’s antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport JR, Shinde Patil VR, Weissleder R. Murine neuronal progenitor cells are preferentially recruited to tumor vasculature via alpha (4)-integrin and SDF-1 alpha-dependent mechanisms. Cancer Biol Ther. 2004;3:838–844. doi: 10.4161/cbt.3.9.1036. [DOI] [PubMed] [Google Scholar]
- Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. [DOI] [PubMed] [Google Scholar]
- Armstrong LC, Bjorkblom B, Hankenson KD, Siadak AW, Stiles CE, Bornstein P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell. 2002;13:1893–1905. doi: 10.1091/mbc.E01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- Bello L, Lucini V, Carrabba G, Giussani C, Machluf M, Pluderi M, Nikas D, Zhang J, Tomei G, Villani RM, Carroll RS, Bikfalvi A, Black PM. Simultaneous inhibition of glioma angiogenesis, cell proliferation, and invasion by a naturally occurring fragment of human metalloproteinase-2. Cancer Res. 2001a;61:8730–8736. [PubMed] [Google Scholar]
- Bello L, Carrabba G, Giussani C, Lucini V, Cerutti F, Scaglione F, Landre J, Pluderi M, Tomei G, Villani R, Carroll RS, Black PM, Bikfalvi A. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001b;61:7501–7506. [PubMed] [Google Scholar]
- Bello L, Lucini V, Costa F, Pluderi M, Giussani C, Acerbi F, Carrabba G, Pannacci M, Caronzolo D, Grosso S, Shinkaruk S, Colleoni F, Canron X, Tomei G, Deleris G, Bikfalvi A. Combinatorial administration of molecules that simultaneously inhibit angiogenesis and invasion leads to increased therapeutic efficacy in mouse models of malignant glioma. Clin Cancer Res. 2004;10:4527–4537. doi: 10.1158/1078-0432.CCR-04-0194. [DOI] [PubMed] [Google Scholar]
- Bertin N, Clezardin P, Kubiak R, Frappart L. Thrombospondin-1 and -2 messenger RNA expression in normal, benign, and neoplastic human breast tissues: Correlation with prognostic factors, tumor angiogenesis, and fibroblastic desmoplasia. Cancer Res. 1997;57:396–399. [PubMed] [Google Scholar]
- Bikfalvi A. Recent developments in the inhibition of angiogenesis: Examples from studies on platelet factor-4 and the VEGF/VEGFR system. Biochem Pharmacol. 2004;68:1017–1021. doi: 10.1016/j.bcp.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- Bogdanov A, Jr, Marecos E, Cheng HC, Chandrasekaran L, Krutzsch HC, Roberts DD, Weissleder R. Treatment of experimental brain tumors with thrombospondin-1 derived peptides: An in vivo imaging study. Neoplasia. 1999;1:438–445. doi: 10.1038/sj.neo.7900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N. PEDF: Anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- Brooks PC. Role of integrins in angiogenesis. Eur J Cancer. 1996;32A:2423–2429. doi: 10.1016/s0959-8049(96)00381-4. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- Chekenya M, Hjelstuen M, Enger PO, Thorsen F, Jacob AL, Probst B, Haraldseth O, Pilkington G, Butt A, Levine JM, Bjerkvig R. NG2 proteoglycan promotes angiogenesis-dependent tumor growth in CNS by sequestering angiostatin. FASEB J. 2002;16:586–588. doi: 10.1096/fj.01-0632fje. [DOI] [PubMed] [Google Scholar]
- Chen H, Strickland DK, Mosher DF. Metabolism of thrombospondin 2. Binding and degradation by 3T3 cells and glycos-aminoglycan-variant Chinese hamster ovary cells. J Biol Chem. 1996a;271:15993–15999. doi: 10.1074/jbc.271.27.15993. [DOI] [PubMed] [Google Scholar]
- Chen H, Sottile J, Strickland DK, Mosher DF. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein: Localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem J. 1996b;318:959–963. doi: 10.1042/bj3180959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Ranalli M, Huang X, Huang L, Volpert O, De Vries GH, Abramson LP, Bouck N. Pigment epithelium-derived factor (PEDF) in neuroblastoma: A multifunctional mediator of Schwann cell antitumor activity. J Cell Sci. 2001;114:4421–4428. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- Dameron KM, Volpert OV, Tainsky MA, Bouck N. The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb Symp Quant Biol. 1994;59:483–489. doi: 10.1101/sqb.1994.059.01.053. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- de Fraipont F, Keramidas M, El Atifi M, Chambaz EM, Berger F, Feige JJ. Expression of the thrombospondin 1 fragment 167–569 in C6 glioma cells stimulates tumorigenicity despite reduced neovascularization. Oncogene. 2004;23:3642–3649. doi: 10.1038/sj.onc.1207438. [DOI] [PubMed] [Google Scholar]
- Ding Q, Stewart J, Jr, Prince CW, Chang PL, Trikha M, Han X, Grammer JR, Gladson CL. Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: Differences in integrin signaling during cell adhesion to osteopontin versus vitronectin. Cancer Res. 2002;62:5336–5343. doi: 10.1100/tsw.2002.247. [DOI] [PubMed] [Google Scholar]
- Emonard H, Bellon G, Troeberg L, Berton A, Robinet A, Henriet P, Marbaix E, Kirkegaard K, Patthy L, Eeckhout Y, Nagase H, Hornebeck W, Courtoy PJ. The low density lipoprotein receptor-related protein mediates endocytic clearance of proMMP-2: TIMP-2 complex through a thrombospondin-independent mechanism. J Biol Chem. 2004;279:54944–54951. doi: 10.1074/jbc.M406792200. [DOI] [PubMed] [Google Scholar]
- Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh L, Cross MJ. Angiostatin and endostatin inhibit endothelial cell migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Fears CY, Sontheimer HW, Bullard DC, Gladson CL. Could labeled neuronal progenitor cells be used to target glioma tumor endothelium? Cancer Biol Ther. 2004;3:845–846. doi: 10.4161/cbt.3.9.1123. [DOI] [PubMed] [Google Scholar]
- Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- Filleur S, Volpert OV, Degeorges A, Voland C, Reiher F, Clezardin P, Bouck N, Cabon F. In vivo mechanisms by which tumors producing thrombospondin 1 bypass its inhibitory effects. Genes Dev. 2001;15:1373–1382. doi: 10.1101/gad.193501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JH, Cnudde SE. What the structure of angiostatin may tell us about its mechanism of action. J Thromb Haemost. 2004;2:23–34. doi: 10.1111/j.1538-7836.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Gingras MC, Roussel E, Bruner JM, Branch CD, Moser RP. Comparison of cell adhesion molecule expression between glioblastoma multiforme and autologous normal brain tissue. J Neuroimmunol. 1995;57:143–153. doi: 10.1016/0165-5728(94)00178-q. [DOI] [PubMed] [Google Scholar]
- Gladson CL. Expression of integrin alpha v beta 3 in small blood vessels of glioblastoma tumors. J Neuropath Exp Neurol. 1996;55:1143–1149. doi: 10.1097/00005072-199611000-00005. [DOI] [PubMed] [Google Scholar]
- Gladson CL, Cheresh DA. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson CL, Wilcox JN, Sanders L, Gillespie GY, Cheresh DA. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci. 1995;108:945–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: A model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C, Perricaudet M, Yeh P, Lu H. Angiostatin gene transfer: Inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrest. Proc Natl Acad Sci USA. 1998;95:6367–6372. doi: 10.1073/pnas.95.11.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groft LL, Muzik H, Rewcastle NB, Johnston RN, Knauper V, Lafleur MA, Forsyth PA, Edwards DR. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br J Cancer. 2001;85:55–63. doi: 10.1054/bjoc.2001.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M, Yam HF, Su B, Chan KP, Pang CP, Liu WW, Zhang WZ, Lu Y. Loss of pigment epithelium derived factor expression in glioma progression. J Clin Pathol. 2003;56:277–282. doi: 10.1136/jcp.56.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan M, Pang CP, Yam HF, Cheung KF, Liu WW, Lu Y. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325–332. doi: 10.1038/sj.cgt.7700675. [DOI] [PubMed] [Google Scholar]
- Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- Hagedorn M, Zilberberg L, Wilting J, Canron X, Carrabba G, Giussani C, Pluderi M, Bello L, Bikfalvi A. Domain swapping in a COOH-terminal fragment of platelet factor 4 generates potent angiogenesis inhibitors. Cancer Res. 2002;62:6884–6890. [PubMed] [Google Scholar]
- Hahn W, Ho SH, Jeong JG, Hahn EY, Kim S, Yu SS, Kim S, Kim JM. Viral vector-mediated transduction of a modified thrombospondin-2 cDNA inhibits tumor growth and angiogenesis. Gene Ther. 2004;11:739–745. doi: 10.1038/sj.gt.3302219. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Sounni NE, Devy L, Grignet-Debrus C, Lewalle JM, Li H, Deroanne CF, Lu H, Colige A, Nusgens BV, Frankenne F, Maron A, Yeh P, Perricaudet M, Chang Y, Soria C, Calberg-Bacq CM, Foidart JM, Noel A. Down-regulation of vascular endothelial growth factor by tissue inhibitor of metalloproteinase-2: Effect on in vivo mammary tumor growth and angiogenesis. Cancer Res. 2001;61:3450–3457. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Harada H, Nakagawa K, Saito M, Kohno S, Nagato S, Furukawa K, Kumon Y, Hamada K, Ohnishi T. Introduction of wild-type p53 enhances thrombospondin-1 expression in human glioma cells. Cancer Lett. 2003;191:109–119. doi: 10.1016/s0304-3835(02)00592-x. [DOI] [PubMed] [Google Scholar]
- Haskell H, Natarajan M, Hecker TP, Ding Q, Stewart J, Jr, Grammer JR, Gladson CL. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin Cancer Res. 2003;9:2157–2165. [PubMed] [Google Scholar]
- Hawighorst T, Velasco P, Streit M, Hong YK, Kyriakides TR, Brown LF, Bornstein P, Detmar M. Thrombospondin-2 plays a protective role in multistep carcinogenesis: A novel host anti-tumor defense mechanism. EMBO J. 2001;20:2631–2640. doi: 10.1093/emboj/20.11.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker TP, Gladson CL. Focal adhesion kinase in cancer. Front Biosci. 2003;8:s705–s714. doi: 10.2741/1115. [DOI] [PubMed] [Google Scholar]
- Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, Chou P, Bouck NP. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56:5684–5691. [PubMed] [Google Scholar]
- Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res Brain Res Rev. 2004;45:143–163. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, Rajopadhye M, Boonstra H, Corstens FH, Boerman OC. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV, Reiher F, Chang L, Munoz A, Karin M, Bouck N. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene. 2001;20:3443–3448. doi: 10.1038/sj.onc.1204464. [DOI] [PubMed] [Google Scholar]
- Joe YA, Hong YK, Chung DS, Yang YJ, Kang JK, Lee YS, Chang SI, You WK, Lee H, Chung SI. Inhibition of human malignant glioma growth in vivo by human recombinant plasminogen kringles 1–3. Int J Cancer. 1999;82:694–699. doi: 10.1002/(sici)1097-0215(19990827)82:5<694::aid-ijc12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Joki T, Machluf M, Atala A, Zhu J, Seyfried NT, Dunn IF, Abe T, Carroll RS, Black PM. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat Biotechnol. 2001;19:35–39. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- Kanamori M, Vanden Berg SR, Bergers G, Berger MS, Pieper RO. Integrin beta3 overexpression suppresses tumor growth in a human model of gliomagenesis: Implications for the role of beta3 over-expression in glioblastoma multiforme. Cancer Res. 2004;64:2751–2758. doi: 10.1158/0008-5472.can-03-3354. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, Chan B, Dhanabal M, Hanai JI, Venkataraman G, Shriver Z, Keiser N, Kalluri R, Zeng H, Mukhopadhyay D, Chen RL, Lander AD, Hagihara K, Yamaguchi Y, Sasisekharan R, Cantley L, Sukhatme VP. Cell surface glypicans are low-affinity endostatin receptors. Mol Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Kaur B, Brat DJ, Calkins CC, Van Meir EG. Brain angiogenesis inhibitor 1 is differentially expressed in normal brain and glioblastoma independently of p53 expression. Am J Pathol. 2003;162:19–27. doi: 10.1016/S0002-9440(10)63794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara N, Ono M, Taguchi K, Okamoto M, Shimada M, Takenaka K, Hayashi K, Mosher DF, Sugimachi K, Tsuneyoshi M, Kuwano M. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512–1517. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- Kawataki T, Naganuma H, Sasaki A, Yoshikawa H, Tasaka K, Nukui H. Correlation of thrombospondin-1 and transforming growth factor-beta expression with malignancy of glioma. Neuropathology. 2000;20:161–169. doi: 10.1046/j.1440-1789.2000.00327.x. [DOI] [PubMed] [Google Scholar]
- Kazuno M, Tokunaga T, Oshika Y, Tanaka Y, Tsugane R, Kijima H, Yamazaki H, Ueyama Y, Nakamura M. Thrombospondin-2 (TSP2) expression is inversely correlated with vascularity in glioma. Eur J Cancer. 1999;35:502–506. doi: 10.1016/s0959-8049(98)00374-8. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000a;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase 2. Cancer Res. 2000b;60:5410–5413. [PubMed] [Google Scholar]
- Kim YM, Hwang S, Kim YM, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J Biol Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- Kragh M, Quistorff B, Tenan M, Van Meir EG, Kristjansen PE. Overexpression of thrombospondin-1 reduces growth and vascular index but not perfusion in glioblastoma. Cancer Res. 2002;62:1191–1195. [PubMed] [Google Scholar]
- Lampert K, Machein U, Machein MR, Conca W, Peter HH, Volk B. Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol. 1998;153:429–437. doi: 10.1016/S0002-9440(10)65586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko T, Bratt A, Arbiser JL, Holmgren L. Angiomotin expression promotes hemangioendothelioma invasion. Oncogene. 2004;23:1469–1473. doi: 10.1038/sj.onc.1207264. [DOI] [PubMed] [Google Scholar]
- Liekens S, De Clercq E, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Lu W, Zhou X, Hong B, Liu J, Yue Z. Suppression of invasion in human U87 glioma cells by adenovirus-mediated co-transfer of TIMP-2 and PTEN gene. Cancer Lett. 2004;214:205–213. doi: 10.1016/j.canlet.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Ma HI, Lin SZ, Chiang YH, Li J, Chen SL, Tsao YP, Xiao X. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9:2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- MacDonald NJ, Shivers WY, Narum DL, Plum SM, Wingard JN, Fuhrmann SR, Liang H, Holland-Linn J, Chen DH, Sim BK. Endostatin binds tropomyosin. A potential modulator of the antitumor activity of endostatin. J Biol Chem. 2001;276:25190–25196. doi: 10.1074/jbc.M100743200. [DOI] [PubMed] [Google Scholar]
- Matsuzawa K, Fukuyama K, Hubbard SL, Dirks PB, Rutka JT. Transfection of an invasive human astrocytoma cell line with a TIMP-1 cDNA: Modulation of astrocytoma invasive potential. J Neuropathol Exp Neurol. 1996;55:88–96. doi: 10.1097/00005072-199601000-00009. [DOI] [PubMed] [Google Scholar]
- Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J Biol Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Aoyagi M, Tamaki M, Yoshino Y, Hori H, Duan L, Yano T, Shibata M, Ohno K, Hirakawa K, Yamaguchi N. Increased levels of tissue endostatin in human malignant gliomas. Clin Cancer Res. 2002;8:2933–2938. [PubMed] [Google Scholar]
- Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci USA. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci USA. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351–358. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994;81:69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- Nishimori H, Shiratsuchi T, Urano T, Kimura Y, Kiyono K, Tatsumi K, Yoshida S, Ono M, Kuwano M, Nakamura Y, Tokino T. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene. 1997;15:2145–2150. doi: 10.1038/sj.onc.1201542. [DOI] [PubMed] [Google Scholar]
- Noh YH, Matsuda K, Hong YK, Kunstfeld R, Riccardi L, Koch M, Oura H, Dadras SS, Streit M, Detmar M. An N-terminal 80 kDa recombinant fragment of human thrombospondin-2 inhibits vascular endothelial growth factor induced endothelial cell migration in vitro and tumor growth and angiogenesis in vivo. J Invest Dermatol. 2003;121:1536–1543. doi: 10.1046/j.1523-1747.2003.12643.x. [DOI] [PubMed] [Google Scholar]
- Nor JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37:209–218. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Parthymou A, Kardamakis D, Pavlopoulos I, Papadimitriou E. Irradiated C6 glioma cells induce angiogenesis in vivo and activate endothelial cells in vitro. Int J Cancer. 2004;110:807–814. doi: 10.1002/ijc.20188. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- Peroulis I, Jonas N, Saleh M. Antiangiogenic activity of endostatin inhibits C6 glioma growth. Int J Cancer. 2002;97:839–845. doi: 10.1002/ijc.10115. [DOI] [PubMed] [Google Scholar]
- Pijuan-Thompson V, Grammer JR, Stewart J, Silverstein RL, Pearce SF, Tuszynski GP, Murphy-Ullrich JE, Gladson CL. Retinoic acid alters the mechanism of attachment of malignant astrocytoma and neuroblastoma cells to thrombospondin-1. Exp Cell Res. 1999;249:86–101. doi: 10.1006/excr.1999.4458. [DOI] [PubMed] [Google Scholar]
- Qiao D, Meyer K, Mundhenke C, Drew SA, Friedl A. Heparan sulfate proteoglycans as regulators of fibroblast growth factor-2 signaling in brain endothelial cells. Specific role for glypican-1 in glioma angiogenesis. J Biol Chem. 2003;278:16045–16053. doi: 10.1074/jbc.M211259200. [DOI] [PubMed] [Google Scholar]
- Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Read TA, Sorensen DR, Mahesparan R, Enger PO, Timpl R, Olsen BR, Hjelstuen MH, Haraldseth O, Bjerkvig R. Local endostatin treatment of gliomas administered by microencapsulated producer cells. Nat Biotechnol. 2001;19:29–34. doi: 10.1038/83471. [DOI] [PubMed] [Google Scholar]
- Rege, T.A., Stewart, J., Jr., Henkin, J., Silverstein, R.L., and Gladson, C.L. (2004) TSP-1 and type 1 repeat domain peptides induce apoptosis of human brain microvascular endothelial cells. Abstract 217. Presented at Second National Meeting of the American Society for Matrix Biology, November 10–13, 2004, San Diego, Calif.
- Reiher FK, Volpert OV, Jimenez B, Crawford SE, Dinney CP, Henkin J, Haviv F, Bouck NP, Campbell SC. Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int J Cancer. 2002;98:682–689. doi: 10.1002/ijc.10247. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rofstad EK, Henriksen K, Galappathi K, Mathiesen B. Antiangiogenic treatment with thrombospondin-1 enhances primary tumor radiation response and prevents growth of dormant pulmonary micrometastases after curative radiation therapy in human melanoma xenografts. Cancer Res. 2003;63:4055–4061. [PubMed] [Google Scholar]
- Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999;18:6240–6248. doi: 10.1093/emboj/18.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NO, Ziu M, Carrabba G, Giussani C, Bello L, Sun Y, Schmidt K, Albert M, Black PM, Carroll RS. Antiangiogenic therapy by local intracerebral microinfusion improves treatment efficiency and survival in an orthotopic human glioblastoma model. Clin Cancer Res. 2004;10:1255–1262. doi: 10.1158/1078-0432.ccr-03-0052. [DOI] [PubMed] [Google Scholar]
- Schrappe M, Klier FG, Spiro RC, Waltz TA, Reisfeld RA, Gladson CL. Correlation of chondroitin sulfate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of astroglial cells. Cancer Res. 1991;51:4986–4993. [PubMed] [Google Scholar]
- Sorensen DR, Read TA, Porwol T, Olsen BR, Timpl R, Sasaki T, Iversen PO, Benestad HB, Sim BK, Bjerkvig R. Endostatin reduces vascularization, blood flow, and growth in a rat gliosarcoma. Neuro-Oncol. 2002;4:1–8. doi: 10.1215/15228517-4-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld R, Van den Berghe H, David G. Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J Cell Biol. 1996;133:405–416. doi: 10.1083/jcb.133.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc Natl Acad Sci USA. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: A potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik HM, Schluesener HJ, Seid K, Meyermann R, Deininger MH. Localization of endostatin in rat and human gliomas. Cancer. 2001;91:1013–1019. [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct anti-angiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci USA. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- Tarui T, Miles LA, Takada Y. Specific interaction of angiostatin with integrin alpha(v)beta(3) in endothelial cells. J Biol Chem. 2001;276:39562–39568. doi: 10.1074/jbc.M101815200. [DOI] [PubMed] [Google Scholar]
- Tenan M, Fulci G, Albertoni M, Diserens AC, Hamou MF, El Atifi-Borel M, Feige JJ, Pepper MS, Van Meir EG. Thrombospondin-1 is downregulated by anoxia and suppresses tumorigenicity of human glioblastoma cells. J Exp Med. 2000;191:1789–1798. doi: 10.1084/jem.191.10.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombran-Tink J, Mazuruk K, Rodriguez IR, Chung D, Linker T, Englander E, Chader GJ. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- Tomii Y, Kamochi J, Yamazaki H, Sawa N, Tokunaga T, Ohnishi Y, Kijima H, Ueyama Y, Tamaoki N, Nakamura M. Human thrombospondin 2 inhibits proliferation of microvascular endothelial cells. Int J Oncol. 2002;20:339–342. [PubMed] [Google Scholar]
- Troyanovsky B, Levchenko T, Månsson G, Matvijenko O, Holmgren L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets acti-vatedendotheliumfordestructionbyanti-angiogenicthrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002a;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Volpert OV, Pili R, Sikder HA, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell. 2002b;2:473–483. doi: 10.1016/s1535-6108(02)00209-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004a;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- Wang S, Herndon ME, Ranganathan S, Godyna S, Lawler J, Argraves WS, Liau G. Internalization but not binding of thrombospondin-1 to low density lipoprotein receptor-related protein-1 requires heparan sulfate proteoglycans. J Cell Biochem. 2004b;91:766–776. doi: 10.1002/jcb.10781. [DOI] [PubMed] [Google Scholar]
- Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3:219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6056. [PubMed] [Google Scholar]
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- Yasui N, Mori T, Morito D, Matsushita O, Kourai H, Nagata K, Koide T. Dual-site recognition of different extracellular matrix components by anti-angiogenic/neurotrophic serpin, PEDF. Biochemistry. 2003;42:3160–3167. doi: 10.1021/bi0206558. [DOI] [PubMed] [Google Scholar]
- Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513–1522. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Fei Z, Gao D, Li X, Liu X, Liang J, Wang X. Angiostatin K(1–3) gene for treatment of human gliomas: An experimental study. Chin Med J. 2000;113:996–1001. [PubMed] [Google Scholar]