Abstract
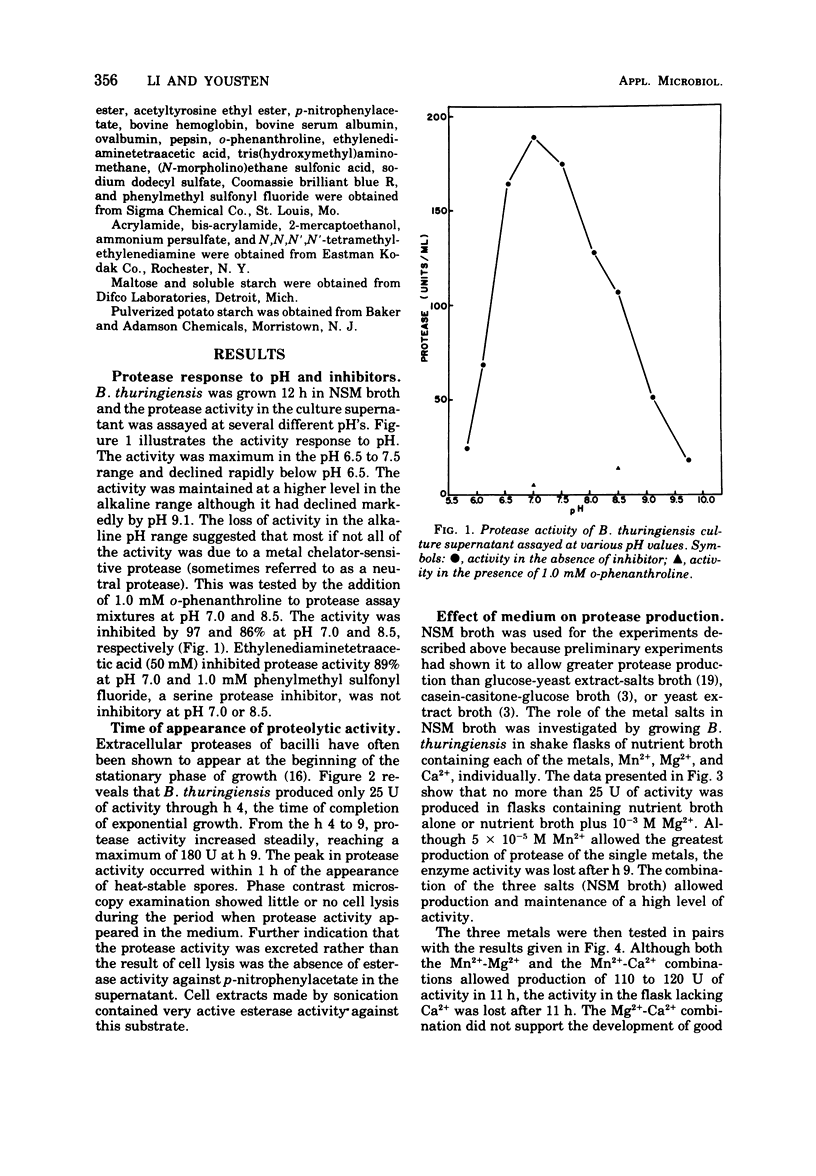
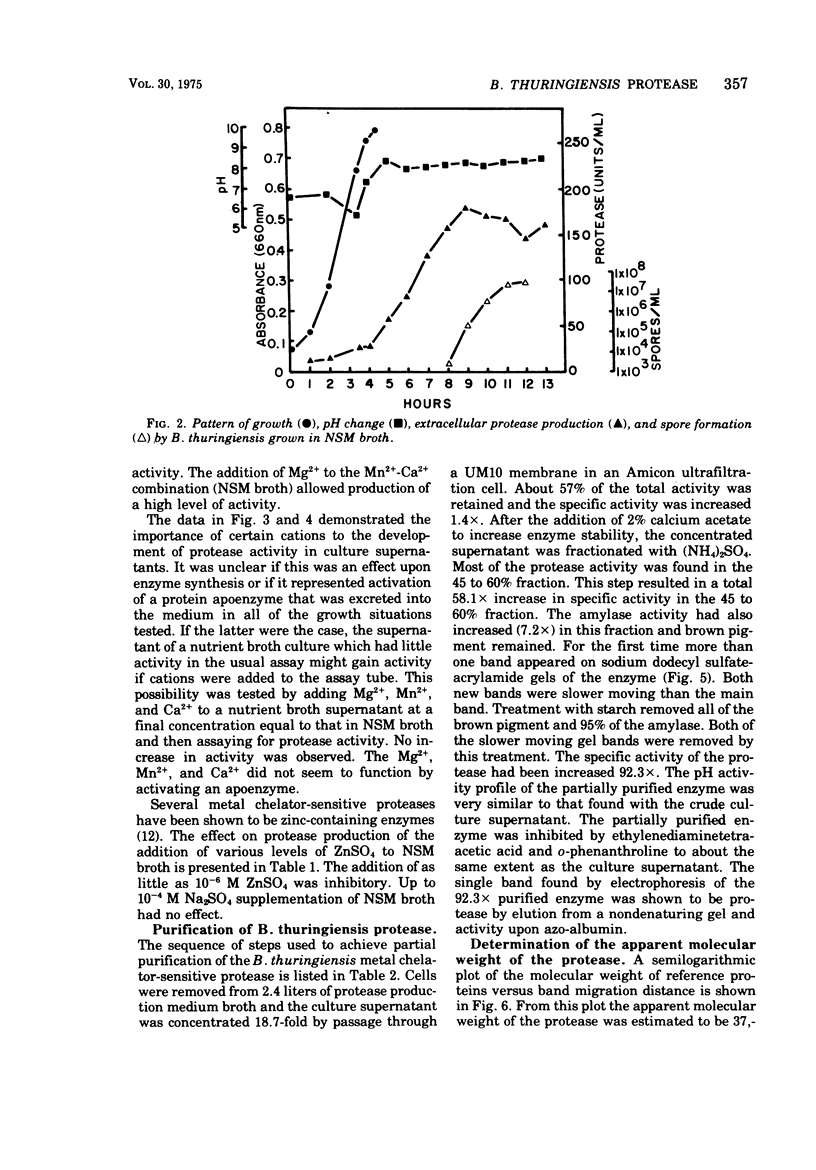
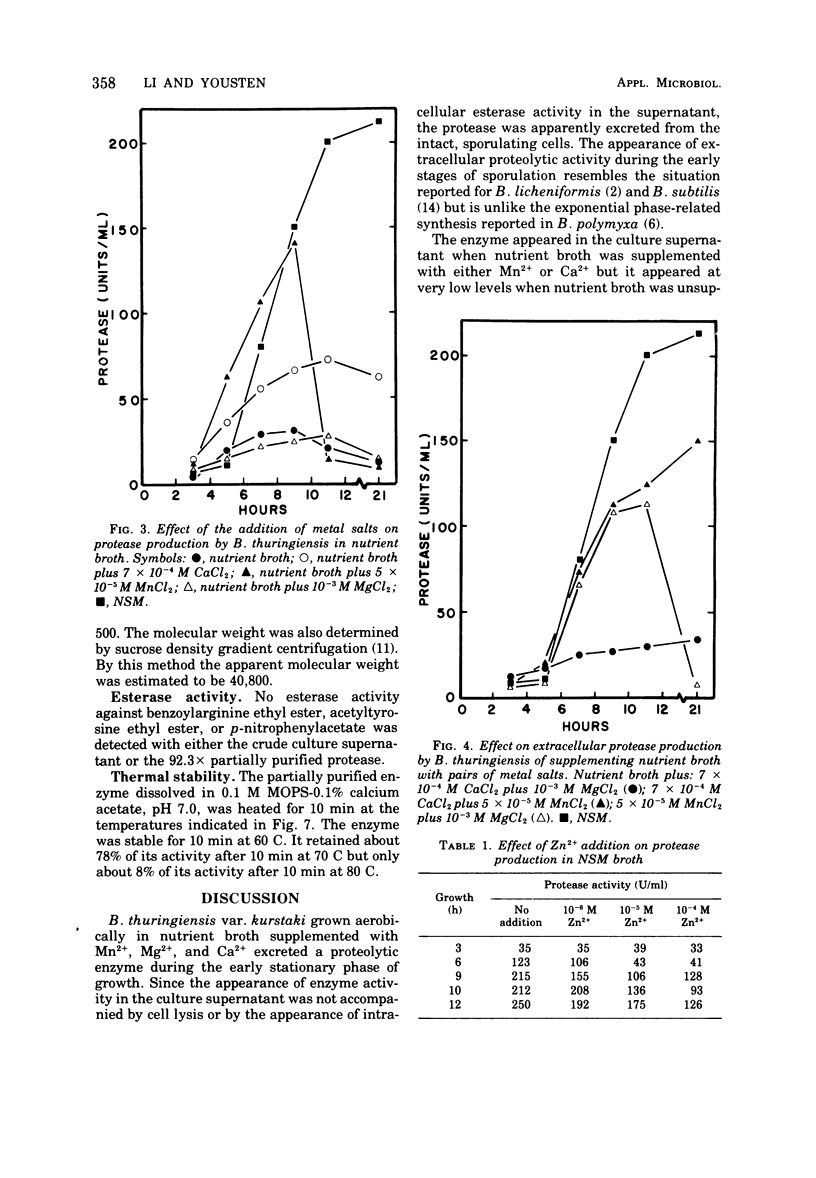
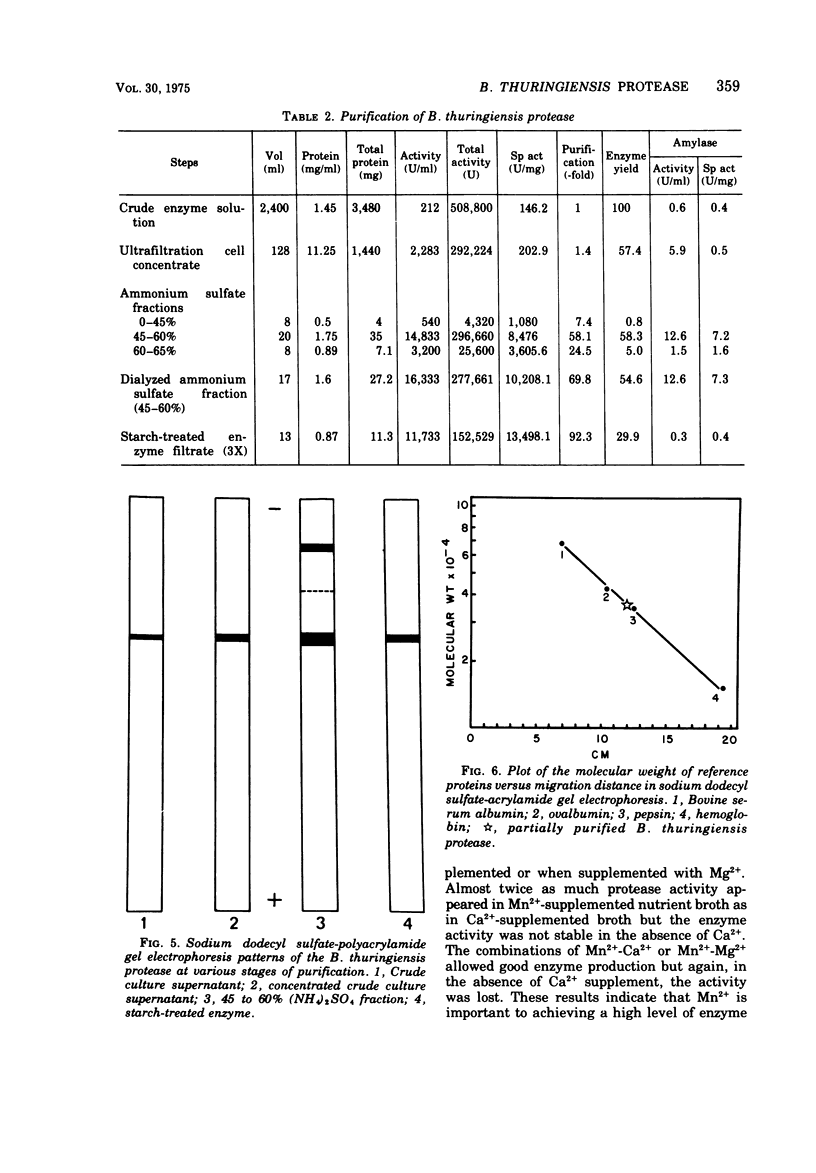
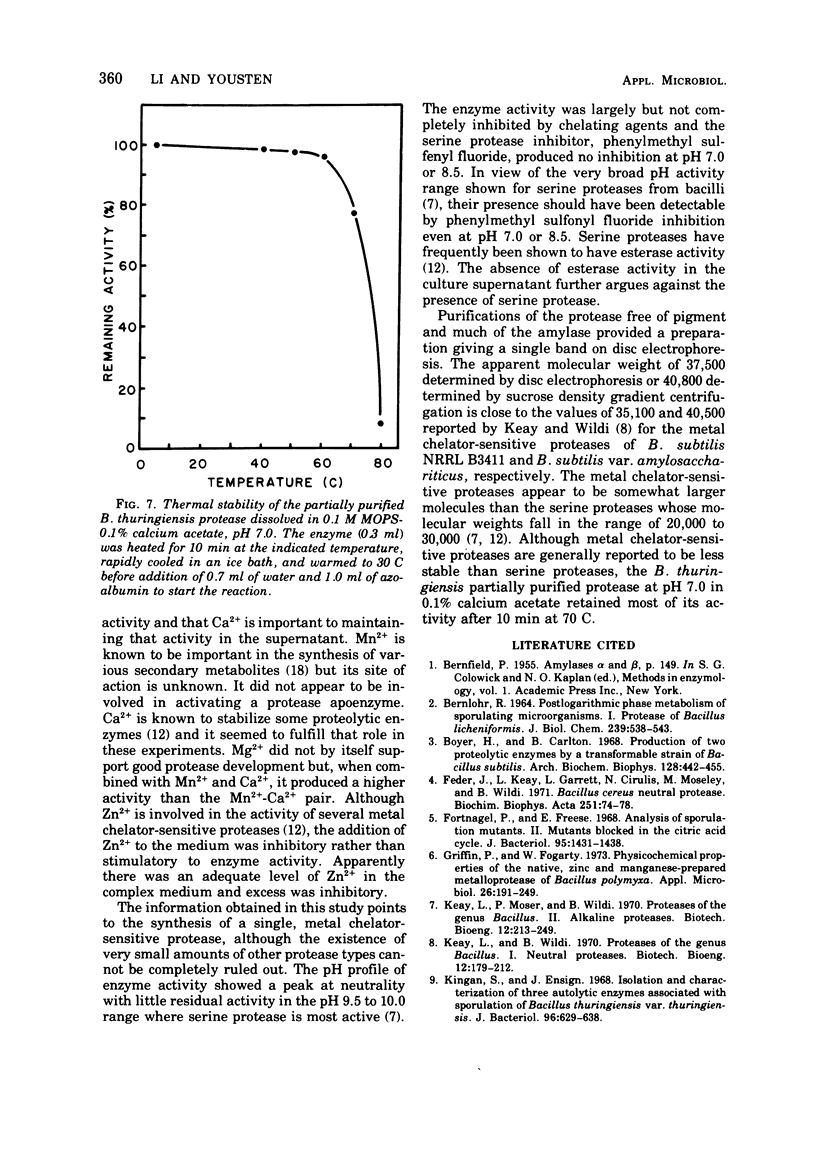
Bacillus thuringiensis var. kurstaki was shown to produce an extracellular, metal chelator-sensitive protease during the early stages of sporulation. Protease production in nutrient broth was dependent upon supplementation with Mn2+ or Ca2+. The addition of Ca2+ was required for enzyme stabilization. Protease production occurred in nutrient broth supplemented with 7 × 10-3 M Ca2+, 5 × 10-4 M Mn2+, and 10-3 M Mg2+. The protease had optimum activity in the pH range 6.5 to 7.5. It was inhibited by chelating agents but not by a serine protease inhibitor. The culture supernatant and the partially purified protease lacked esterase activity. Partial purification of the enzyme (92.3 ×) by (NH4)2SO4 fractionation and starch adsorption yielded an enzyme whose molecular weight was estimated to be 37,500 by acrylamide gel-sodium dodecyl sulfate electrophoresis or 40,800 by sucrose density gradient centrifugation. In the presence of Ca2+, the partially purified enzyme retained 78% of its activity after heating at 70 C for 10 min but only 8% of its activity after heating at 80 C for 10 min.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- Feder J., Keay L., Garrett L. R., Cirulis N., Moseley M. H., Wildi B. S. Bacillus cereus neutral protease. Biochim Biophys Acta. 1971 Oct;251(1):74–78. doi: 10.1016/0005-2795(71)90061-4. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P. J., Fogarty W. M. Physiochemical properties of the native, zinc- and manganese-prepared metalloprotease of Bacillus polymyxa. Appl Microbiol. 1973 Aug;26(2):191–195. doi: 10.1128/am.26.2.191-195.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L., Moser P. W., Wildi B. S. Proteases of the genus Bacillus. II. Alkaline proteases. Biotechnol Bioeng. 1970 Mar;12(2):213–249. doi: 10.1002/bit.260120206. [DOI] [PubMed] [Google Scholar]
- Keay L., Wildi B. S. Proteases of the genus Bacillus. I. Neutral proteases. Biotechnol Bioeng. 1970 Mar;12(2):179–212. doi: 10.1002/bit.260120205. [DOI] [PubMed] [Google Scholar]
- Kingan S. L., Ensign J. C. Isolation and characterization of three autolytic enzymes associated with sporulation of Bacillus thuringiensis var. thuringiensis. J Bacteriol. 1968 Sep;96(3):629–638. doi: 10.1128/jb.96.3.629-638.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogoff M. H., Yousten A. A. Bacillus thuringiensis: microbiological considerations. Annu Rev Microbiol. 1969;23:357–386. doi: 10.1146/annurev.mi.23.100169.002041. [DOI] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Yousten A. A., Rogoff M. H. Metabolism of Bacillus thuringiensis in relation to spore and crystal formation. J Bacteriol. 1969 Dec;100(3):1229–1236. doi: 10.1128/jb.100.3.1229-1236.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



