Abstract
We studied the activity of T138067-sodium in patients with malignant gliomas. T138067-sodium is a unique new chemotherapy agent that inhibits microtubule formation by binding irreversibly and specifically to β1, β2, and β4 isotypes of β-tubulin, causing cell arrest at G2/M and inducing apoptosis. Patients with recurrent anaplastic astrocytoma or glioblastoma multiforme were treated intravenously with 330 mg/m2 of T138067-sodium weekly. Treatment was continued until the patient experienced either unacceptable toxicity or progressive disease. Patients had to have histologically proven glioma, have bidimensionally measurable disease at least 1 cm × 1 cm, and have received no more than one prior adjuvant chemotherapy. No chemotherapy or radiotherapy for recurrent disease was permitted. Nineteen patients entered the trial. One patient was found to be ineligible. There were two patients with anaplastic astrocytoma and 16 with glioblastoma multiforme. Only two patients had received prior adjuvant chemotherapy. The first seven patients had full pharmacokinetic sampling. No dose-limiting toxicity was seen, and pharmacokinetic results were consistent with those from non-glioma patients. The most common drug-related effects were fatigue (33%), nausea (28%), neutropenia (28%), and anorexia (17%). No patients stopped the study because of toxicity. No responses were seen in the 15 eligible patients who completed at least one cycle. Three patients had stable disease with a median duration of 2.6 months. Our results suggest that given in this dose and schedule T138067-sodium does not have activity in this population of anaplastic astrocytoma and glioblastoma multiforme.
Malignant astrocytomas are incurable tumors with median survivals ranging from 4.7 to 58.6 months depending on age, histology, and Karnofsky performance status (Curran et al., 1993). Initial radiotherapy usually provides temporary control, and adjuvant chemotherapy provides some improvement in survival (GMT Group, 2002; Stupp et al., 2004), but at recurrence, the response rate is low and usually of brief duration (Yung et al., 2000). Thus more efficacious agents are desperately needed.
T138067-sodium is a novel chemotherapeutic agent that covalently binds to cysteine-239 on β-tubulin iso-forms 1, 2, and 4, inhibiting microtubule polymerization (Shan et al., 1999). In tumor-derived cells, T138067-sodium causes microtubule dysfunction inducing cyto-skeletal collapse, cell-cycle arrest at the G2/M checkpoint, and eventually apoptosis. It also evades common cellular mechanisms of drug resistance, including P-glycoprotein drug pump overexpression (Shan et al., 1999), unlike the taxanes and vinca alkaloids. Intravenous T138067-sodium crosses the blood-brain barrier in mice with a plasma-to-brain ratio of 8:1 at 5 min postinjection and labels brain tubulin (Rubenstein et al., 2001). In a nude mouse model with intracerebrally implanted glioblastoma multiforme (GBM),3 T138067-sodium had a small but statistically significant effect on survival.
Phase 1 trials of T138067-sodium were conducted by using a 3-h infusion of drug given weekly or every 21 days (Donehower et al., 2001; Preston et al., 2001). Dose-limiting neurotoxicity consisting of encephalopathy, headache, hearing loss, and ataxia was seen in two patients, one treated with 585 mg/m2 every 21 days and the other treated with 440 mg/m2 per week. One patient treated with 440 mg/m2 per week developed dose-limiting neutropenia. Patients treated with 330 mg/m2 per week tolerated it well except for two patients who had hepatocellular carcinoma with prior low platelet counts and who developed dose-limiting thrombocytopenia. The 3-h weekly infusion schedule was selected for further evaluation, and the recommended starting dose was 330 mg/m2 per week.
We studied the efficacy and pharmacokinetics (PK) of T138067-sodium given in this schedule in patients with recurrent anaplastic astrocytoma (AA) and GBM in a phase 2 trial of the National Cancer Institute of Canada Clinical Trials Group.
Methods
Eligibility criteria were as follows: age ⩾18 years, histologically proven GBM or AA, recurrent or progressive disease following primary surgery and radiation treatment, no more than one prior chemotherapy regimen in the adjuvant setting, stable dose of steroids for at least 14 days prior to baseline CT or MRI scan and registration, enhancing lesions on CT or MRI that were ⩾1 cm × 1 cm in at least one lesion, Karnofsky performance status at ⩾60, adequate hematological and hepatic function, negative pregnancy test, and for patients capable of reproduction, willingness to use contraception. Written informed consent on the local Research Ethics Board–approved consent form was obtained from all patients prior to registration in the study.
Exclusion criteria included any prior treatment for recurrent disease including surgery within six weeks of study treatment, previous malignancies except curatively treated in situ carcinoma of the cervix or nonmelanoma skin cancer, known defect in glutathione metabolism, need for at least 4 g of acetaminophen per day, Gilbert’s syndrome, and patients with organ allograft.
This was a multicenter, nonrandomized, nonblinded phase 2 study. The initial six patients were scheduled to receive T138067-sodium at a dose of 330 mg/m2 weekly. Full PK sampling was planned for the first six patients to rule out any substantial changes in drug clearance and/or toxic effects in this population of patients who often receive steroids and anti-epileptic medications. If dose-limiting toxicity (defined as grade 4 neutropenia, or platelets <25 × 109/liter or grade 3 organ toxicity) was seen in no more than one patient of the six during cycle 1, then all subsequent patients would be enrolled at the same dose. Otherwise, all further patients would be enrolled at a dose of 220 mg/m2. Limited PK sampling was done on day 1 in the remainder of the patients. Using a Fleming two-stage design (Fleming, 1982), assuming a 20% response rate would be of interest, we planned to enroll 15 response-assessable patients. If no responses were seen, the trial would be closed. If one or more responses were observed, enrollment would continue to a maximum of 30 patients. The power (1-beta) associated with this design was 86.5%, and the significance level (alpha) was 5.8%.
T138067-sodium was administered weekly as a 3-h intravenous infusion that was based on the patient’s actual calculated body surface area at the beginning of each three-week cycle and that was capped at 2.0 m2. Patients were not routinely premedicated for nausea or vomiting. Treatment was withheld for patients with absolute neutrophil count <0.75 × 109/liter, platelets <50 × 109/liter, or grade 3 or greater neurotoxicity or other major organ toxicity for a maximum of two weeks. Otherwise, treatment was discontinued. Patients with a grade 2 toxicity had a 50% dose reduction.
After the full PK analyses were performed on the first six patients, limited sampling was to be done on the remainder of the patients to determine peak concentration (Cmax) and estimate clearance. We calculated PK parameters from the plasma samples using a proprietary validated LC/MS/MS method by Alta Analytical Laboratory (El Dorado Hills, Calif.).
Each patient had a complete history, neurological and physical examination, and serum biochemistries within 14 days of initiating therapy and on day 1 of each cycle. Complete blood cell counts were done weekly. A CT or an MRI brain scan was done at baseline and every six weeks. Toxicities were evaluated after every cycle and recorded and graded according to the NCI Common Toxicity Criteria version 2.0 (Cancer Therapy Evaluation Program, 1998).
Patients who received at least one full cycle of therapy and had their disease reevaluated were assessable for response. Assessment of response was based on scan changes of enhancing tumor and clinical neurological assessment. Scans were graded by using a modification of the MacDonald criteria (MacDonald et al., 1990). “Definite improvement” was defined as an estimated 50% or more decrease, “equivocal/no change” was defined as <50% decrease and <25% increase, and “definite progression” was defined as an estimated ⩾25% increase in the size of the enhancing lesions as calculated by the product of cross-sectional diameters or appearance of new lesions.
Clinical neurological assessment was performed every three weeks and classified by the criteria of Levin et al. (1977) as “better” (Levin +2, +3), “equivocal/no change” (Levin +1, 0, −1), and “worse” (Levin −2, −3). Any patient who required an increased dose of corticosteroids to maintain a “no change” status was considered to have deteriorated clinically and was classified as “worse.” Those patients who worsened with a decreased dose of corticosteroids were considered “equivocal/no change” until they were reassessed at their original corticosteroid dose. This was to avoid “pseudo-responses” and “pseudo-progression” caused by changes in corticosteroid doses.
Results
Nineteen patients were registered for the trial. One patient was ineligible according to both central pathology review (mixed oligoastrocytoma) and radiographic review (enhancing tumor size <1 cm × 1 cm). Sixteen patients had GBM and two had AA. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics (n = 18 patients)
| Characteristic | Value or Number |
|---|---|
| Median Value (range) | |
| Age | 62 (41–75) |
| Months since diagnosis | 9.1 (5.9–30.5) |
| Number of Patients | |
| Gender | |
| Female | 4 |
| Male | 14 |
| Karnofsky performance status | |
| 60 | 2 |
| 70 | 8 |
| 80 | 3 |
| 90 | 3 |
| 100 | 2 |
| Prior therapy | |
| Adjuvant chemotherapy | 2 |
| Radiotherapy | 18 |
| Histology | |
| Anaplastic astrocytoma | 2 |
| Glioblastoma multiforme | 16 |
Thirty-eight cycles of T138067-sodium were administered. The median number of cycles given was 2 (range, 1–8). Five patients required a dose reduction. Eighteen patients were assessable for toxicity (Tables 2 and 3). There were no dose-limiting toxicities in the first six patients fully treated at 330 mg/m2, and thus accrual continued at the same dose.
Table 2.
Related* nonhematologic toxicities, worst by patient (n = 18 patients)
| Grade** | |||||||
|---|---|---|---|---|---|---|---|
| Toxicity | 1 | 2 | 3 | 4 | 5 | Total | % Points |
| Flu-like symptoms | |||||||
| Fever | 1 | 1 | (5.6) | ||||
| Fatigue | 4 | 2 | 6 | (33.3) | |||
| Rigors, chills | 1 | 1 | (5.6) | ||||
| Gastrointestinal | |||||||
| Anorexia | 1 | 2 | 3 | (16.7) | |||
| Diarrhea | 1 | 1 | (5.6) | ||||
| Dyspepsia/heartburn | 1 | 1 | (5.6) | ||||
| Nausea | 3 | 2 | 5 | (27.8) | |||
| Vomiting | 1 | 1 | (5.6) | ||||
| Renal | |||||||
| Urine frequency/urgency | 1 | 1 | (5.6) | ||||
| Neurology | |||||||
| Confusion | 1 | 1 | 2 | (11.1) | |||
| Neuropathy-cranial | 1 | 1 | (5.6) | ||||
| Speech impairment | 1 | 1 | (5.6) | ||||
| Memory loss | 1 | 1 | (5.6) | ||||
| Neuropathy-motor | 1 | 1 | 2 | (11.1) | |||
| Seizure(s) | 1 | 1 | (5.6) | ||||
| Depressed conscious | 2 | 2 | (11.1) | ||||
| Tremor | 2 | 2 | (11.1) | ||||
| Pain | |||||||
| Headache | 1 | 2 | 3 | (16.7) | |||
| Pulmonary | |||||||
| Dyspnea | 1 | 1 | (5.6) | ||||
| Dermatology | |||||||
| Alopecia | 2 | 2 | (11.1) | ||||
| Injection site reaction | 1 | 1 | (5.6) | ||||
| Pruritus | 1 | 1 | (5.6) | ||||
| Rash/desquamation | 1 | 1 | (5.6) | ||||
| Any | 9 | 10 | 4 | 0 | 0 | 12 | (66.7) |
Considered by investigator to be possibly, probably, or definitely related to protocol treatment.
Toxicity graded according to National Cancer Institute Common Toxicity Criteria version 2.0.
Table 3.
Hematologic toxicity, worst by patient (n = 17 assessable patients)*
| Toxicity Grade** | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Granulocytes | 6 | 2 | 6 | 3 | |
| Hgb | 7 | 10 | |||
| Platelets | 14 | 3 | |||
| WBC | 6 | 5 | 5 | 1 | |
Includes eligible patients who could be evaluated for at least one cycle (blood count done between days 7 and 16).
Toxicity graded according to National Cancer Institute Common Toxicity Criteria version 2.0.
Nonhematological toxicity (Table 2) was generally mild, with only two patients reporting grade 3 fatigue and one patient reporting grade 3 diarrhea. One patient had reversible grade 3 neurotoxicity consisting of grade 3 confusion, expressive dysphasia, and grade 2 tremors, and a second patient developed grade 3 weakness. The toxicity in both patients was thought possibly to be related to T13867-sodium. Hematological toxicity (Table 3) in 17 assessable patients was moderate, with no grade 4 toxicity and no febrile neutropenia. Biochemical effects were minimal. One patient had grade 4 elevation in creatinine (normal at baseline), but this was thought unlikely to be drug related. No patients were withdrawn because of toxicity.
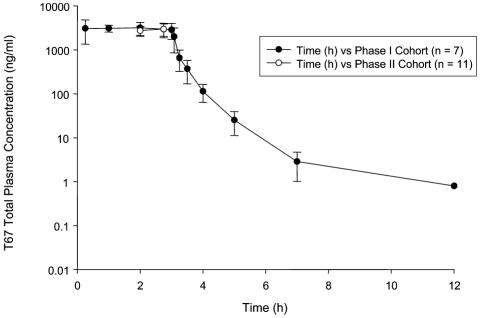
Full PK analysis was carried out on seven patients (cohort I). All seven were receiving enzyme-inducing anticonvulsants and/or corticosteroids. Results are shown in Table 4. A plot of mean concentration versus time for those patients with full PK sampling and those with limited sampling (cohort II) is shown in Fig. 1.
Table 4.
Pharmacokinetic results. Mean pharmacokinetic parameters (n = 18 patients)
| Patients | Number Evaluated | AUC mg/h/m | Cmaxng/ml | Vol. Dist. liters/m2 | Half-life h | Clearance liters/m2/h |
|---|---|---|---|---|---|---|
| 1–7 | 7 | Mean 9653.7 | 4046 | 9.4 | 0.6 | 35.9 |
| SD 2057.4 | 1325 | 3.8 | 0.1 | 9.7 | ||
| 8–18 | 11 | Mean | 3201 | 40.2 | ||
| SD | 911.2 | 9.1 | ||||
| All | 18 | Mean | 3529 | 38.5 | ||
| SD | 1135 | 9.3 |
Abbreviations: AUC, area under the curve; Cmax, peak concentration; Vol. Dist., volume of distribution.
Fig. 1.
Plot of plasma T138067-sodium concentration versus time (mean ± standard deviation) for patients having full pharmacokinetic sampling (•) and those with limited sampling (○).
Fifteen patients completed cycle 1 and were assessable for response (Table 5). Three patients did not complete the cycle and were not assessable. There were no partial or complete responses. Three patients had stable disease with a median duration of 2.6 months. The ineligible patient with a mixed oligoastrocytoma had a partial response in a solitary lesion. However, the trial was closed because no responses were seen in the 15 eligible patients.
Table 5.
Confirmed response (n = 18 eligible patients)
| Duration in Months | |||
|---|---|---|---|
| Response | Number of Patients | Median | (Range) |
| Complete response | 0 | ||
| Partial response | 0 | ||
| Stable disease | 3 | 2.6 | (1.3–5.5) |
| Progressive disease | 12 | ||
| Not assessable | 3 | ||
| Response Rate = 0.0% (95% CI, 0.0–15.3%) | |||
Discussion
T138067-sodium was well tolerated in this patient population. Pharmacokinetic studies yielded results similar to those reported in two other studies: The mean clearance was 38.5 liters/m2 per hour in this trial, and in the other studies it was 52.9 ± 21.97 and 42.1 ± 16.1 liters/m2 per hour (Donehower et al., 2001; Preston et al., 2001). The mean half-life in the seven patients with full PK studies was 0.6 h, also similar to that in other trials (0.58 ± 0.2 h and 0.49 ± 0.19 h) (Donehower et al., 2001; Preston et al., 2001).
Thus the PK behavior of the drug in this glioma patient population did not appear to be affected by concomitant medications, although no specific analysis of T138067-sodium clearance in the same patients before and after dosing with steroids or anticonvulsants was undertaken. Furthermore, the similarity of the toxicity profile for the dose and schedule used to that reported in other studies is suggestive of no significant interactions. However, the number of patients was very small, and so firm conclusions about drug interactions cannot be drawn.
Despite the preclinical data suggesting this agent might have promising activity, given in this dose and schedule, T138067-sodium showed no evidence of objective antitumor activity in this population of patients with recurrent malignant AA or GBM. The PK data and toxicity profile suggest that inadequate dosing is unlikely to have been a factor.
Unlike the taxanes, T138067-sodium is unaffected by the multidrug resistance profile of the tumor cells or the presence of TP53 mutations. Additional mechanisms of resistance for tubulin-binding drugs are suspected. Expression of β3 isotypes of β-tubulin has been associated with increased resistance to paclitaxel in some tumor cell lines (Burkhart et al., 2001; Hari et al., 2003). In normal astrocytes, β3-tubulin is not expressed. But in astrocytomas, expression of increasing amounts of β3-tubulin has been reported to be associated with an increasing degree of anaplasia (Katsetos et al., 2001). If aberrant β3-tubulin expression by tumor cells is a marker for another mechanism of resistance to tubulin-binding drugs, this may be a possible explanation for the lack of efficacy seen in our patient population of high-grade astrocytomas.
The response seen in the ineligible patient with a mixed oligoastrocytoma raises the possibility that T138067-sodium may be effective in oligodendrogliomas and mixed oligoastrocytomas. Further study may be warranted in these tumors.
Acknowledgments
We thank the following who, in addition to the authors, contributed to this trial: David MacDonald, London Regional Cancer Centre, who contributed patients; Wendy Walsh, National Cancer Institute of Canada Clinical Trials Group, who provided statistical support; and Greg Cairncross, Chair of the Brain Disease Site Committee of the National Cancer Institute of Canada Clinical Trials Group, for his leadership and support.
Footnotes
This study was supported by grants from the National Cancer Institute of Canada and Tularik, Inc.
Abbreviations used are as follows: AA, anaplastic astrocytoma; Cmax, peak concentration; GBM, glioblastoma multiforme; PK, pharmacokinetic(s).
References
- Burkhart CA, Kavallaris M, Horwitz SB. The role of α-tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta. 2001;1471:O1–O9. doi: 10.1016/s0304-419x(00)00022-6. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program (1998) Common Toxicity Criteria, version 2.0. National Institutes of Health, National Cancer Institute, Bethesda, Md.
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, Nelson DF. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Donehower, R.C., Schwartz, G.H., Wolff, A.C., Olivo, N., Burks, K.L., Wright, M., Walling, J., and Rowinsky, E.K. (2001) A phase I pharmacokinetic study of T138067 administered as a weekly 3-hour infusion. Abstract 438. Paper presented at the American Society of Clinical Oncology 2001 ASCO Annual Meeting. Available at www.asco.org
- Fleming T. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- GMT Group. Glioma Meta-Analysis Trialists Group (2002) Chemotherapy for high-grade glioma. Cochrane Database Syst Rev. 4:CD003913. doi: 10.1002/14651858.CD003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III β-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil Cytoskeleton. 2003;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Del Valle L, Geddes JF, Assimakopoulou M, Legido A, Boyd JC, Balin B, Parikh NA, Maraziotis T, de Chadarevian JP, Varakis JN, Matsas R, Spano A, Frankfurter A, Herman MM, Khalili K. Aberrant localization of the neuronal class III β-tubulin in astrocytomas. Arch Pathol Lab Med. 2001;125:613–624. doi: 10.5858/2001-125-0613-ALOTNC. [DOI] [PubMed] [Google Scholar]
- Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47:329–335. doi: 10.3171/jns.1977.47.3.0329. [DOI] [PubMed] [Google Scholar]
- MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- Preston, G., Schwartz, G., Garrison, M., Olivio, N., Wright, M., Walling, J., Gallagher, M., Stevenson, J., Rowinsky, E.K., and O’Dwyer, P. (2001) A phase I pharmacokinetic study of T138067, a synthetic microtubule depolymerizing agent, administered as a 3-hour infusion daily × 5 every 3 weeks. Abstract 443. Paper presented at the American Society of Clinical Oncology 2001 ASCO Annual Meeting. Available at www.asco.org
- Rubenstein SM, Baichwal V, Beckmann H, Clark DL, Frankmoelle W, Roche D, Santha E, Schwender S, Thoolen M, Ye Q, Jaen JC. Hydrophilic, pro-drug analogues of T138067 are efficacious in controlling tumor growth in vivo and show a decreased ability to cross the blood brain barrier. J Med Chem. 2001;44:3599–3605. doi: 10.1021/jm000478d. [DOI] [PubMed] [Google Scholar]
- Shan B, Medina JC, Santha E, Frankmoelle WP, Chou TC, Learned RM, Narbut MR, Stott D, Wu P, Jaen JC, Rosen T, Timmermans PB, Beckman H. Selective, covalent modification of β-tubulin residue Cys-239 by T138067, an antitumor agent with in vivo efficacy against multi-drug resistant tumors. Proc Natl Acad Sci USA. 1999;96:5686–5691. doi: 10.1073/pnas.96.10.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn M, Brandes AA, Cairncross G, Lacombe D, Mirimanoff RO. Concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM). Conclusive results of a randomized phase III trial by the EORTC Brain & RT Groups and NCIC Clinical Trials Group. J. Clin. Oncol. 2004;22(14S 2 (suppl.)) [Google Scholar]
- Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]