Abstract
Interleukin-8 (IL-8, or CXCL8), which is a chemokine with a defining CXC amino acid motif that was initially characterized for its leukocyte chemotactic activity, is now known to possess tumorigenic and proangiogenic properties as well. In human gliomas, IL-8 is expressed and secreted at high levels both in vitro and in vivo, and recent experiments suggest it is critical to glial tumor neovascularity and progression. Levels of IL-8 correlate with histologic grade in glial neoplasms, and the most malignant form, glioblastoma, shows the highest expression in pseudopalisading cells around necrosis, suggesting that hypoxia/anoxia may stimulate expression. In addition to hypoxia/anoxia stimulation, increased IL-8 in gliomas occurs in response to Fas ligation, death receptor activation, cytosolic Ca2+, TNF-α, IL-1, and other cytokines and various cellular stresses. The IL-8 promoter contains binding sites for the transcription factors NF-κB, AP-1, and C-EBP/NF-IL-6, among others. AP-1 has been shown to mediate IL-8 upregulation by anoxia in gliomas. The potential tumor suppressor ING4 was recently shown to be a critical regulator of NF-κB-mediated IL-8 transcription and subsequent angiogenesis in gliomas. The IL-8 receptors that could contribute to IL-8-mediated tumorigenic and angiogenic responses include CXCR1 and CXCR2, both of which are G-protein coupled, and the Duffy antigen receptor for cytokines, which has no defined intracellular signaling capabilities. The proangiogenic activity of IL-8 occurs predominantly following binding to CXCR2, but CXCR1 appears to contribute as well through independent, small-GTPase activity. A precise definition of the mechanisms by which IL-8 exerts its proangiogenic functions requires further study for the development of effective IL-8-targeted therapies.
Tumorigenesis is a complex, multistep process that includes cellular neoplastic transformation, resistance to apoptosis, autonomous growth signaling, emergence of a vascular supply, evasion of immunologic surveillance, and the acquisition of invasive/metastatic properties. Soluble factors in the tumoral environment—derived not only from neoplastic cells but also from stroma, inflammatory cells, and endothelial cells—are critical determinants of many of these neoplastic processes. Direct and indirect evidence strongly implicates a subset of soluble factors, the chemokines, as key regulators of tumorigenesis (Balkwill, 2004; Vicari and Caux, 2002). One of these, interleukin-8 (IL-8,3 or CXCL8), is best known for its leukocyte chemotactic properties and associated role in inflammatory and infectious diseases (Harada et al., 1994), although its tumorigenic and proangiogenic activities that were first suggested in the early 1990s are now widely accepted (Xie, 2001). This review focuses on our current knowledge of IL-8 and its receptors, with an emphasis on the role of IL-8 signaling in gliomagenesis and angiogenesis.
IL-8 Is a CXC Chemokine
Interleukin-8 is a member of the chemokine family. The chemokines are specialized cytokines produced and secreted by a variety of normal and neoplastic human cell types, which have been defined by their ability to cause directed migration of leukocytes. They are generally secreted in response to growth factors, inflammatory cytokines, and pathophysiologic conditions (Matsushima and Oppenheim, 1989; Walz et al., 1987; Yoshimura et al., 1987). Among the first chemokines discovered, IL-8 was identified as a chemotactic factor secreted by activated monocytes and macrophages that promotes the directional migration of neutrophils, basophils, and T lymphocytes (Baggiolini et al., 1989; Rossi and Zlotnik, 2000). It was later found to play an important role in autoimmune, inflammatory, and infectious diseases (Harada et al., 1994; Koch et al., 1992; Smyth et al., 1991). Because of its potent pro-inflammatory properties, IL-8 is tightly regulated, and its expression is low or undetectable in normal tissues.
Like all chemokines, IL-8 is a small, soluble peptide (8–10 kDa). The chemokine family has been divided into four major groups, CXC, CC, C, and CX3C, with individual classification based on the number and location of conserved cysteines within the N-terminal amino acid sequence (Baggiolini et al., 1994, 1997). More than 50 human chemokines have been defined thus far (Balkwill, 2004), with the majority belonging to either the CC subfamily, in which the first two cysteines are consecutive, or the CXC family, in which the first two cysteines are separated by a single amino acid. Interleukin-8 is prototypical of the CXC group, which also includes growth-regulated oncogene (GRO)-α, -β, and -γ (CXCL1–3), neutrophil-activating protein-2 (NAP-2; CXCL7), epithelial-cell-derived neutrophil-activating protein (ENA78; CXCL5), granulocyte chemotactic protein-2 (GCP-2; CXCL6), γ-interferon-inducible protein 10 (IP-10; CXCL10), monokine induced by γ-interferon (Mig; CXCL9), interferon-inducible T cell α-chemoattractant (I-TAC; CXCL11), stromal-cell-derived factor-1 (SDF-1; CXCL12), platelet factor 4 (PF4; CXCL4), BCA-1 (CXCL13), and BRAK (CXCL14). Interleukin-8 has sequence identity ranging from 24% to 46% with the other members of the CXC family. All of the human CXC genes are clustered on chromosome 4 between 4q12 and 4q21, which suggests that they may all be derived from a common ancestral gene.
Interleukin-8 is synthesized primarily as a 99 amino acid peptide that is processed through signal sequence cleavage and N-terminal proteolysis to yield several active IL-8 isoforms (Hebert and Baker, 1993), with peptides of 72 and 77 amino acids being most biologically relevant (Strieter et al., 1989). The 72 amino acid peptide is the major form secreted by monocytes and macrophages in culture, whereas the 77 amino acid variant is the most abundant secretory product of nonimmune cells. The monomer peptide structure of IL-8 contains an NH2-terminal loop, three antiparallel beta-strands connected by loops, and a C-terminal alpha helix. While IL-8 readily forms dimers in solution, the monomer is believed to be the biologically relevant and active form of this chemokine (Horcher et al., 1998; Rajarathnam et al., 1994). Expression of IL-8 can be induced, in some cases up to 100-fold, by IL-1, TNF-α, IL-6, interferon-γ, lipopolysaccharide, phytohemagglutinin, phorbol myristate acetate, reactive oxygen species, and other cellular stresses (Baggiolini et al., 1994; DeForge et al., 1993). Potent inhibitors of IL-8 production include dexa-methasone, IL-4, and IL-10 (Mukaida et al., 1994; Xie, 2001). Protein secretion appears to be directly related to cellular levels of mRNA, and both transcriptional rates of the IL-8 gene and its mRNA half-life are believed to be responsible for dictating steady-state mRNA levels (Mukaida et al., 1990; Roebuck, 1999). Transcriptional regulation of the IL-8 gene is mediated by its 5′ flanking region (Fig. 1).
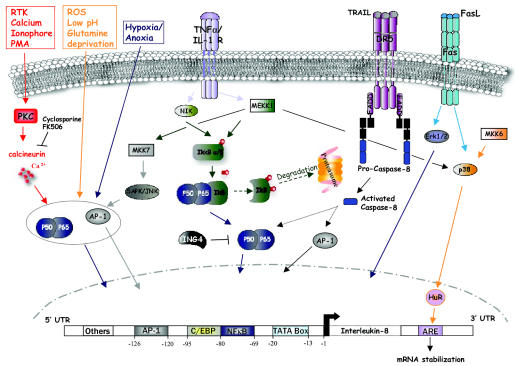
Fig. 1.
Regulation of IL-8 expression in cancer cells. Expression of IL-8 is low in normal tissue and can be induced by a variety of stimuli, such as IL-1, TNF-α, hypoxia/anoxia, death receptors (Fas, DR5), and a variety of cellular stresses. The regulation of IL-8 gene expression is highly dependent on its 5′ flanking region. The best characterized sequence in the IL-8 promoter contains binding sites for the transcription factors NF-κB, AP-1, and C-EBP/NF-IL-6. NF-κB is required for transcriptional activity of the IL-8 promoter. AP-1 and C-EBP/NF-IL-6 also regulate transcription in a cell-dependent manner under certain pathophysiologic conditions. Maximal activation occurs with the interaction of the three transcription factors. The 3′ untranslated region of the gene is also implicated in the regulation of IL-8. This region contains an AU-rich RNA instability element (ARE) regulated by MEKK1, MKK6, and p38 MAP kinase and is critical for determining the half-life of IL-8 mRNA.
To date, regulatory sequences have been characterized from −1 to −2500 of the gene’s 5′ region, with the most thoroughly characterized portion at −425 to −70, which contains binding sites for the transcriptional modulators AP-1, NF-κB, and C-EBP/NF-IL-6 (Fig. 1), as well as putative binding sites for the glucocorticoid receptor, hepatocyte nuclear factor-1, and interferon regulatory factor-1 (Hoffmann et al., 2002; Kunsch and Rosen, 1993; Mukaida et al., 1990; Roebuck, 1999). NF-κB binding appears to be required for transcriptional activity at the IL-8 promoter in all cell types, whereas AP-1 and C-EBP/NF-IL-6 binding contribute to transcriptional activity in a cell-dependent manner (Mahe et al., 1991; Mukaida et al., 1990). Maximal activation at the IL-8 promoter occurs in the setting of synergistic interaction of transcription factors. For example, the physical interaction of AP-1 or NF-IL-6 with NF-κB enhances promoter activity (Matsusaka et al., 1993).
Transcriptional regulation of IL-8 synthesis incorporates signals from diverse intracellular signaling pathways and appears to differ according to whether the stimulus for upregulation is a cytokine or cellular stress (Holtmann et al., 1999, 2001). Transcriptional stimulation of the IL-8 gene by the cytokines IL-1 or TNF-α involves a small fragment of the promoter (−1 to −133) that includes the NF-κB and NF-IL-6 binding sequences and the TATA box. Transcription of the IL-8 gene is also upregulated by oxidative stress, and this type of stimulation appears to depend largely on the AP-1 binding site (Lakshminarayanan et al., 1998). In some cell types, especially those that are neoplastically transformed, IL-8 expression is constitutive (Xie, 2001) but requires the sequence between −133 and −85. Mutations within the AP-1 or NF-κB binding sequences can abolish constitutive IL-8 promoter activity. Epigenetic regulation of IL-8 transcription is less extensively studied, although differential methylation of two CpG sites in IL-8 was identified at a position approximately 1.2 kb upstream of the IL-8 promoter in breast carcinoma cell lines (De Larco et al., 2003). However, unlike most examples of epigenetic regulation, methylation at these sites was associated with increased IL-8 expression and secretion.
One of the critical regulators of TNF-α-mediated NF-κB activity is the phosphorylation status of its natural cytosolic inhibitor IκB (Fig. 1). Phosphorylation of two adjacent serines on IκB by IκB kinase (IKK)-α and -β leads to proteosomal degradation of IκB and to the release of the p50/p65 heterodimeric NF-κB to the nucleus to initiate transcription (Bobrovnikova-Marjon et al., 2004; Yamamoto and Gaynor, 2004). IκB kinase-α and -β are activated as a result of their phosphorylation by NF-κB-inducing kinase (NIK) or through the activity of mitogen-activated protein (MAP) kinase kinase kinase 1 (MEKK1). Both NIK and MEKK1 strongly upregulate transcriptional activity at the IL-8 promoter (Holtmann et al., 1999). MAP kinase kinase 7 (MKK7) was also found to increase the transcription of the IL-8 gene through its activation of the stress-activated protein kinase/c-jun N-terminal kinase, and MKK7 shows additive effect with NIK on IL-8 transcription. Tran scriptional activation by both NIK and MKK7 requires a minimal IL-8 promoter with functional AP-1 and NF κB binding sites. Neither MKK7 nor NIK have an appre ciable effect on mRNA stability. However, there is adenosine- and uridine (AU)-rich RNA instability element (AUUUA) present in the 3′ untranslated region of the IL-mRNA that contributes to its having a short half-life. An important modulator of this AU-rich element in the IL-transcript is HuR, a protein associated with RNA stabili zation that is upregulated in response to TNF-α (Nabors et al., 2003). The role of the AU-rich element in IL-transcript stability is also influenced by MEKK1, MKK6, and p38 MAP kinase (Tebo et al., 2003; Winzen et al. 1999, 2004). Taken together, it appears that activation NF-κB, stress-activated protein kinase/c-jun N-terminal kinase, and p38 MAP kinases all interact to increase IL-8 mRNA levels, either by increased transcription by increased transcript stability. MEKK1 activates three of these signaling mediators and results in maxi mal IL-8 levels (Holtmann et al., 1999; Winzen et al. 1999).
An independent mechanism for upregulating IL-transcription may involve agents that increase cytosolic Ca2+ mobilization, such as protein kinase C activation (Kuhns et al., 1998; Wakabayashi et al., 2004). Mechanisms that cause increased intracellular Ca2+ in gliomas could include the activation of receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor. A recent investigation demonstrated that exposure of U251 glioblastoma (GBM) cells to Ca2+ ionophores and phorbol-myristate-acetate leads to dramatic increases in IL-8 production (Wakabayashi et al., 2004) and that Ca2+-induced IL-8 expression could be suppressed by the calcineurin inhibitors cyclosporine and FK506. However, TNF-α-induced IL-8 expression was not suppressed by these inhibitors, which suggests divergent intracellular signaling mechanisms for controlling IL-8 expression. Both the increased expression of IL-8 due to Ca2+ mobilization and its inhibition by cyclosporine required the presence of intact NF-κB and AP-1 binding sites in the IL-8 promoter. The inhibition of Ca2+-dependent mechanisms at the NF-κB promoter by cyclosporine and FK506 raised the possibility that calcineurin could be a critical intermediary. The authors suggested that calcineurin mediates the degradation of IκB-α, perhaps through the activation of IκB kinases, resulting in increased nuclear translocation of NF-κB and transcription at the IL-8 promoter (Trushin et al., 1999).
IL-8 Binds to Specific CXC Receptors
CXC chemokines mediate their biological functions by interacting with specific G-protein-coupled CXC chemokine receptors (CXCRs) (Fig. 2). CXCRs are seven-transmembrane, rhodopsin-like receptors that range in size from 339 to 373 amino acids and have 25% to 80% sequence identity within their family (Murphy et al., 2000; Rossi and Zlotnik, 2000). At least 18 human chemokine receptors have been identified to date (Balkwill, 2004). The binding of CXC chemokines to their receptors (i.e., CXCRs) shows limited overlap, although it is clear that some receptors bind to more than one chemokine, and some chemokines bind to more than one receptor. Most chemokine receptors were initially described in association with the study of leukocytes, but it has become evident that their expression is more ubiquitous, having been shown for endothelial cells, epithelium, neurons, astrocytes, and microglia in the brain, as well as for a variety of tumor cells (Flynn et al., 2003; Murdoch et al; 1999, Murphy et al., 2000).
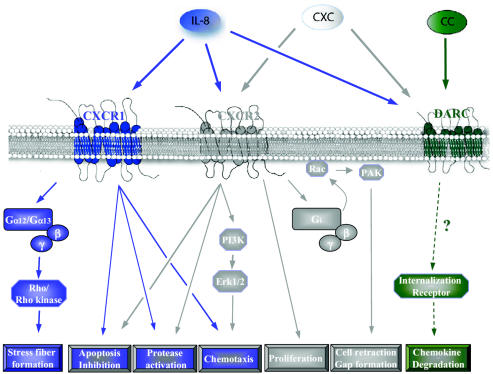
Fig. 2.
IL-8 signaling pathways in endothelial cells. CXCR1 and CXCR2 bind IL-8 with high affinity. CXCR1 binds only IL-8 and GCP-2, while CXCR2 is able to bind IL-8 and several other CXC chemokines with similar affinity. Binding of IL-8 to these seven-transmembrane receptors (G-protein-coupled receptors) induces angiogenic properties: endothelial cell proliferation, chemotaxis, survival, and protease activation. Duffy antigen receptor for chemokines binds both CXC (including IL-8) and CC chemokines but has not been shown to induce any intracellular signaling and may be implicated in the degradation of chemokines through receptor internalization.
Two homologous chemokine receptors, CXCR1 (IL-8RA) and CXCR2 (IL-8RB), bind to IL-8 with high affinity (Holmes et al., 1991; Murphy and Tiffany, 1991) (Fig. 2 and Table 1). They share 78% sequence identity and are currently considered to be the only biologically significant IL-8 receptors. Their genes, IL-8RA and IL-8RB, each of which is encoded by a single exon, are located 20 kb apart on chromosome 2q35, and they are associated with an inactive CXCR2 pseudogene, IL-8RP (Ahuja et al., 1992, 1994). At the cell surface, CXCR1 shows binding only for IL-8 and GCP-2, without appreciable affinity for other CXC chemokines (Wolf et al., 1998). In contrast, CXCR2 binds to IL-8 as well as other CXC chemokines with similar high affinity, including ENA-78; GRO-α, -β, and -γ; neutrophil-activating protein-2; and GCP-2 (Ahuja and Murphy, 1996). Another chemokine receptor, Duffy antigen receptor for cytokines (DARC), is a 40- to 45-kDa glycosylated protein expressed on red blood cells, and to a lesser extent on endothelial and epithelial cells, which was first recognized as the erythrocyte receptor for malaria parasites (Horuk et al., 1993; Neote et al., 1993; Tournamille et al., 2004). Duffy antigen receptor for cytokines binds to both CXC and CC cytokines (including IL-8) with relative promiscuity. However, DARC lacks the protein motif in its second intracellular loop that is required for coupling to G-proteins, and it has not yet been linked to any intracellular signaling pathways. It is possible that this receptor acts as a molecular sink for binding and degradation of chemokines through receptor internalization or that it participates in chemokine transport. Interleukin-8 also displays low-affinity binding for glycosaminoglycans, but the biological significance of this interaction is still unclear.
Table 1.
IL-8 receptor properties
| Property | CXCR1 | CXCR2 | DARC |
|---|---|---|---|
| Cellular expression | Neutrophils
Monocyte/macrophages Basophils T-lymphocytes Endothelial cells |
Neutrophils
Monocyte/macrophages Basophils T-lymphocytes Endothelial cells CNS neurons |
Erythrocytes
Endothelial cells Cerebellar Purkinje cells |
| Biologic function | Neutrophil recruitment
Angiogenesis |
Neutrophil recruitment
Macrophage accumulation Angiogenesis |
Erythrocyte binding of Plasmodium vivax |
| Ligand binding | IL-8, GCP-2 | IL-8; ENA-78; GRO-α, -b, and -γ NAP-2; GCP-2 | CXC: IL-8, NAP-2, GRO-α
CC: RANTES, MCP-1 |
| Signaling properties | Coupled to Gi, Gα
Rho/Rho kinase Phospholipase D |
Coupled to Gi, Gα,
ERK 1/2, PI-3-K Rac/PAK |
None |
Abbreviations: CC, CC chemokines; CXCR, CXC chemokine receptor; DARC, Duffy antigen receptor for cytokines; ERK 1/2, extracellular signal–regulated kinase 1 and 2; GCP, granulocyte chemotactic protein; GRO, growth-regulated oncogene; IL-8, interleukin-8; NAP, neutrophil-activating protein; PI-3-K, phosphoinositide 3 kinase.
IL-8 Has Proangiogenic Activity
Interleukin-8 possesses biologic functions in addition to and distinct from its well-recognized role in regulating inflammatory responses (Strieter, 2001). Of particular relevance to tumorigenesis, IL-8 is a potent mediator of angiogenesis (Hu et al., 1993; Koch et al., 1992; Strieter et al., 1992). Angiogenesis is the process by which new blood vessels are formed from preexisting ones and follows a complex sequence of events that occurs in response to proangiogenic and antiangiogenic factors (Brat and Van Meir, 2001). This process is tightly regulated and is activated only in pathologic states such as wound healing and neoplasia, and in limited physiologic settings such as menstrual cycle events. The beginning phase of angiogenesis is associated with increased vascular permeability of parent vessels that leads to extravasation of plasma and deposition of proangiogenic matrix proteins. In response to the mitogenic effects of proangiogenic cytokines, endothelial cells proliferate and then migrate along a chemotactic gradient into the extracellular matrix. Once established, endothelial cells form tubes with a central lumen, elaborate a new basement membrane, and eventually recruit pericytes and smooth muscle cells to surround the mature vessels.
The proangiogenic properties of IL-8 were first demonstrated in the early 1990s, when recombinant human IL-8 was shown to have chemotactic properties on human umbilical vein endothelial cells (HUVEC) and human aortic endothelial cells (Koch et al., 1992; Szekanecz et al., 1994). The biologic significance of this relationship in human disease was shown by demonstrating that the angiogenic effect of conditioned media from inflamed human tissue could be blocked by antibodies directed specifically at IL-8 (Koch et al., 1992). Similarly, angiogenic properties of conditioned media from activated monocytes and macrophages were attenuated by IL-8 antisense oligonucleotides. Interleukin-8 was later confirmed to have proangiogenic properties in vivo through use of the rat mesenteric window assay, the rat and rabbit corneal assays, and a subcutaneous sponge model (Hu et al., 1993; Koch et al., 1992; Norrby, 1996; Strieter et al., 1992). The angiogenic activity of IL-8 stimulates both endothelial proliferation and capillary tube formation in vitro in a dose-dependent manner, and both of these effects can be blocked by monoclonal antibodies to IL-8 (Li et al., 2003; Shono et al., 1996). Results from the majority of investigations point to the angiogenic effects of IL-8 as being independent of its chemotactic activity for neutrophils and other pro-inflammatory effects, since IL-8 promotes angiogenesis in the absence of inflammatory cells (Hu et al., 1993; Strieter et al., 1992). The proangiogenic nature of IL-8 is presumed to be related to the Glu-Leu-Arg (ELF) motif that immediately precedes its first N-terminal cysteine residue, as all CXC chemokines with this motif are known to promote angiogenesis (Moore et al., 1998; Strieter et al., 1995). These include IL-8, ENA-78, GCP2, and GRO-α, -β, and -γ. Those CXCs without the ELF motif (ELF-), including platelet factor 4, Mig, and γ-interferon inducible protein 10, do not demonstrate angiogenic activity. In fact, these ELF- CXC chemokines were even found to block angiogenesis induced by ELF+ chemokines, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (Loetscher et al., 1996; Maione et al., 1990; Strieter et al., 1995). The presence of ELF in the amino acid sequence of CXCs is believed to modify the interaction of chemokines with their cell surface receptors on effector cells including endothelial cells and neutrophils (Clark-Lewis et al., 1993). The significance of the ELF domain to the proangiogenic properties of IL-8 has been shown most convincingly by using mutant forms of IL-8 that lack the ELF motif. These mutant forms have no appreciable angiogenic activity in assays of endothelial cell migration or in rat corneal assays. Importantly, when an ELF motif is introduced into an ELF-negative chemokine, such as Mig, in vivo angiogenic properties are conferred. Thus, the ELF domain appears to be critical to the proangiogenic properties of CXCs.
The proangiogenic effects of IL-8 additionally stem from its ability to inhibit the apoptosis of endothelial cells (Li et al., 2003). In serum-free medium, which typically induces programmed endothelial cell death, IL-8 inhibits HUVEC apoptotic response, and this inhibition is associated with increased levels of the antiapoptotic factors Bcl-xl and Bcl-2 as well as with decreased levels of Bax.
Finally, as concerns IL-8-mediated angiogenesis, it was further shown that IL-8 stimulates increased endothelial cell mRNA expression of matrix metalloproteinases (MMPs) MMP-2 and -9 as well as modest increases in gelatinase activity (Li et al., 2003). These MMP activities are required for the proteolytic modifications of basement membranes and extracellular matrices during angiogenesis. In total, IL-8 has at least four distinct proangiogenic properties, each of which is manifested through an endothelial cell response: the enhancement of endothelial cell proliferation, chemotaxis, survival, and protease activation.
Endothelial Cell Expression of CXCR1 and CXCR2 Mediates IL-8 Angiogenic Activity
The ability of IL-8 to elicit angiogenic activity depends on the endothelial cell expression of its receptors (Fig. 2). Recent studies based on fluorescence-activated cell sorting analysis and confocal microscopy indicate that CXCR1 and CXCR2 are highly and moderately expressed, respectively, on human microvascular endothelial cells (HMEC) (Salcedo et al., 2000), whereas HUVEC cells show low levels of CXCR1 and CXCR2 expression. These findings are consistent with the ability of IL-8 to induce HMEC chemotaxis to a much greater extent than HUVEC. Antibodies directed at CXCR1 and CXCR2 are capable of inhibiting IL-8-induced migration of HMEC, which indicates that these two receptor subtypes are critical for the IL-8 angiogenic response (Salcedo et al., 2000).
Since CXCR2 binds to all of the ELF+ CXCs that induce angiogenesis, including IL-8, whereas CXCR1 binds with high affinity only to IL-8 and GCP-2, it is reasonable to speculate that CXCR2 is a more likely candidate for mediating the proangiogenic effects of IL-8. In support of this speculation, antibodies directed at CXCR2 effectively inhibit chemotaxis of HMEC induced by the ELF+ chemokines, including IL-8 and ENA-78, whereas antibodies directed at CXCR1 have no anti-chemotactic effect. Antibodies directed at CXCR2 also inhibit neovascularization induced by ELF+ chemokines in a rat corneal model of angiogenesis (Addison et al., 2000), and this result is consistent with the diminished ELF+ chemokine–associated corneal angiogenesis in CXCR2 knockout mice. Similar observations regarding the importance of CXCR2 to IL-8-mediated angiogenesis have been made in association with investigations utilizing human intestinal microvasculature endothelial cells (HIMEC), which undergo proliferation, directed migration, and stress fiber assembly in response to IL-8, but not in the presence of antibodies directed at CXCR2 (Heidemann et al., 2003). In total, these data strongly support a role for CXCR2 in angiogenesis induced by ELF+ chemokines (Addison et al., 2000).
The intracellular signaling mechanisms that follow IL-8 binding to CXCRs on endothelial cells have been only partially characterized (Fig. 2). One of the earliest events that occurs after IL-8 exposure to endothelial cells is the increased phosphorylation of extracellular signal–regulated kinase 1 and 2 (ERK 1/2), which can be noted within 15 min in HIMEC and is sustained for more than 60 min (Heidemann et al., 2003). Inhibitors of both MAPK kinase (PD98059) and phosphoinositide 3 kinase (PI-3-K) (LY294002 and wortmannin) are capable of blocking the spontaneous formation of tubelike structures and IL-8-dependent chemotaxis of HIMEC, which indicates that both signaling mediators are downstream effectors of CXCR2. In addition, the IL-8-mediated activation of ERK 1/2 is believed to be downstream of PI-3-K, since PI-3-K inhibitors are capable of blocking ERK 1/2 phosphorylation (Sotsios and Ward, 2000). It has been suggested that the activation of the MAPK signaling cascade by binding of IL-8 to CXCR2 on endothelial cells may depend on the activation status of EGFR (Schraufstatter et al., 2003).
Downstream of cell surface receptor activation and intracellular signaling, the angiogenic response of endothelium to IL-8 requires coordinated cytoskeletal rearrangement to initiate and sustain migration. A detailed analysis of cytoskeletal reorganization induced by IL-8 in HMEC indicates that both CXCR1 and CXCR2 contribute to this activity, albeit within different time frames (Schraufstatter et al., 2001). Specifically, the use of antibodies against each receptor reveals that the early phase (1–5 min) of IL-8-stimulated actin stress fiber formation is dependent on the activation of CXCR1, whereas later cytoskeletal events, which include cell retraction and the formation of gaps between adjacent cells, depend primarily on CXCR2. The early stress fiber formation events mediated by CXCR1 can be blocked by inhibitors of Rho (C3 botulinum toxin) and Rho kinase (Y-27632), as well as by dominant negative forms of Rho. Pertussis toxin, which blocks Gi-coupled events, does not inhibit these early events. On the basis of the inhibitory profile, the authors suggest that CXCR1 couples to either Gα12 or Gα13, which in turn activates the Rho/Rho kinase cascade. The late IL-8-CXCR2-mediated cytoskeletal events are sensitive to pertussis toxin, which implicates receptor coupling to Gi. Moreover, CXCR2 activation is associated with Rac translocation to the cell membrane, where it associates with p21-activated kinase and promotes endothelial cell retraction (del Pozo et al., 2000). These late IL-8-associated events can be inhibited by dominant negative forms of Rac, which suggests that Rac is a critical downstream mediator of CXCR2. Thus, these studies suggest that each receptor couples to distinct G proteins and initiates intracellular signaling cascades that are coordinately regulated to allow for cellular reorganization of the cytoskeleton.
IL-8 Promotes Gliomagenesis and Tumoral Angiogenesis
Results from recent investigations suggest that IL-8 and its receptors are critical to the development and progression of numerous malignancies, including those that arise in the brain (Xie, 2001). Glioblastoma is a common, high-grade, infiltrative form of astrocytoma that is rapidly fatal. The presence of necrosis with pseudo-palisading and microvascular proliferation, a specialized form of angiogenesis, signals the onset of aggressive growth. Angiogenesis is most conspicuous in the regions surrounding necrosis and is due in large part to the local expression of proangiogenic factors combined with the loss of angiogenesis inhibitors. Most angiogenesis research in GBM has focused on VEGF and its regulation by hypoxia and genetic alterations (Brat et al., 2003). The possibility that chemokines may be relevant to glioma biology was raised by the findings that the majority of human GBM cell lines secrete factors that have chemotactic properties on neutrophils and that these effects were enhanced by exposure of tumor cells to TNF-α (Van Meir, 1995; Van Meir et al., 1992). Many GBM cell lines constitutively express IL-8 mRNA and secrete the 77 amino acid peptide isoform into conditioned media at concentrations that could account for this chemotactic effect on neutrophils (Tada et al., 1993; Van Meir et al., 1992). In addition, IL-8 gene expression and peptide secretion by glioma cells can be dramatically enhanced by TNF-α and IL-1 (Kasahara et al., 1991, Morita et al., 1993; Tada et al., 1993). In human brain tumor specimens, IL-8 mRNA and protein are expressed in grades II, III, and IV astrocytomas and anaplastic oligodendroglioma, which indicates that IL-8 expression is not an artifact of cell culturing. Interleukin-8 can be detected in the cyst fluid of primary astrocytic tumors, but only rarely in the cerebrospinal fluid (CSF) of astrocytoma patients, which suggests that the peptide either is secreted at low levels in the CSF or has a short half-life once in the CSF (Van Meir et al., 1992).
Glioblastomas are biologically complex and composed not only of neoplastic glioma cells but also non-neoplastic glia and neurons, endothelial cells, macrophages, microglia, lymphocytes, and neutrophils. Secretion of IL-8 could arise from any of these cellular compartments and contribute to gliomagenesis. In particular, macrophages are known to produce high levels of IL-8 (Matsushima and Oppenheim, 1989), and the brain has a resident population of macrophages that lie relatively dormant in the perivascular space. In CNS diseases, including high-grade gliomas, activated macrophages migrate away from their perivascular residence to the site of pathology, where they participate in the inflammatory response. Macrophages are not frequently encountered in low-grade (WHO grade II) astrocytomas, but their numbers increase with increasing histologic grade of the tumor, being highest in GBM (Nishie et al., 1999), and IL-8 levels in astrocytic neoplasms roughly correlate with the number of macrophages in the specimen. Moreover, the density of the macrophage infiltrates correlates with the degree of microvascular proliferation.
Microglia are a second population of primary CNS cells that are derived from the monocyte/macrophage lineage and express IL-8, especially following activation, although their role in tumorigenesis is not established (Lee et al., 2002). In addition to their abilities to directly release IL-8, macrophages and microglia also secrete TNF-α and IL-1, which could potentially act on tumor cells to induce the release of additional IL-8 (Kasahara et al., 1991). Thus, macrophages and microglia within GBMs likely have protumorigenic mechanisms related to their production of IL-8; the secretion of other factors by these cells may also promote tumor growth and angiogenesis (Chen et al., 2003; Sunderkotter et al., 1994).
In experiments on human astrocytoma specimens that included in situ hybridization and immunohistochemistry, IL-8 expression was highest in pseudopalisading cells surrounding necrosis and was also noted in the perivascular region, likely due to inflammatory cells (Desbaillets et al., 1997; Van Meir et al., 1992). The localization of IL-8 expression to pseudopalisading cells, which are known to be hypoxic and express high levels of hypoxia-inducible factor (HIF)-1α, suggested that hypoxia might lead to increased IL-8 expression. Indeed, enhanced IL-8 expression can be demonstrated in the hypoxic centers of glioma spheroids grown in culture and in glioma cells grown under anoxic conditions. While both IL-8 and VEGF expression are induced by anoxia in glioma cell lines, and both are expressed in the regions of pseudopalisades in GBM specimens, the kinetics of IL-8 mRNA expression following anoxia are distinct from those of VEGF, suggesting different roles in the tumor angiogenic response (Desbaillets et al., 1999). Further, the pattern of IL-8 mRNA expression in pseudopalisades, as demonstrated by in situ hybridization, is punctate, while that of VEGF is more uniform. Thus, IL-8 expression in response to anoxia appears to be distinct from VEGF, in terms of both its spatial and its temporal characteristics.
The results of both nuclear run-on experiments and transcriptional inhibition indicate that increased IL-8 levels in anoxic gliomas are due to increased transcriptional activity. Binding of the transcription factor AP-1 to the IL-8 promoter increases dramatically under anoxia in glioma cells, while that of NF-κB and C-EBP/ NF-IL-6 does not, implicating AP-1 as a critical oxygen-sensitive transcriptional factor complex in IL-8 production (Desbaillets et al., 1999). Others have found IL-8 transcription to be upregulated by hypoxia/anoxia in normal and cancerous cells (Shi et al., 1999), though a conventional hypoxia-responsive element, such as that recognized by the HIF family of transcription factors, has not been identified in the IL-8 promoter, and consequently, HIF-mediated transcription is probably not directly involved in IL-8 expression. Results from some studies suggest that NF-κB might also participate in the hypoxia-IL-8 expression response (Huang et al., 2001). Unlike VEGF expression, the oxygen-sensitive transcription of IL-8 is independent of TP53 status in glioma cells and is thus unlikely to be related to hypoxic induction of p53 (Desbaillets et al., 1997). In addition to stimulation by low oxygen, IL-8 expression is promoted by other conditions in the tumor microenvironment, such as reduced glucose levels, amino acid deprivation, low pH, and reactive oxygen species, all of which are associated with nutrient deprivation (Bobrovnikova-Marjon et al., 2004; Shi et al., 2000; Tanaka et al., 1997).
The exact role of IL-8 in the biology of astrocytomas is not clear. Among many potential roles, it could act as (1) an inflammatory chemoattractant as part of the host response to neoplasia, (2) a more general pro-inflammatory factor released in response to tissue stress and necrosis, (3) a proangiogenic factor that promotes new vessel growth, or (4) an autocrine growth factor secreted by tumor cells to promote their own growth. Whether IL-8 has direct effects on tumor cells themselves is a contentious issue, since it is not clear if tumor cells express the specific IL-8 receptors CXCR1 and CXCR2. Interleukin-8 has been reported to have direct growth-stimulating effects on glioma cell lines, such as NP-1 and U251MG (Wakabayashi et al., 2004; Yamanaka et al., 1995). IL-8 has also been reported to have chemoattractant properties on glioma cells by promoting directed migration assays (Wakabayashi et al., 2004). RT-PCR investigations have demonstrated high levels of CXCR1 and low levels of CXCR2 mRNA in U251 glioma cells as well as primary astrocytes isolated from the human brain (Flynn et al., 2003; Wakabayashi et al., 2004), whereas low-level expression of CXCR1 and CXCR2 has been demonstrated by RNase protection assays in five of 16 glioma cell lines (Zhou et al., 2002). To further complicate this matter, fluorescence-activated cell sorting analysis indicates that the majority of glioma cell lines lack detectable levels of CXCR1 and CXCR2 (Benedetti and Van Meir, unpublished data). With respect to the analysis of tumor tissues, RT-PCR studies for CXCR1 and CXCR2 have demonstrated transcripts in astrocytoma specimens of grade II, III, and IV, though in situ hybridization results suggest that the expression may be in lymphocytes and macrophages rather than in tumor cells (Desbaillets et al., 1997). RNase protection assays have not revealed the presence of CXCR1 and CXCR2 mRNA in GBMs (Zhou et al., 2002). Interleukin-8 does bind to glioma cells, but this may be due to its interactions with glycosaminoglycans (Benedetti and Van Meir, unpublished data).
While the details of its mechanism of action remain to be worked out, the importance of IL-8 in gliomagenesis was recently demonstrated in a study of its transcriptional regulation by ING4 (Garkavtsev et al., 2004). ING4 is a nuclear factor expressed in all normal human tissues, including the brain, but its expression is markedly reduced in astrocytic neoplasms, with levels inversely correlated with tumor grade. Inhibition of ING4 expression by antisense techniques strongly promoted the growth of U87MG glioma in vivo, whereas ING4 overexpression led to growth suppression. Intravital microscopy demonstrated that those tumors lacking ING4 expression showed increased neovascularization compared with ING4-expressing tumors, thereby suggesting that ING4 may be a regulator of angiogenesis. Among the genes most strongly upregulated in tumors treated with antisense ING4 was IL-8, which was increased nearly 10-fold. ING4 was shown to exert its influence over IL-8 expression through its physical interaction with the p65 subunit of NF-κB. The formation of an ING4/NF-κB complex reduced NF-κB transcriptional activity, as determined by gel mobility shift assays and NF-κB-dependent luciferase reporter assays. Finally, siRNA directed at IL-8 transcripts was shown to reverse the effects suppressing ING4, which implies that IL-8 is a critical mediator of the ING4/NF-κB interaction in U87MG. In summary, ING4 was found to form a transcriptional complex that represses NF-κB-mediated expression of IL-8. When ING4 expression is reduced, IL-8 expression increases, which leads to enhanced glioma growth and neovascularization. While these findings will need to be reproduced in other glioma cell lines before they can be generalized, they are the first to demonstrate that IL-8 is a critical proangiogenic factor in gliomas.
Despite the studies mentioned above that define a paracrine role for tumor cell–secreted IL-8 in glioma neovascularization, the precise mechanisms by which IL-8 exerts its angiogenic effects are still being defined. It is quite possible that glial tumor IL-8 is derived from both tumor cells and macrophages but exerts its biologic effects on blood vessels and inflammatory cells within human brain tumors. Because CXCR1 and CXCR2 have been described in a perivascular distribution in human glioma specimens, it seems likely that receptor expression is associated with infiltrating leukocytes rather than endothelial cells (Desbaillets et al., 1997). IL-8 could participate in the recruitment of leukocytes to the tumor, whereby these inflammatory cells secrete protumorigenic factors. The DARC receptor, on the other hand, which shows binding for IL-8 as well as other CXC and CC chemokines, showed relatively high and specific staining by immunohistochemistry on microvascular cells within astrocytoma specimens. While the DARC receptor is widely regarded as a chemokine receptor that is not linked to intracellular signaling, its role in glioma tumorigenesis and angiogenesis is not entirely clear.
IL-8 Expression and Apoptotic Signaling in Gliomas
For reasons that are not entirely clear, IL-8 expression in gliomas also appears to be regulated by signaling pathways that are best known for inducing apoptosis. Both activation of Fas/FasL (CD95/CD95L) and the binding of tumor necrosis factor–related, apoptosis-inducing ligand (TRAIL) to death receptors 4 and 5 (DR4, DR5) are well known to induce apoptosis in subsets of glioma cell lines (Gratas et al., 1997; Song et al., 2003). Other cell lines remain resistant to these apoptotic mechanisms, which raises the possibility that alternative functions may result from activation of Fas and DR. In the case of Fas, both activating anti-Fas antibodies and FasL promote a dramatic, dose-dependent increase in IL-8 mRNA and protein levels in glioma cell lines (Choi et al., 2001; Hor et al., 2003). Fas ligation leads to activation of ERK 1/2 and p38 MAPK signaling pathways, and the pharmacologic inhibition of these pathways blocks the secretion of IL-8. In surgically resected human tissues, Fas protein levels were found to be more than twice as high in GBMs than in the normal brain, and the Fas protein concentration correlated well with IL-8 tissue levels. Fas is normally expressed in human gliomas and may trigger a FasL-mediated T-cell apoptotic response that has been identified as a possible means for gliomas to evade immune destruction (Saas et al., 1997). The fact that Fas activation induces IL-8 secretion may also indicate that Fas activation has pro-inflammatory or proangiogenic properties in gliomas (Biancone et al., 1997).
In a similar manner, activation of DR5, either by TRAIL or by activating antibodies, leads to apoptosis in a subset of glioma cells; in those glioma cells that survive, IL-8 is strongly and specifically upregulated (Choi et al., 2002). Both TRAIL-induced apoptosis and IL-8 secretion are dependent on Fas-activated death domain recruitment and downstream activation of caspases 1 and 8. While the DR5-mediated apoptotic pathway could be blocked by caspase 3 inhibitors, IL-8 secretion could not, which indicates that signaling upstream of caspase 3 is responsible for IL-8 production. Increased IL-8 mRNA expression following TRAIL ligation of DR5 was shown to be due to activation of the transcription factors AP-1 and NF-κB, as assessed by electrophoretic mobility shift assays. Transcription factor activation could also be blocked by inhibitors of caspase 1 and 8, which indicates that these two caspases mediate the DR5 effects on IL-8. The reason for such a strong and consistent expression of IL-8 by glioma cells that survive DR5 or Fas activation is not known. Secretion of IL-8 could be an adaptive response by gliomas by which the proapoptotic signaling via DR5 and Fas activation is suppressed, in a manner similar to that shown by reactive astrocytes (Saas et al., 1999). The lack of constitutive expression of CXCR1 and CXCR2 on glioma cells and in GBM specimens suggests that this effect may not involve autocrine stimulation through these receptors. However, IL-8 secretion through these mechanisms could play an active role in angiogenesis or inflammatory response (Chen et al., 1998).
Conclusions and Therapeutic Implications
In summary, there is strong evidence that IL-8 secretion is associated with glioma formation and malignant progression. The precise cell population(s) responsible for the production of IL-8 in gliomas and the IL-8 receptors involved in transducing IL-8-initiated signaling still needs to be defined. While IL-8 could contribute to tumor growth through several mechanisms, the strongest evidence to date suggests a direct role in angiogenesis. This is an important finding, as it indicates that IL-8 might act in an additive or alternative manner to VEGF for glioma angiogenesis. If these recent findings can be substantiated in multiple glioma models, it will be important to consider new therapeutic approaches to antagonize IL-8 action in the clinical setting (Van Meir et al., 2003; Wakabayashi et al., 2004). Such therapeutic strategies might work best in conjunction with anti-VEGF/VEGFR treatment.
Footnotes
Supported in part by the U.S. Public Health Service National Institutes of Health (NIH) grants CA-86335 (E.G.V.M.) and NS-42934 (D.J.B.), the Musella Foundation (E.G.V.M.), and the Pediatric Brain Tumor Foundation of the U.S. (E.G.V.M.).
Abbreviations used are as follows: AU, adenosine and uridine; CSF, cerebrospinal fluid; CXCR, CXC chemokine receptor; DARC, Duffy antigen receptor for cytokines; DR, death receptor; EGFR, epidermal growth factor receptor; ELF, Glu-Leu-Arg; ENA, epithelial-cell-derived neutrophil-activating protein; ERK 1/2, extracellular signal–regulated kinase 1 and 2; GBM, glioblastoma; GCP, granulocyte chemotactic protein; GRO, growth-regulated oncogene; HIF, hypoxia-inducible factor; HIMEC, human intestinal microvasculature endothelial cells; HMEC, human microvascular endothelial cell; HUVEC, human umbilical vein endothelial cells; IL-8, interleukin-8; MAP, mitogen-activated protein; MEKK1, MAP kinase kinase kinase 1; Mig, monokine induced by γ-interferon; MKK, MAP kinase kinase; MMP, matrix metalloproteinase; NIK, NF-κB-inducing kinase; PI-3-K, phosphoinositide 3 kinase; TRAIL, tumor necrosis factor–related, apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
References
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Ozcelik T, Milatovitch A, Francke U, Murphy PM. Molecular evolution of the human interleukin-8 receptor gene cluster. Nat Genet. 1992;2:31–36. doi: 10.1038/ng0992-31. [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Shetty A, Tiffany HL, Murphy PM. Comparison of the genomic organization and promoter function for human interleukin-8 receptors A and B. J Biol Chem. 1994;269:26381–26389. [PubMed] [Google Scholar]
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Human chemokines: An update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Biancone L, Martino AD, Orlandi V, Conaldi PG, Toniolo A, Camussi G. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J Exp Med. 1997;186:147–152. doi: 10.1084/jem.186.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: Role of nuclear factor-kappaB and activating protein-1. Cancer Res. 2004;64:4858–4869. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: A new world of angiogenesis research. Am J Pathol. 2001;158:789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Kaur B, Van Meir EG. Genetic modulation of hypoxia induced gene expression and angiogenesis: Relevance to brain tumors. Front Biosci. 2003;8:D100–D116. doi: 10.2741/942. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- Choi C, Xu X, Oh JW, Lee SJ, Gillespie GY, Park H, Jo H, Benveniste EN. Fas-induced expression of chemokines in human glioma cells: Involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 2001;61:3084–3091. [PubMed] [Google Scholar]
- Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002;22:724–736. doi: 10.1128/MCB.22.3.724-736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA. 1993;90:3574–3577. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268:25568–25576. [PubMed] [Google Scholar]
- De Larco JE, Wuertz BR, Yee D, Rickert BL, Furcht LT. Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells. Proc Natl Acad Sci USA. 2003;100:13988–13993. doi: 10.1073/pnas.2335921100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo MA, Price LS, Alderson NB, Ren X.-D, Schwartz MA. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 2000;19:2008–2014. doi: 10.1093/emboj/19.9.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbaillets I, Diserens AC, de Tribolet N, Hamou MF, Van Meir EG. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene. 1999;18:1447–1456. doi: 10.1038/sj.onc.1202424. [DOI] [PubMed] [Google Scholar]
- Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Garkavtsev I, Kozin SV, Chernova O, Xu L, Winkler F, Brown E, Barnett GH, Jain RK. The candidate tumour suppressor protein ING4 regulates brain tumour growth and angiogenesis. Nature. 2004;428:328–332. doi: 10.1038/nature02329. [DOI] [PubMed] [Google Scholar]
- Gratas C, Tohma Y, Van Meir EG, Klein M, Tenan M, Ishii N, Tachibana O, Kleihues P, Ohgaki H. Fas ligand expression in glioblastoma cell lines and primary astrocytic brain tumors. Brain Pathol. 1997;7:863–869. doi: 10.1111/j.1750-3639.1997.tb00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- Hebert CA, Baker JB. Interleukin-8: A review. Cancer Invest. 1993;11:743–750. doi: 10.3109/07357909309046949. [DOI] [PubMed] [Google Scholar]
- Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Holtmann H, Winzen R, Holland P, Eickemeier S, Hoffmann E, Wallach D, Malinin NL, Cooper JA, Resch K, Kracht M. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol Cell Biol. 1999;19:6742–6753. doi: 10.1128/mcb.19.10.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann H, Enninga J, Kalble S, Thiefes A, Dorrie A, Broemer M, Winzen R, Wilhelm A, Ninomiya-Tsuji J, Matsumoto K, Resch K, Kracht M. The MAPK kinase kinase TAK1 plays a central role in coupling the interleukin-1 receptor to both transcriptional and RNA-targeted mechanisms of gene regulation. J Biol Chem. 2001;276:3508–3516. doi: 10.1074/jbc.M004376200. [DOI] [PubMed] [Google Scholar]
- Hor WS, Huang WL, Lin YS, Yang BC. Cross-talk between tumor cells and neutrophils through the Fas (APO-1, CD95)/FasL system: Human glioma cells enhance cell viability and stimulate cytokine production in neutrophils. J Leukoc Biol. 2003;73:363–368. doi: 10.1189/jlb.0702375. [DOI] [PubMed] [Google Scholar]
- Horcher M, Rot A, Aschauer H, Besemer J. IL-8 derivatives with a reduced potential to form homodimers are fully active in vitro and in vivo. Cytokine. 1998;10:1–12. doi: 10.1006/cyto.1997.0251. [DOI] [PubMed] [Google Scholar]
- Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: The erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- Hu DE, Hori Y, Fan TP. Interleukin-8 stimulates angiogenesis in rats. Inflammation. 1993;17:135–143. doi: 10.1007/BF00916100. [DOI] [PubMed] [Google Scholar]
- Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Mukaida N, Yamashita K, Yagisawa H, Akahoshi T, Matsushima K. IL-1 and TNF-alpha induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology. 1991;74:60–67. [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kuhns DB, Young HA, Gallin EK, Gallin JI. Ca2+-dependent production and release of IL-8 in human neutrophils. J Immunol. 1998;161:4332–4339. [PubMed] [Google Scholar]
- Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayanan V, Drab-Weiss EA, Roebuck KA. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J Biol Chem. 1998;273:32670–32678. doi: 10.1074/jbc.273.49.32670. [DOI] [PubMed] [Google Scholar]
- Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: Structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, Murakami S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor kB and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem. 1991;266:13759–13763. [PubMed] [Google Scholar]
- Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247:77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: Novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Moore BB, Arenberg DA, Addison CL, Keane MP, Strieter RM. Tumor angiogenesis is regulated by CXC chemokines. J Lab Clin Med. 1998;132:97–103. doi: 10.1016/s0022-2143(98)90004-x. [DOI] [PubMed] [Google Scholar]
- Morita M, Kasahara T, Mukaida N, Matsushima K, Nagashima T, Nishizawa M, Yoshida M. Induction and regulation of IL-8 and MCAF production in human brain tumor cell lines and brain tumor tissues. Eur Cytokine Netw. 1993;4:351–358. [PubMed] [Google Scholar]
- Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–13295. [PubMed] [Google Scholar]
- Murdoch C, Monk PN, Finn A. CXC chemokine receptor expression on human endothelial cells. Cytokine. 1999;11:704–712. doi: 10.1006/cyto.1998.0465. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Nabors LB, Suswam E, Huang Y, Yang X, Johnson MJ, King PH. Tumor necrosis factor alpha induces angiogenic factor up-regulation in malignant glioma cells: A role for RNA stabilization and HuR. Cancer Res. 2003;63:4181–4187. [PubMed] [Google Scholar]
- Neote K, Darbonne W, Ogez J, Horuk R, Schall TJ. Identification of a promiscuous inflammatory peptide receptor on the surface of red blood cells. J Biol Chem. 1993;268:12247–12249. [PubMed] [Google Scholar]
- Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- Norrby K. Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif. 1996;29:315–323. doi: 10.1111/j.1365-2184.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994;264:90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, French L, Van Meir EG, de Tribolet N, Tschopp J, Dietrich PY. Fas ligand expression by astrocytoma in vivo: Maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saas P, Boucraut J, Quiquerez AL, Schnuriger V, Perrin G, Desplat-Jego S, Bernard D, Walker PR, Dietrich PY. CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: A key role in brain inflammation? J Immunol. 1999;162:2326–2333. [PubMed] [Google Scholar]
- Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Pow-ell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6714–6722. doi: 10.4049/jimmunol.171.12.6714. [DOI] [PubMed] [Google Scholar]
- Shi Q, Le X, Abbruzzese JL, Wang B, Mujaida N, Matsushima K, Huang S, Xiong Q, Xie K. Cooperation between transcription factor AP-1 and NF-kappaB in the induction of interleukin-8 in human pancreatic adenocarcinoma cells by hypoxia. J Interferon Cytokine Res. 1999;19:1363–1371. doi: 10.1089/107999099312821. [DOI] [PubMed] [Google Scholar]
- Shi Q, Le X, Wang B, Xiong Q, Abbruzzese JL, Xie K. Regulation of interleukin-8 expression by cellular pH in human pancreatic adenocarcinoma cells. J Interferon Cytokine Res. 2000;20:1023–1028. doi: 10.1089/10799900050198471. [DOI] [PubMed] [Google Scholar]
- Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, Kohno K, Kuwano M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Zachariae CO, Norihisa Y, Ortaldo JR, Hishinuma A, Matsushima K. IL-8 gene expression and production in human peripheral blood lymphocyte subsets. J Immunol. 1991;146:3815–3823. [PubMed] [Google Scholar]
- Song JH, Song DK, Pyrzynska B, Petruk KC, Van Meir EG, Hao C. TRAIL triggers apoptosis in human malignant glioma cells through extrinsic and intrinsic pathways. Brain Pathol. 2003;13:539–553. doi: 10.1111/j.1750-3639.2003.tb00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotsios Y, Ward SG. Phosphoinositide 3-kinase: A key biochemical signal for cell migration in response to chemokines. Immunol Rev. 2000;177:217–235. doi: 10.1034/j.1600-065x.2000.17712.x. [DOI] [PubMed] [Google Scholar]
- Strieter RM. Chemokines: Not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001;2:285–286. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, Marks RM. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989;243:1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284. [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, Chan S.-Y, Roczniak S, Shanafelt AB. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z, Shah MR, Harlow LA, Pearce WH, Koch AE. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth, Pathobiology. 1994;62:134–139. doi: 10.1159/000163891. [DOI] [PubMed] [Google Scholar]
- Tada M, Suzuki K, Yamakawa Y, Sawamura Y, Sakuma S, Abe H, van Meir E, de Tribolet N. Human glioblastoma cells produce 77 amino acid interleukin-8 (IL-8(77)) J Neurooncol. 1993;16:25–34. doi: 10.1007/BF01324831. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Kamata H, Takeshita H, Yagisawa H, Hirata H. Redox regulation of lipopolysaccharide (LPS)-induced interleukin-8 (IL-8) gene expression mediated by NF kappa B and AP-1 in human astrocytoma U373 cells. Biochem Biophys Res Commun. 1997;232:568–573. doi: 10.1006/bbrc.1997.6264. [DOI] [PubMed] [Google Scholar]
- Tebo J, Der S, Frevel M, Khabar KS, Williams BR, Hamilton TA. Heterogeneity in control of mRNA stability by AU-rich elements. J Biol Chem. 2003;278:12085–12093. doi: 10.1074/jbc.M212992200. [DOI] [PubMed] [Google Scholar]
- Tournamille C, Blancher A, Le Van Kim C, Gane P, Apoil PA, Nakamoto W, Cartron JP, Colin Y. Sequence, evolution and ligand binding properties of mammalian Duffy antigen/receptor for chemokines. Immunogenetics. 2004;55:682–694. doi: 10.1007/s00251-003-0633-2. [DOI] [PubMed] [Google Scholar]
- Trushin SA, Pennington KN, Algeciras-Schimnich A, Paya CV. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J Biol Chem. 1999;274:22923–22931. doi: 10.1074/jbc.274.33.22923. [DOI] [PubMed] [Google Scholar]
- Van Meir EG. Cytokines and tumors of the central nervous system. Glia. 1995;15:264–288. doi: 10.1002/glia.440150308. [DOI] [PubMed] [Google Scholar]
- Van Meir EG, Ceska M, Effenberger F, Walz A, Grouzmann E, Desbaillets I, Frei K, Fontana A, de Tribolet N. Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer Res. 1992;52:4297–4305. [PubMed] [Google Scholar]
- Van Meir, E.G., Hao, C., Post, D.E., Liau, L.M., and Brat, D.J. (2003) Therapeutic targeting of the pathways that induce brain tumor development. Chapter 18 in Zhang, W., and Fuller, G.N. (Eds.) Genomic and Molecular Neuro-Oncology Sudbury, Mass.: Jones and Bartlett Publishers, pp. 303–332.
- Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002;13:143–154. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Kambe F, Cao X, Murakami R, Mitsuyama H, Nagaya T, Saito K, Yoshida J, Seo H. Inhibitory effects of cyclosporin A on calcium mobilization-dependent interleukin-8 expression and invasive potential of human glioblastoma U251MG cells. Oncogene. 2004;23:6924–6932. doi: 10.1038/sj.onc.1207778. [DOI] [PubMed] [Google Scholar]
- Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzen R, Gowrishankar G, Bollig F, Redich N, Resch K, Holtmann H. Distinct domains of AU-rich elements exert different functions in mRNA destabilization and stabilization by p38 mitogen-activated protein kinase or HuR. Mol Cell Biol. 2004;24:4835–4847. doi: 10.1128/MCB.24.11.4835-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Delgado MB, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. Eur J Immunol. 1998;28:164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. IκB kinases: Key regulators of the NF-κB pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Tanaka R, Yoshida S, Saitoh T, Fujita K. Growth inhibition of human glioma cells modulated by retrovirus gene transfection with antisense IL-8. J Neurooncol. 1995;25:59–65. doi: 10.1007/BF01054723. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]