Abstract
Glioblastomas, like other solid tumors, have extensive areas of hypoxia and necrosis. The importance of hypoxia in driving tumor growth is receiving increased attention. Hypoxia-inducible factor 1 (HIF-1) is one of the master regulators that orchestrate the cellular responses to hypoxia. It is a heterodimeric transcription factor composed of α and β subunits. The α subunit is stable in hypoxic conditions but is rapidly degraded in normoxia. The function of HIF-1 is also modulated by several molecular mechanisms that regulate its synthesis, degradation, and transcriptional activity. Upon stabilization or activation, HIF-1 translocates to the nucleus and induces transcription of its downstream target genes. Most important to gliomagenesis, HIF-1 is a potent activator of angiogenesis and invasion through its upregulation of target genes critical for these functions. Activation of the HIF-1 pathway is a common feature of gliomas and may explain the intense vascular hyperplasia often seen in glioblastoma multiforme. Activation of HIF results in the activation of vascular endothelial growth factors, vascular endothelial growth factor receptors, matrix metalloproteinases, plasminogen activator inhibitor, transforming growth factors α and β, angiopoietin and Tie receptors, endothelin-1, inducible nitric oxide synthase, adrenomedullin, and erythropoietin, which all affect glioma angiogenesis. In conclusion, HIF is a critical regulatory factor in the tumor microenvironment because of its central role in promoting proangiogenic and invasive properties. While HIF activation strongly promotes angiogenesis, the emerging vasculature is often abnormal, leading to a vicious cycle that causes further hypoxia and HIF upregulation.
Gliomas are the most common primary tumors arising in the central nervous system. After decades of advances in detection, surgery, and therapies, the median survival after initial diagnosis of their most aggressive form, glioblastoma multiforme (GBM)3 (WHO grade IV), is still only 50 weeks (Salvati et al., 1998), and less than 2% of patients survive three years postdiagnosis (Senger et al., 2003). Glioblastoma multiforme can occur as the result of progression from lower grade astrocytomas or can arise de novo. Pathological examination of low-grade astrocytomas (WHO grade II) demonstrates diffusely infiltrating tumor cells in the normal brain, which begin as nonangiogenic tumors that have the ability to co-opt a blood supply from existing vasculature. When grade II astrocytomas progress to grade III (anaplastic astrocytoma), tumor cell density increases, nuclear anaplasia and cellular proliferation are apparent, and there is a mild increase in vascular density. The most dramatic histological changes occur with the transition to GBM and reflect a profound alteration in the tumor’s vascular biology. Tufted aggregates of rapidly dividing endothelial cells, referred to as glomeruloid bodies, and areas of necrosis surrounded by pseudopali-sading cells develop in the tumor (Brat and Van Meir, 2001; Brat et al., 2004). On MRI scans, GBMs appear as contrast-enhancing spheres with a central necrotic center, while microscopic analysis exposes more widespread invasion and multiple hypoxic regions in the growing periphery of these tumors. Recent investigations indicate that hypoxia is responsible for the appearance of necrosis associated with the pseudopalisading cells seen in GBM (Fig. 1). Hypoxia-initiated angiogenesis leads to the elaborate microvascular proliferation that heralds a phase of more malignant tumor growth (Barker et al., 1996). Central to the cascade of events that occur as gliomas progress is the response of a tumor cell to low-oxygen conditions, which is elicited via the stabilization and activation of hypoxia-inducible factor (HIF), a transcription factor critical for adaptive response to reduced oxygen. Both hypoxic and HIF staining can be observed best at a distance from blood vessels and is absent immediately adjacent to the vasculature, where tissue oxygenation is adequate (Fig. 1). Activation of HIF leads to upregulation of factors essential for blood vessel formation and is one of the primary forces driving both physiological and pathological angiogenesis.
Fig. 1.
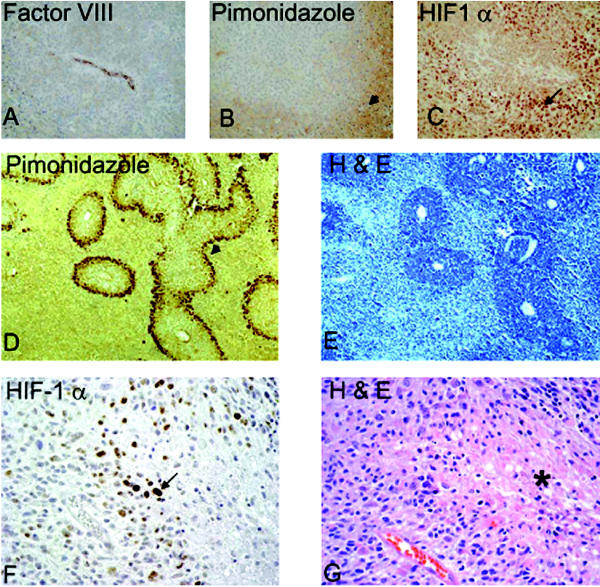
HIF stabilization in hypoxic areas. HIF-1α is stabilized in cells distant from a blood vessel. A–C: Adjacent sections of subcutaneously grown tumor xenografts of human LN229 glioma cells. Panel A shows stain Factor VIII, highlighting a vessel. Panel B is pimonidazole staining showing hypoxic areas in brown (arrowhead). Panel C is HIF-1α immunostain (arrow), showing nuclear staining of HIF. D–E: U87 glioma xenografts from a rat orthotopic brain tumor model. Panel D is pimonidazole staining that shows a rim of viable hypoxic cells at the periphery of vascularized regions (arrowhead). Panel E shows the corresponding H & E staining. Note large areas of necrosis (light blue). F–G: Human GBM specimen. Panel F highlights the HIF-1α-positive staining cells localized in the pseudopalisading cells (arrow), and Panel G is the corresponding H & E of the adjacent section showing the necrotic area (asterisk).
Hypoxia-Inducible Factor
One of the main early cellular events evoked upon exposure to hypoxia is activation of HIF-1, a key heterodimeric transcription factor. In reduced oxygen conditions, HIF-1 binds to hypoxia-responsive elements (HREs) and induces transcription of various target genes involved in tumor angiogenesis, invasion, cell survival, and glucose metabolism. The concept of a transcription factor being activated in limiting oxygen conditions was put forth in 1992 to explain upregulation of erythropoietin (Epo), a hormone stimulating red blood cell production in response to hypoxia (Semenza and Wang, 1992). HIF-1α and HIF-1β were identified as proteins that contain a basic helix-loop-helix and a Per/ARNT/Sim (PAS) domain and were determined to be responsible for hypoxic induction of Epo. These subunits must associate to form the active HIF heterodimer responsible for transcriptional activation (Wang et al., 1995). HIF-1β is the aryl hydrocarbon receptor nuclear translocator (ARNT) and is constitutively expressed. ARNT2 and ARNT3 are highly homologous proteins to ARNT, and all three are implicated in forming dimers with the various HIF-α subunits (Maynard and Ohh, 2004). HIF-1α has two closely related homologs, HIF-2α and HIF-3α. HIF-2α (also known as endothelial PAS domain protein, or EPAS1) is 48% identical to HIF-1α, is induced by hypoxia, and binds to HIF-1β to activate transcription of hypoxia-responsive genes (Tian et al., 1997). HIF-3α appears to be a dominant negative regulator of HIF, as it dimerizes with HIF-1β to generate a transcription-ally inactive heterodimer. Knockout mice homozygously deleted for HIF-1α exhibit embryonic lethality, dying at postcoitus day 10 with gross abnormalities in cardiac development and vasculature, underscoring the importance of HIF-1α in vascular development (Kline et al., 2002). Mice lacking HIF-2α die mid-gestation and show defects of cardiac development and reduced cat-echolamine levels (Tian et al., 1998). In normoxic conditions, HIF-1α is expressed ubiquitously at low levels in all organs, and HIF-2α is most abundantly expressed in the lung, followed by the heart, brain, liver, and various other organs. Despite their similarities in mediating transcriptional responses to hypoxia, HIF-1α and HIF-2α have distinct, nonredundant functions (reviewed in Semenza [2004]).
Domain Structure of the α Subunits of HIF
The domain structures of HIF-1α and its homologs are depicted in Fig. 2. Both HIF-1α and HIF-2α have a similar domain organization containing an N-terminal transactivation domain and a C-terminal transactivation domain (CAD). Six different splice variants of HIF-1α have been reported. Both HIF-1α785 and HIF-1α736 have the ability to transactivate downstream targets. HIF-1α785 is induced by 6-phorbol-12-myristate-13-acetate and heat shock and is relatively stable in normoxic conditions (Chun et al., 2003). HIF-1α736 is regulated by oxygen conditions in a manner similar to the way in which the full-length HIF-1α is regulated (Gothie et al., 2000). HIF-1α557 and HIF-1α516 function as dominant negative regulators of HIF activity by binding to and sequestering HIF-1β from the functionally active HIF-1α (Chun et al., 2001, 2002). HIF-1α417, the shortest variant, lacks a transcriptional activation domain but can bind to HIF-1β and then promote its nuclear translocation and transactivation by using the trans-activation domain within HIF-1β (Lee et al., 2004). HIF-3α is the least-studied member of the family and has multiple splice variants (HIF-3α-1–6) (reviewed in Maynard and Ohh [2004]). All of the HIF-3α isomers contain the N-terminal transactivation domain but lack the CAD domain (Hara et al., 2001). HIF-3α-1 was the first identified human HIF-3α isoform and has been shown to repress the hypoxic activation of target genes by sequestering HIF-1β from HIF-1/2α (Hara et al., 2001). HIF-3α-1 is notable among the other HIF-3α isoforms by the presence of a leucine zipper domain (LZIP) at its C-terminal in place of a CAD. Leucine zippers are motifs involved in DNA binding and protein-protein interactions, which indicates that this isoform may be involved in the activation of other yet-unknown genes (Maynard and Ohh, 2004). HIF-3α-2, also called inhibitory PAS domain protein (IPAS), binds to HIF-1α and prevents the formation of a functional HIF-1α/β complex, and it thus prevents HIF target gene activation (Makino et al., 2001). HIF-3α-2 (IPAS) is primarily expressed in the brain and eye and may have a specific role in negatively regulating HIF-induced gene expression in these tissues. The function of the other HIF-3α variants remains to be elucidated.
Fig. 2.
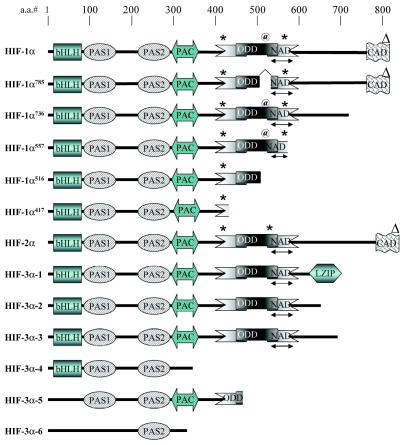
Domain structure of HIF-1α1–6 (shown as HIF-1α–HIF-1α417), HIF-2α, and HIF-3α1–6. Functional domains are abbreviated as follows: bHLH, basic helix-loop-helix; PAS, Per/Arnt/Sim; PAC, PAS-associated motifs; NAD, N-terminal transactivation domain; ODD, oxygen-dependent domain; CAD, C-terminal activation domain; and LZIP, leucine zipper domain. Position of modified amino acids are indicated as follows: *, hydroxylated proline; @, acetylated lysine;Δ, hydroxylated asparagines. (Adapted from Maynard et al. [2003] and Lee et al. [2004].)
Regulation of HIF-1/2α Protein Stability Under Hypoxia
In response to changes in oxygen availability, mammalian cells launch a host of responses, most of which are mediated by HIF. Regulation of HIF by partial oxygen pressure is orchestrated by many molecular players that affect HIF-1/2α protein stability or the ability of these proteins to bind to cofactors essential for transcriptional activity. In normoxia, HIF α subunits carrying an oxygen-dependent degradation (ODD) domain are highly labile proteins that are rapidly ubiquitinated and degraded by the proteasome (Fig. 3) (Crews, 1998). This ubiquitination is mediated by the von Hippel-Lindau protein (pVHL), the recognition component of an E3 ubiquitin ligase (Semenza, 2002). Mutations in the VHL gene result in the autosomal dominant von Hippel-Lindau syndrome that is characterized by the presence of highly vascularized tumors overexpressing vascular endothelial growth factor (VEGF) (Kaelin, 2002). The recognition of HIF-1/2α by pVHL is augmented by hydroxylation of two proline residues (P402 and P564) within the ODD domain by specific prolyl hydroxylases (PHD1, PHD2, PHD3) (Bruick and McKnight, 2001; Epstein et al., 2001). The PHDs are iron-dependent enzymes also requiring oxygen, 2-oxoglutarate, and ascorbate for activity. The catalytic activity of all three PHDs is reduced in hypoxia, with their respective rates of catalysis in normoxia being PHD2 = PHD3 > PHD1 (Tuckerman et al., 2004). In hypoxia, PHD1 and PHD3 are rapidly degraded by the proteasome pathway, which adds another layer of control to the system (Nakayama et al., 2004). On the contrary, PHD2 levels are upregulated by HIF-1 in hypoxic conditions and may be a mechanism to rapidly stop hypoxic signaling upon tissue reoxygenation (Metzen et al., 2004). Overexpression of any of the three PHDs destabilizes HIF-1α protein in COS-1 cells (Tuckerman et al., 2004). On the contrary, short interfering RNA studies have demonstrated that specific silencing of only PHD2 and not PHD1 or PHD3 in a battery of immortalized human cell lines and primary cell cultures led to increased HIF stability, which suggests that PHD2 may be the only physiologically relevant hydroxylase involved in HIF regulation (Berra et al., 2003). This dilemma may be explained if the contribution of each isoform to HIF hydroxylation depends on its relative abundance in a given cell type and a given culture condition, and if all three function in a nonredundant fashion (Appelhoff et al., 2004). Clearly, transgenic and knockout studies currently ongoing will help verify this assumption.
Fig. 3.
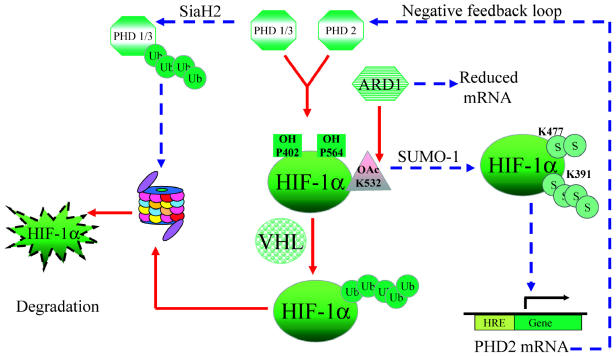
Factors affecting HIF-1α protein stability. PHD-mediated hydroxylations and ARD-mediated acetylation of specific residues within HIF-1α increase its affinity for pVHL, which leads to its ubiquitination (Ub) and degradation by the proteasomal pathway under normoxia (solid arrows). PHD1, 2, and 3 have a reduced catalytic activity in the absence of oxygen. Further, PHD1 and 3 and ARD have reduced levels in hypoxia (dashed arrows), adding another level of control. SUMO-1-mediated sumoylation in hypoxia leads to HIF-1α stabilization (S) and activation, causing transactivation of specific downstream target genes. PHD2 is induced by HIF, which indicates a negative feedback loop.
The arrest defective 1 protein (ARD1) is an acetyl-transferase that acetylates HIF-1α at Lys532 within the ODD domain. ARD1 stimulates HIF-1α–pVHL association, ubiquitination, and subsequent proteasomal degradation (Jeong et al., 2002). Unlike HIF-1α hydroxylation, the acetylation reaction itself is not thought to be an oxygen-dependent process. However, the level of HIF acetylation is still influenced by hypoxia, as ARD1 mRNA levels are reduced in hypoxia (Jeong et al., 2002). Thus, ARD1-mediated acetylation adds to the regulation of HIF-1α protein stability in response to oxygenation.
Small ubiquitin-like modifier-1 (SUMO-1) is an 18-kDa protein that shares 18% identity with ubiquitin and uses an ubiquitin-like conjugation system to affect protein localization. In certain circumstances, sumoylation may counter the effects of ubiquitination (Seeler and Dejean, 2003). SUMO-1 has been shown to co-localize and interact with HIF-1α in response to hypoxia in neurons and cardiomyocytes (Shao et al., 2004). SUMO-1 induces sumoylation of HIF-1α at Lys391/Lys477, leading to its stabilization and increased transcriptional activity (Shao et al., 2004). Given that HIF-1 activation increases VEGF expression and that VEGF is a survival factor for neurons, this sumoylation may have a neuro-protective function in the CNS (Wang et al., 2004a).
Hypoxia-Mediated HIF Activation
Besides regulating HIF stability, pO2 concentrations also affect HIF transcriptional activity (Fig. 4). In addition to hydroxylation of key proline residues by PHDs, HIF-1/2α are hydroxylated at asparagine residues (803 in HIF-1α and 851 in HIF-2α). This is mediated by an asparaginyl hydroxylase, FIH-1, or factor-inhibiting HIF-1, which is an Fe(II)-dependent enzyme that requires molecular oxygen to modify its substrate (Lando et al., 2002a, b). Hydroxylation at this residue decreases the ability of HIF to bind to its transcriptional coactivators CREB-binding protein (CBP)/p300, resulting in decreased transcriptional activity in the presence of molecular oxygen (Lando et al., 2002a, b). CBP, steroid receptor coactivator-1, transcription intermediary factor-2, and p300 are proteins that act as general transcriptional coactivators. HIF-1α binds to the cysteine/histidine-rich (CH1) domain of CBP/p300, and this interaction is necessary for HIF transcriptional activity (Paul et al., 2004). Inhibition of HIF interaction with CBP/p300 using small molecules (chetomin) interferes with the induction of HIF target genes under hypoxia and inhibits tumor growth (Kung et al., 2004). The interaction between HIF and CBP/p300 is under further physiological regulation by the CBP/p300-interacting transactivator with Glu/Asp-rich- C-terminal domain 2 (CITED2). CITED2 was identified as a 35-kDa protein that associates with the CH1 region of CBP/p300 and is also referred to as p35srj (Bhattacharya et al., 1999). CITED2/p35srj can negatively regulate HIF-1 activity under hypoxia by competing with HIF-1α for binding to CBP/p300 (Freedman et al., 2003). CITED2/p35srj likely represents a negative feedback regulatory loop, as it is upregulated by hypoxia and HIF (Bhattacharya et al., 1999).
Fig. 4.
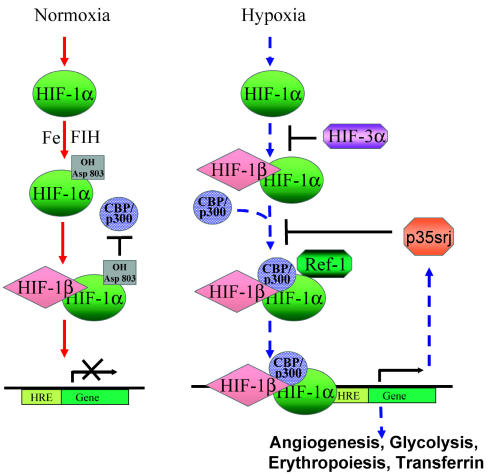
HIF-1α is differentially regulated under normoxia versus hypoxia. In normoxia (solid arrows), hydroxylation of HIF-1α mediated by asparaginyl hydroxylase FIH-1 interferes with its ability to bind co-activator CBP/p300, which is necessary to form an active HIF complex. Under hypoxia (dashed arrows), HIF-1α is stabilized and translocates to the nucleus after binding to HIF-1β, where Ref-1 aids in the recruitment of CBP/p300 to the HIF-1α complex, leading to transcriptional activation of genes containing the HREs. CITED2/p35srj negatively regulates HIF activity by competing with HIF-1α for binding to the CH1 region of CBP/p300. It is also upregulated by HIF, which indicates a possible negative feedback regulation.
Another level of regulation of HIF activity is mediated by Ref-1, a dual-function protein having both DNA endonuclease– and cysteine–reducing activities. Ref-1 reduces a unique cysteine in the basic helix-loop-helix domain of HIF-2α, which leads to its increased activation (Lando et al., 2000). Ref-1 also positively modulates the transactivation ability of HIF-1α, possibly by enhancing the recruitment of its coactivator complex (Carrero et al., 2000). Ref-1 has been shown to be a critical component of the HIF transcriptional complex required for the high-affinity association between HIF-1 and VEGF HRE (Ziel et al., 2004).
Signaling Pathways Affecting HIF-1α Regulation
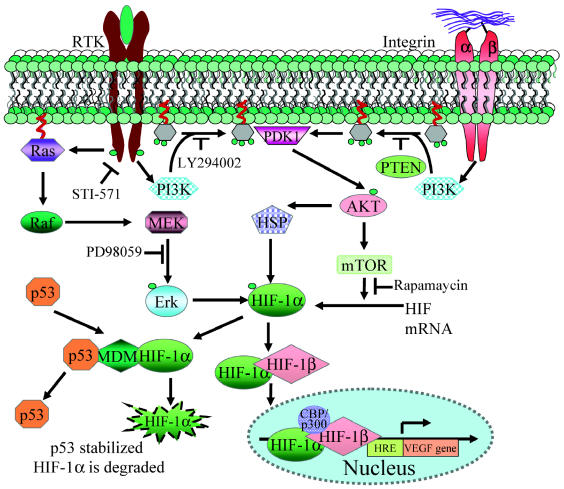
Hypoxia-inducible factor can be activated by physiological or pathological activation of growth factor and cell adhesion pathways (Fig. 5). Growth-factor-induced activation of receptor tyrosine kinases (RTKs) leads to HIF1-α stabilization and activation. Upon ligand binding, these receptors dimerize and autophosphorylate, which leads to their activation. Activated RTKs interact with p85, the regulatory subunit of phosphatidylinositol 3-kinase (PI3K), which leads to its activation. PI3K is a lipid kinase that generates the signaling molecule phosphatidylinositol 3,4,5-triphosphate by phosphorylating its precursor phosphatidylinositol 4,5-biphosphate. Activated PI3K triggers a phosphorylation cascade that results in the phosphorylation/activation of AKT, a serine/threonine kinase that promotes antiapoptotic and pro-survival responses of a cell (Newton, 2004). Activation of AKT has been shown to lead to an increase in HIF-1α protein translation by the AKT/FRAP/mTOR pathway (Fig. 5) (Laughner et al., 2001; Zhong et al., 2000). Inhibition of this pathway using LY294002, a selective inhibitor of PI3K, and with rapamycin, a selective inhibitor of mTOR, a downstream target of AKT, causes a reduction in HIF-1α amount and activity (Blancher et al., 2001).
Fig. 5.
Molecular signals affecting HIF-1α regulation. Induction of Ras, PI3K, and AKT phosphorylation mediated by RTK activation or integrin ligation leads to increased HIF-1α by modulating its stability and increased translation by the PI3K/AKT/mTOR pathway. TP53 negatively modulates this process by inducing MDM2, which can ubiquitinate and lead to HIF-1α degradation by the proteasome pathway.
Induction of HIF by growth factor receptors such as epidermal growth factor receptor (EGFR) or Her 2 (neu) is blocked by inhibitors of PI3K (LY294002 and wortmannin), which indicates the requirement of the PI3K pathway (Zhong et al., 2000). Activated RTKs also signal through the MAPK pathway, and phosphorylated p38 and extracellular-signal-regulated kinase 1/2 (ERK1/2) can further phosphorylate and activate HIF-1α (Wang et al., 2004b). Inhibition of ERK activity leads to inhibition of HIF activity without affecting HIF stabilization (Fig. 5) (Hur et al., 2001).
In addition to growth factor–mediated RTK activation, the PI3K/AKT pathway is also activated by extra-cellular matrix (ECM) adhesion mediated by integrins (Friedrich et al., 2004). Integrin ligation causes an activation of the integrin-linked kinase (ILK) leading to increased HIF-1α, as well as increased VEGF production by the PI3K/AKT/FRAP/mTOR pathway (Tan et al., 2004). Increased activity of integrin-linked kinase has been reported in gliomas (Obara et al., 2004). Additionally, activation of PI3K/AKT also leads to an increase in steady-state concentrations of heat shock proteins 90 and 70, both of which interact with and stabilize HIF-1α (Zhou et al., 2004).
Genetic Alterations That Lead to HIF Activation in Gliomas
Both the activation of oncogenes (EGFR) and the loss of tumor suppressor function (p53, PTEN) that are common in gliomas can affect HIF expression through several mechanisms. EGFR gene amplification and/or overexpression is seen frequently in a variety of tumors, including GBM, and is associated with a poor prognosis (Frederick et al., 2000). The most common EGFR gene mutation (EGFRvIII) is a deletion of exons 2–7, resulting in a truncated but constitutively active, ligand-independent receptor (Holland et al., 1998). Activation of EGFR (EGFR/EGFRvIII) by ligand binding or gene amplification results in activation of the PI3K pathway, which increases HIF-1α by the PI3K/AKT/FRAP/mTOR pathway (Clarke et al., 2001).
Glioblastomas have a 20% to 40% incidence of PTEN (phosphatase and tensin homolog deleted on chromosome 10) mutations, including both gene deletions and point mutations. PTEN loss of function has been experimentally shown to be associated with increased HIF-1α expression and tumor vascularization in gliomas. Over-expression of recombinant PTEN in glioma cells leads to marked reduction in HIF-1α expression (Zundel et al., 2000). PTEN is a dual-specificity phosphatase that can dephosphorylate both protein and lipid substrates. It dephosphorylates 3,4,5-triphosphate, the molecular messenger generated by PI3K and thus opposes its activity. Thus the loss of PTEN function during glioma progression results in dysregulation of the PI3K/AKT pathway as well as loss of control over HIF-regulated transcription (reviewed in Sansal and Sellers [2004]).
The tumor suppressor p53 is a transcription factor that is frequently mutated in low-grade astrocytomas. Expression of wild-type p53 leads to an inhibition of angiogenesis, in part because of downregulation of the expression of VEGF (Hunter et al., 2003). It has been proposed that p53 may lead to inhibition of HIF activity in hypoxia by promoting MDM2-mediated ubiquitination and degradation of HIF-1α (Ravi et al., 2000).
HIF as a Proangiogenic Master Switch
Through multiple regulatory mechanisms, HIF acts as a delicate sensor enabling a cell to respond rapidly to changes in levels of oxygenation in the environment. It acts as a potent activator of angiogenesis by stimulating the production of VEGF and many other factors that initiate endothelial cell proliferation, invasion, and migration. Loss-of-function studies using HIF-1α null embryonic stem cells and gain-of-function studies using a constitutively active form of HIF-1α have demonstrated that apart from VEGF, HIF-1 controls the expression of many other angiogenic factors, such as placenta-like growth factor, platelet-derived growth factorβ, and angiopoietin (Ang)-1 and -2 (Kelly et al., 2003). Apart from its role in angiogenesis, HIF-1α also promotes invasion by regulating the expression of cathepsin D; matrix metalloproteinase (MMP)-2; urokinase plasminogen activator receptor; fibronectin 1; keratins 14, 18, and 19; vimentin; transforming growth factor (TGF)-α; and autocrine motility factor (Krishnamachary et al., 2003). In fact, HIF-1α expression is found at the invasive front of glioblastomas and correlates with glioma grade and vessel density, which emphasizes its role in brain tumor progression and angiogenesis (Zagzag et al., 2000). These features have rendered HIF an attractive target for anticancer therapy (Post, 2004; Tan et al., 2005). Following is a discussion of the angiogenic factors that are induced by HIF in response to hypoxia and their contributions to glioma angiogenesis.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor belongs to a family of growth factors that includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta-like growth factor (reviewed in Kaur et al. [2004]). VEGF-A was the first secreted growth factor that was found to have endothelial-cell-specific mitogenic effects. It has five different isoforms: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206, which are produced by alternative splicing. In addition to endothelial cell proliferation, it also promotes endothelial cell migration, vascular permeability, and invasiveness, which are required for angiogenesis. Targeted gene disruption experiments in mice have revealed that loss of even a single allele of VEGF (hemizygosity) induces an embryonic lethal phenotype with gross vascular abnormalities demonstrating a dose-dependent requirement for VEGF in vasculogenesis (Ferrara, 2004).
Expression of VEGF is increased in response to hypoxia, and this is mediated by two mechanisms. First, hypoxia induces the activation of VEGF gene transcription through an HIF-dependent mechanism, mediated by HIF-1 binding to an HRE within the VEGF promoter, resulting in increased gene transcription (Forsythe et al., 1996). The second mechanism upregulates VEGF mRNA levels by regulating mRNA stability. This effect is mediated by the 3′ untranslated region of the VEGF mRNA (Onesto et al., 2004). Hypoxic induction of VEGF is considered to be the major driving force behind new vessel development, both during embryogenesis and in tumor progression. The production of VEGF in gliomas is significant, VEGF levels having been found in the cyst fluid of GBM patients that are 200- to 300-fold higher than those present in the serum (Takano et al., 1996). In situ hybridization of tumor tissue has revealed VEGF mRNA to be localized in the pseudopalisading cells surrounding hypoxic/necrotic foci in GBM, which is likely due to hypoxic induction. The high levels of VEGF produced around areas of pseudopalisading necrosis are believed to be responsible for the florid glomeruloid microvascular proliferation that is characteristic of GBM (reviewed in Brat and Van Meir [2001]). In fact, inhibition of VEGF signaling leads to an inhibition of tumor growth and angiogenesis.
VEGF Receptors
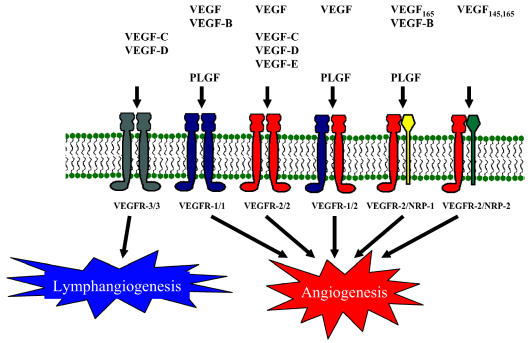
Vascular endothelial growth factor binds five different receptors: VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR), VEGFR-3 (Flt-4), neuropilin-1 (NRP-1), and neuropilin-2 (NRP-2). Each has unique binding properties for the various VEGF family members (Fig. 6). VEGFR-1 and VEGFR-2 are the major receptors expressed on endothelial cells, whereas VEGFR-3 is expressed mainly on lymphatic endothelial cells and is thought to be primarily involved in lymphangiogenesis (Fig. 6). VEGFR-2 is considered to be the main mitogenic signaling receptor for VEGF. The precise function of VEGFR-1 has been controversial, and it was originally considered to function as a decoy receptor important for sequestering VEGF from VEGFR-2. More recent results, however, indicate that VEGFR-1 is important for migration and differentiation of endothelial cells, a process that is required for angiogenesis. The importance of VEGFR-1 and VEGFR-2 is highlighted by the embryonic lethal phenotype displayed by mice lacking either VEGFR-1 or VEGFR-2 (reviewed in Ferrara [2004]). VEGFR-1 is induced by HIF-1, and an HRE has been located within an enhancer element (Gerber et al., 1997). VEGFR-2 and VEGFR-3 are also induced by HIF in hypoxia in endothelial cells of vascular and lymphatic origin, respectively (Nilsson et al., 2004). Recent studies on glioma cell lines and glioma-derived cells revealed a low-level expression of VEGFR-1 and -2 on tumor cells, but the significance of this is yet unclear (Mentlein et al., 2004). The expression of VEGFRs on endothelial cells derived from glioma samples indicates that their expression correlates with glioma grade and vascularization and that antagonizing VEGF receptor signaling inhibits glioma growth (Broholm and Laursen, 2004; Millauer et al., 1994; Osada et al., 2004).
Fig. 6.
VEGF and VEGF-receptor interactions. The various family members of VEGF bind differentially to a variety of receptors. The VEGFR-1 (Flt-1) and VEGFR-2 (Flk-1/KDR) have been predominately found in endothelial cells, but expression has also been reported in glioma tissues and glioma cell lines. VEGFR-3 is mainly expressed on the endothelial cells in the lymphatics and is thought to be involved in lymphangiogenesis. Both NRP-1 and NRP-2 are neuronal and endothelial cell surface glycoproteins that dimerize with VEGFR-2 and are expressed in some GBMs and to a lesser extent in low-grade astrocytomas.
Neuropilin-1 and -2 are isoform-specific receptors of VEGF, which function as co-receptors with VEGFR-2 (Fig. 6) (reviewed in Kaur et al. [2004]). In addition to functioning as a co-receptor for VEGFR-2, NRP-1 can independently mediate endothelial cell adhesion to ECM (Murga et al., 2004). The role of NRP-2 in gliomagenesis is not yet clear and needs further elucidation. Both endothelial and astrocytic cells express NRP-1, with an increase in expression seen in the endothelium and neoplastic astrocytes of GBM (Broholm and Laursen, 2004). Overexpression of NRP-1 in a rat prostate xenograft model demonstrated increased tumor growth and angiogenesis (Miao et al., 2000). Expression of NRP-1 in gliomas correlates with grade and predicts poor prognosis, which underscores the importance of NRP-1 in gliomagenesis (Osada et al., 2004). Targeted disruption of either of the NRP-1 or NRP-2 genes also results in an embryonic lethal phenotype with vascular defects, which demonstrates a key role for all of the VEGF receptors in blood vessel development (Takashima et al., 2002).
Angiopoietin and Tie Receptors
Tie-1 and Tie-2 are RTKs expressed almost exclusively on endothelial cells and are implicated as potent angiogenic regulators involved in vessel remodeling, vessel maturation, and endothelial cell survival (Ziegler et al., 1993). Angiopoetin-1 is the ligand for Tie-2, and both components are required for vascular formation, as demonstrated by similar phenotypes and the resulting embryonic lethality of mice engineered to lack either Ang-1 or Tie-2 (Suri et al., 1996). Ang-2, Ang-3 (in mice), and Ang-4 (in humans) are other related proteins that bind to Tie-2 (Valenzuela et al., 1999). Ang-1 binding causes Tie-2 receptor phosphorylation and protects endothelial cells from apoptosis, and this effect is antagonized by Ang-2/Ang-3 (Kim et al., 2000; Xu et al., 2004). Ang-4 binding to Tie-2 can function similarly to Ang-1 binding to induce angiogenesis (Yamakawa et al., 2003). While Ang-1 is believed to stabilize and prevent leakiness of vessels, Ang-2 destabilizes the existing vasculature, causing vessel sprouting and new vessel growth in the presence of VEGF, but resulting in regression of vessels without the presence of VEGF (Holash et al., 1999).
The levels of angiopoietins and their receptors are barely detectable in the normal brain (Tait and Jones, 2004). Ang-1, Ang-2, and Tie-2 levels are greatly elevated in GBM samples, with Ang-1 protein and mRNA found to be expressed mainly by the tumor cells and Ang-2 and Tie-2 expression to be localized to the tumor vasculature and invasive edge of the tumor. Tie-2 expression and activation correlate with increasing grade of astrocytomas, and inhibition of Tie-2 signaling decreased glioma cell tumorigenicity in mice (Zadeh et al., 2004a, b). Expression of Ang-1 in glioma cells resulted in increased tumor vascularization in both subcutaneous and intracranial xenografts (Zadeh et al., 2004a, b). While little is known of Ang-1 regulation, Tie-2, Ang-2, and Ang-3/4 mRNA are induced by hypoxia, and overexpression of HIF-1 induced the expression of Ang-2 and Ang-4, demonstrating the significance of angiopoietins in the hypoxic angiogenic response of endothelial cells (Nilsson et al., 2004; Yamakawa et al., 2003; Zagzag et al., 1999). The role of both Ang-1 and Ang-2 in tumor angiogenesis remains to be fully understood, with studies reporting the overexpression of these proteins to yield both proangiogenic and antiangiogenic effects in different tissue tumor model systems. Using a rat glioma model for tumor growth, however, the overexpression of Ang-1 was found to cause a significant increase in tumor growth marked by enhanced angiogenesis, while the overexpression of Ang-2 inhibited the growth of tumors (Machein et al., 2004). Even though both angiopoietins are frequently upregulated in gliomas, it appears to be the Ang-2:Ang-1 ratio that swings the balance in favor of angiogenesis (Tait and Jones, 2004; Zadeh and Guha, 2003).
Matrix Metalloproteinase
Matrix metalloproteinases are a large family of zinc-binding endopeptidases that play a major role in ECM degradation. They are initially expressed as inactive zymogens and become activated by a proteolytic cleavage, releasing their active subunit (Osenkowski et al., 2004). The MMP-mediated digestion of ECM sets the stage for the proliferating endothelial cells to migrate and invade through the matrix, an essential step for the development of new blood vessels. In addition to ECM, MMPs can fragment growth factors and cytokines into smaller molecules that display an enhanced or reduced biological activity on endothelial and tumor cell growth and migration. For example, MMP-2 mediates the release of fibronectin fragments from fibronectin, and domain DIII from laminin-5, both of which have potent angiogenic properties (Grant et al., 1998; Schenk et al., 2003).
MMP-2, as well as membrane-type MMP-1, MMP-7, and MMP-9, is upregulated by HIF-1-dependent pathways in hypoxia (Lolmede et al., 2003). Furthermore, in human glioma cells HIF activation induces TGF-β2, which further modulates MMP activity by upregulating MMP-1, -2, and -9 and suppressing tissue inhibitor of metalloprotease expression (Wick et al., 2001). Numerous studies have examined the role of these HIF-1-induced MMPs during cancer progression. Membrane type 1 MMP (MMP-14) is upregulated in various tumor types, including glioma, where it activates MMP-2 (Van Meter et al., 2004). Expression of MMP-2 correlates with tumor grade, prognosis, and vascularity in human astrocytic and other tumor types (Thorns et al., 2003). Pharmacological inhibition of MMPs in mouse glioma as well as rat orthotopic models showed decreased tumor angiogenesis (Lakka et al., 2004; Yoshida et al., 2004). Taken together, these studies underscore the importance of HIF-induced MMPs in glioma vascularization/angiogenesis.
Plasminogen Activator Inhibitor-1
Human plasminogen activator inhibitor type 1 (PAI-1) is a 45-kDa single-chain glycoprotein, a member of the serpin (serine proteinase inhibitors) superfamily, and the primary negative regulator of plasminogen activation (Mottonen et al., 1992). Plasminogen plays an important role in promoting angiogenesis by activating MMPs and by releasing growth factors and cytokines trapped within the ECM (Rakic et al., 2003). PAI-1 acts as an inhibitor of tissue (t-PA) and urokinase (u-PA) plasminogen activators, resulting in downregulation of plasminogen under normal physiological conditions.
Expression of PAI-1 is increased under hypoxic conditions, and this is HIF-1 mediated. The PAI-1 gene contains an HRE within its promoter (Kietzmann et al., 2003). Hypoxia also leads to a rapid and transient activation of PI3K/AKT and ERK1/2 that ultimately leads to increased PAI-1 expression (Zhang et al., 2004).
Concomitant with its inhibition of plasminogen activation, PAI-1 inhibits in vivo angiogenesis in the sprout formation and in the chicken chorioallantoic membrane (CAM) assays (Brodsky et al., 2001; Isogai et al., 2001), and PAI-1-deficient mice have increased angiogenesis (Ploplis et al., 2004). In contrast, in tumors PAI-1 regulates invasion, metastasis, and tumor-related angiogenesis. It stimulates expression and release of VEGF in human glioma cell line models that would lead to increased angiogenesis in vivo (Hjortland et al., 2004). In tumor studies, absence of PAI-1 markedly impairs tumor invasion and vascularization (Bajou et al., 2004). Additionally, high PAI-1 levels are strongly associated with high histologic grade and increased necrosis in adult glioma tumors (Muracciole et al., 2002). Further studies are needed to elucidate the mechanism by which PAI-1 enhances glioma vascularization and migration in order to shed light on its seemingly opposing role in angiogenesis under physiological versus pathological conditions.
Endothelin-1
Endothelins are small, 21 amino acid peptides produced by the proteolytic processing of larger precursor proteins by endothelin-converting enzymes-1 and -2 (reviewed in Bagnato and Spinella [2003]). To date, three endothelins have been identified, of which endothelin-1 (ET-1) has been extensively linked to the promotion of angiogenesis. A potent vasoconstrictor produced by vascular smooth muscle cells and endothelial cells, ET-1 elicits its effects by binding to two specific G-protein-coupled receptors, ETRA and ETRB. In a dose-dependent manner, ET-1 has been shown to enhance the proliferation, migration, and invasion of endothelial cells and to promote the formation of vascular structures in Matrigel plugs (BD Biosciences, San Jose, Calif.) (Salani et al., 2000).
Hypoxia stimulates expression of ET-1, and HIF-1 binding sites have been identified in the promoter region of the ET-1 gene. For induction of ET-1 in hypoxia, the transcription modulators AP-1, neurofibromin-1, and GATA-2 were found to be required for the stabilization of HIF-1 and recruitment of CBP/p300 (Hu et al., 1998). Conversely, ET-1 also regulates HIF-1. In ovarian carcinoma cell lines, ET-1 caused an increase in HIF-1α accumulation through protein stabilization and was shown to activate the HIF-1 transcription complex (Spinella et al., 2002).
Various studies show the expression of ET-1 in different glioma cell lines and significantly associate an increased expression of ET-1 with poor differentiation and higher-grade astrocytomas (Li et al., 2002a; Sone et al., 2000; Spinella et al., 2002). In human GBMs, ET-1 and its receptors were consistently found to be expressed in the tumor vasculature, but expression of the ETRB receptor was the only component localized specifically on individual tumor cells (Egidy et al., 2000). VEGF and MMP-2 have been shown to be upregulated by ET-1, and overexpression of ET-1 in a CAM assay produced highly vascular nodules, which are greatly reduced in the presence of inhibitors targeting either the ET-1 receptors or the converting enzyme endothelin-converting enzyme-1 (Cruz et al., 2001; Salani et al., 2000; Spinella et al., 2002).
ET-1 exhibits both growth-factor-like mitogenic effects and antiapoptotic protective effects in endothelial, astrocytic, and glioma cell lines; these effects have been attributed to the ET-1-induced increase in both PI3K activity and Ca2+ concentration (Asano et al., 1994; Egidy et al., 2000). However, the relationship between ET-1 and HIF activation in GBM remains to be fully understood and warrants further study.
Inducible Nitric Oxide Synthase
Nitric oxide is a highly reactive free-radical compound with a short half-life known to affect many cellular processes, including vasodilation, cytotoxicity in immunological responses, and neurotransmission. Three identified forms of nitric oxide synthase (NOS) are responsible for the production of NO from L-arginine, and these enzymes exist either as the cell-specific constitutively active forms of neuronal NOS and endothelial NOS or as inducible NOS (iNOS), which produces the substantial, sustained amounts of NO associated with its pathological effects (Kroncke et al., 1997). Expression of iNOS can be detected after the treatment of mammalian cells with cytokines such as tumor necrosis factor-α or interferons and in the presence of bacterial endotoxins such as lipopolysaccharide (Xie et al., 1992).
In hypoxia, HIF-1α induces the expression of iNOS, leading to the increase in NO concentration often found in hypoxic environments (Jung et al., 2000; Nilsson et al., 2004; Palmer et al., 1998). Nitric oxide has been shown to exhibit tumoricidal activity by inducing tumor cell cytolysis but paradoxically may also contribute to tumor growth by promoting neovascularization of tumor masses. Rat C6 glioma cells express high levels of iNOS, and iNOS activity has been implicated as a critical factor for the growth and maintenance of these tumors (Yamaguchi et al., 2002). Hypoxia-responsive elements have been identified in both murine and rat iNOS promoters but have yet to be described for the human iNOS gene (Yamaguchi et al., 2002). While HIF-1α activates iNOS expression and causes an increase in NO concentration, the complex relationship between HIF-1α and NO has yet to be fully elucidated. Nitric oxide can also regulate HIF activity. Nitric oxide–releasing compounds have been shown to both inhibit (sodium nitroprusside) and activate (S-nitroso-N-acetyl-penicillamine and S-nitroso-glutathione) HIF-1α, and these conflicting results have been attributed to different pharmacological activities of the various NO donor compounds. However, NO produced by iNOS has also been shown to inhibit the DNA binding activity of HIF-1 in a suggested negative feedback loop (Yin et al., 2000). The regulation of HIF activity by NO was found to be concentration dependent: At low concentrations, NO destabilizes HIF-1α by increasing local oxygen concentration through inhibition of mitochondrial respiration, whereas high NO concentrations stabilize HIF-1α through a mitochondrial-independent pathway in both high- and low-oxygen concentrations (Mateo et al., 2003). The relationship between HIF-1α and NO is quite complex and in need of further clarification, especially in relation to growth of gliomas.
Adrenomedullin
Adrenomedullin (ADM) is a secreted protein that is a potent vasodilatory agent with proangiogenic effects (Zhao et al., 1998). It also has natriuretic and diuretic effects, reduces blood pressure after infusion, inhibits bronchodilation, inhibits proliferation in response to platelet-derived growth factor, and suppresses apoptosis (Jougasaki and Burnett, 2000). Adrenomedullin is upregulated by hypoxia in human glioma cell lines (Kitamuro et al., 2000), and the human ADM gene promoter region contains three HIF-1 binding consensus sites (Garayoa et al., 2000). Multiple tumor cell lines, including carcinomas of the lung, breast, prostate, colon, and a glioma cell line, demonstrate ADM induction ranging from 1.3- to 25-fold after hypoxia or CoCl2 treatment (reviewed in Zudaire et al. [2003]).
Adrenomedullin has proangiogenic effects and has been shown to stimulate proliferation of human umbilical vein endothelial cells (HUVECs) and to stimulate blood vessel growth in a CAM assay (Zhao et al., 1998). The finding that homozygous ADM knockout mice die at embryonic day 14 because of severe vascular defects supports a role for ADM in vessel formation (Shindo et al., 2001). Expression of ADM correlates with vascularity in renal cell, breast, and endometrial carcinomas and has been demonstrated to stimulate tumor angiogenesis (Oehler et al., 2002). Proadrenomedullin NH2-terminal peptide is a 20 amino acid, N-terminal peptide derived from the ADM precursor. It is expressed in the adrenal gland, vascular system, and the central nervous system, with binding sites located in a number of places, including the brain (Iwasaki et al., 1996). It displays a more potent angiogenic effect, inducing angiogenesis at lower concentrations, than ADM (Martinez et al., 2004).
The mechanism of ADM-induced angiogenesis has recently been examined in HUVECs. Adrenomedullin induces the phosphorylation of AKT, ERK1/2, and focal adhesion kinase (p125FAK), in a dose-dependent manner, which is partially suppressed by ADM22-52, an inhibitor of the ADM receptor (Kim et al., 2003). Additionally, levels of AKT and NO are increased in a dose-dependent fashion by ADM expression (Iimuro et al., 2004). Adrenomedullin also binds to a complex of calcitonin- receptor-like receptor and receptor-activity-modifying protein-2 and -3 (CRLR/RAMP2; CRLR/RAMP3), and the angiogenic activity of ADM can be blocked by neutralizing antibodies to these receptors.
Adrenomedullin receptors have been identified in GBM samples and multiple glioma cell lines, and ADM has been shown to be secreted by various glial cell tumors (Takahashi et al., 2002; Zimmermann et al., 1996). In immunohistochemical studies of human glioma, all tumors were positive for the ADM receptor, with staining being particularly intense in tumor endothelial cells (Takahashi et al., 2002). Expression of ADM mRNA is increased in higher-grade glioma tumors and cell lines in comparison with normal brain tissue. The addition of ADM-neutralizing antibodies or the ADM inhibitor AM22–52 in vitro to U87 cells causes an inhibition of growth, indicating that ADM may function in an autocrine manner to stimulate GBM cell proliferation. Additionally, in a xenograft tumor model, treatment with anti-ADM antibody greatly reduced both tumor growth and vascularity (Ouafik et al., 2002).
Current evidence indicates that there is a high level of expression of ADM in gliomas and that it may be acting in an autocrine fashion to promote growth and in a paracrine fashion to stimulate angiogenesis. Xenograft tumor data indicates that inhibiting the actions of ADM may have a potent antitumor effect and that treatments targeting ADM in the brain may have efficacy against gliomas.
Erythropoietin
Erythropoietin is a 30-kDa hormone that is produced and secreted by the fetal liver and adult peritubular cells of the kidneys (Schuster et al., 1992). The best characterized function of Epo is its stimulation of erythropoiesis by preventing apoptosis and inducing differentiation of erythrocytic precursor cells in the bone marrow. While classically believed to be isolated to hematopoietic tissues, functional Epo and its receptor, EpoR, are expressed in multiple tissues, including the brain, where they are found on neurons, astrocytes, glia, and endothelial cells (Siren et al., 2001). Epo expression is controlled at the level of mRNA transcription and is strongly upregulated by hypoxia and HIF-1 (Siren et al., 2001). There is a 50-bp enhancer in the 3′ flanking region of the Epo gene that is responsible for the regulation of transcription by hypoxia (Semenza et al., 1991). This enhancer has three binding sites, one of which is a conserved HIF-1 binding HRE sequence.
There is considerable evidence to suggest a role for Epo as a proangiogenic factor. Erythropoietin stimulates capillary outgrowth in adult myocardial tissue comparable to the effect of VEGF (Jaquet et al., 2002), recombinant human Epo (rhEpo) elicits a strong angiogenic response in the chick CAM assay (Ribatti et al., 1999), and EpoR stimulates the proliferation and migration of endothelial cells in vitro (Jelkmann and Wagner, 2004). In accordance with its proangiogenic effect, functional Epo/EpoR complexes have been linked to increased growth rates and vascularization in certain neoplasms. For example, human renal carcinoma cells have been shown to express Epo and EpoR and to proliferate in a dose-dependent response to rhEpo treatment (Westenfelder and Baranowski, 2000). Also, EpoR levels have been correlated to the degree of vascularization and stage in gastric carcinoma (Ribatti et al., 2003) and chemically induced murine hepatic tumors. It is unknown whether Epo and EpoR are expressed by gliomas, and not many studies have examined Epo and its effect on gliomas. In a study on hemangioblastomas, four GBM samples tested negative for EpoR mRNA expression, but the matter has not been studied extensively (Krieg et al., 1998). Since the Epo/EpoR system has been implicated as a proangiogenic and proliferative factor in other cancers, is expressed in astrocytes, and is a known target of HIF-1α, it would be interesting to investigate the changes in Epo/EpoR status in the progression from normal astrocytes to GBMs and to investigate possible correlations between EpoR expression and glioma vascularity.
Transforming Growth Factor α
Transforming growth factor α is a cytokine that shares about 40% homology with epidermal growth factor (EGF) and also binds to the EGF-receptor (EGFR) to produce its biologic effects. TGF-α is expressed by tumor cells in a large number of carcinomas and has a more potent proangiogenic effect than EGF (Schreiber et al., 1986).
Renal cell carcinoma cells that are deficient for pVHL rely on EGFR activation mediated by HIF-induced TGF-α for proliferation and survival (Gunaratnam et al., 2003). Upon binding to TGF-α, EGFR is activated, which can then activate PI3K (Bjorge et al., 1990; Hu et al., 1992). Activation of the PI3K/AKT pathway further increases HIF-1 expression and activity (Zhong et al., 2000). Hence, TGF-α could lead to the activation of HIF-1-dependent gene transcription through the PI3K pathway.
Expression of TGF-α correlates with vascularity and stage in a number of neoplasms, including gliomas (Cai et al., 1997; Eggert et al., 2000; Li et al., 2000b; Schlegel et al., 1990). Increased expression of TGF-α and EGFR has been reported in a number of neoplasms (Gerosa et al., 1989; Nister et al., 1988; Waha et al., 1996; Yung et al., 1990). Cells treated with TGF-α induce expression of VEGF via the transcription factor AP-2 (Detmar et al., 1994). TGF-α increases cell motility (El-Obeid et al., 1997), proliferation (Kurimoto et al., 1994), and invasiveness (Mori et al., 2000) in glioma cells. Additionally, blocking TGF-α expression inhibits cell growth in vitro and also results in a reduced tumorigenicity in vivo (Rubenstein et al., 2001; Tang et al., 1999). The direct role of TGF-α as a regulator of angiogenesis in GBM formation needs to be further elucidated.
Transforming Growth Factor β
Transforming growth factor β is a cytokine with three different isoforms encoded by three separate genes. All of the TGF-β isoforms are secreted as inactive proteins associated with a latency-associated peptide (McMahon et al., 1996). TGF-β is secreted by gliomas (Leitlein et al., 2001) and has a wide variety of both tumor-suppressive and tumor-promoting effects (Van Meir, 1995; Wieser, 2001). TGF-β ligands transmit signals by binding their serine/threonine kinase receptors, thus causing the phosphorylation and activation of SMAD family proteins. Upon ligand binding, dimers of TGF-β type II receptors join and phosphorylate TGF-β type I receptor dimers, which in turn phosphorylate the SMAD proteins. Upon activation, SMADs translocate to the nucleus, where they require interaction with various DNA binding cofactors to specify the cell’s diverse responses to TGF-β (Massague and Wotton, 2000).
HIF-1α stimulates TGF-β production in a cell-type and isoform-specific manner. HIF-responsive elements near the start site of the TGF-β3 gene were identified and demonstrated to be responsive to hypoxia and to bind HIF-1α (Schaffer et al., 2003). The invasive phenotype of trophoblasts in the low-oxygen conditions at early pregnancy is mediated by HIF-1-induced TGF-β3 (Caniggia et al., 2000). HIF-1α regulation of TGF-β1 is less clear, where depending on the cell type, TGF-β1 is either induced (Leungwattanakij et al., 2003; Norman et al., 2000) or reduced (Scheid et al., 2000; Zhang et al., 2003) in hypoxia and/or the presence of HIF-1α. To date, HREs have not been identified or examined in the TGF-β1 promoter. HUVECs express increased levels of TGF-β2 in hypoxia, but HREs in the TGF-β2 promoter have yet to be identified, so it is unclear whether this is a direct, HIF-dependent effect (Zhang et al., 2003). The relationships of TGF-β1, 2, and 3 and HIF-1α have not yet been studied in gliomas.
All three isoforms of TGF-β have been found in glioma cell cultures, in cerebrospinal fluid samples, and in brain tumor biopsies. Glioblastoma multiforme release active TGF-β1 and TGF-β2 (Leitlein et al., 2001), and TGF-β1 and TGF-β2 are secreted by both astrocytes and GBM. The receptors for TGF-β are also upregulated in GBM, especially as compared to nontumor glial tissue (Yamada et al., 1995). TGF-β1 and TGF-β2 can stimulate the proliferation of some glioma cell lines (reviewed in Van Meir [1995]).
In gliomas, both TGF-β1 and TGF-β2 have been shown to stimulate VEGF production, and in hypoxic situations, cooperation between HIF-1α and SMAD proteins induced by TGF-β signaling leads to the induction of VEGF expression (Sanchez-Elsner et al., 2001). HRE and SMAD binding elements were identified in the VEGF gene promoter, and strong induction of VEGF resulted from the activation of both HIF-1 and SMAD3 (Sanchez-Elsner et al., 2001). TGF-β has been shown to have other proangiogenic activities in gliomas, including the upregulation of metalloproteinases, the downregulation of metalloproteinase inhibitors (Wick et al., 2001), and the secretion of ECM (Rich et al., 1999). Inhibition of TGF- β activity by a novel small-molecule inhibitor (SB-431542) prevented the expression of VEGF and PAI-1 and inhibited proliferation of glioma cells in vitro (Hjelmeland et al., 2004). Such an inhibitor could prove useful in treatment of GBM by preventing some of the tumor-promoting effects of TGF-β.
Conclusions
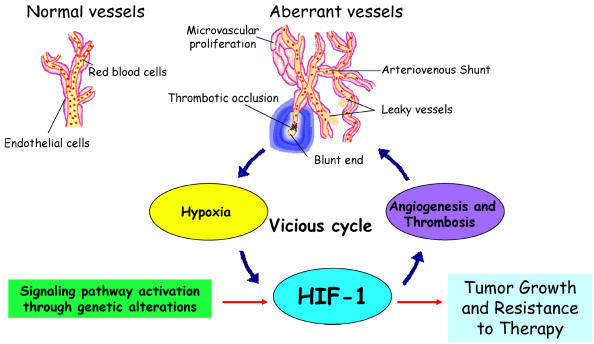
HIF is a transcription factor rapidly activated in cells under conditions of low partial oxygen pressure or hypoxia. HIF activates adaptation to oxygen deprivation, including enzymes of the glycolytic cascade and proangiogenic cytokines. Some of the HIF-induced proangiogenic factors such as VEGF/VEGFR and Ang-1/Tie-2 elicit specific mitogenic effects on endothelial cells, and HIF induction of MMPs promotes endothelial cell migration by degradation of the ECM. Malignant gliomas contain multiple hypoxic regions, which exhibit elevated HIF activity, resulting in augmented expression of many HIF target genes and contributing to the growth and highly vascularized nature of these tumors. Activation of HIF expression in GBM appears to be initiated through a vicious cycle of induction of poorly functioning vasculature perpetuating the development of a hypoxic microenvironment throughout the tumor (Fig. 7) (Brat and Van Meir, 2004; Rong et al., 2005). The expression and activation of HIF are tightly regulated through molecular pathways, which are attractive targets for therapeutic manipulation of tumor growth.
Fig. 7.
Vicious HIF activation cycle in glioma. Genetic alterations (such as loss of PTEN or EGFR mutation) affect critical signaling pathways that lead to HIF-1 stabilization and activation. Transcriptional activity by HIF-1 directly upregulates genes that promote angiogenesis, such as VEGF. Although the upregulation and secretion of proangiogenic factors by tumor cells leads to a strong angiogenic response, the resulting tumor vasculature is abnormal, displaying leakiness due to excessive vascular permeability, focal thickening of vascular walls, and the formation of glomeruloids arising from excessive microvascular proliferation. The tumor vasculature also exhibits abnormal vessel branching, arteriovenous shunts, poor blood flow, and low structural integrity due to inappropriate strengthening of nascent vessels by smooth muscle/pericyte lining. Thrombotic events that arise from poor blood flow and increased tissue factor expression by the neoplasm lead to vascular occlusion. Combined, the vascular phenomena lead to further escalating levels of hypoxia, which in turn stimulates more HIF-1 expression and activation. These events take place at the growing edge of the tumor and lead to a constellation of microregions of hypoxia that develop into pseudopalisading necrosis fueling further HIF expression, angiogenesis, and peripheral tumor expansion. All of these factors lead to tumor growth and resistance to therapy, with HIF-1 as a main component of this vicious cycle.
Acknowledgments
We thank N.S. Devi for technical assistance in generating the pictures for Fig. 1.
Footnotes
We acknowledge support by the U.S. National Institutes of Health, grants CA 86335, and CA 87830 (to E.G.V.M.) and NS 42943 (to D.J.B).
Abbreviations used are as follows: ADM, adrenomedullin; Ang, angiopoietin; ARD1, arrest defective 1 protein; ARNT, aryl hydrocarbon receptor nuclear translocator; CAD, C-terminal transactivation domain; CAM, chicken chorioallantoic membrane; CBP, CREB-binding protein; CH1, cysteine/histidine rich; CITED2, CBP/p300-interacting transactivator with Glu/Asp-rich C-terminal domain; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; Epo, erythropoietin; ERK1/2, extracellular signal–regulated kinase 1/2; ET-1, endothelin-1; GBM, glioblastoma multiforme; HIF, hypoxia-inducible factor; HRE, hypoxia-responsive element; HUVEC, human umbilical vein endothelial cell; iNOS, inducible NOS; MMP, matrix metalloproteinase; NOS, nitric oxide synthase; NRP, neuropilin; ODD, oxygen-dependent degradation; PAI-1, plasminogen activator inhibitor-1; PAS, Per/ARNT/Sim; PHD, prolyl hydroxylase; PI3K, phosphatidylinositol 3-kinase; pVHL, von Hippel-Lindau protein; RTK, receptor tyrosine kinase; SUMO-1, small ubiquitin-like modifier-1; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
References
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Asano T, Aoyagi M, Hirakawa K, Ikawa Y. Effect of endothelin-1 as growth factor on a human glioma cell line; its characteristic promotion of DNA synthesis. J Neurooncol. 1994;18:1–7. doi: 10.1007/BF01324597. [DOI] [PubMed] [Google Scholar]
- Bagnato A, Spinella F. Emerging role of endothelin-1 in tumor angiogenesis. Trends Endocrinol Metab. 2003;14:44–50. doi: 10.1016/s1043-2760(02)00010-3. [DOI] [PubMed] [Google Scholar]
- Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, Carmeliet P, Foidart JM, Noel A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–6990. doi: 10.1038/sj.onc.1207859. [DOI] [PubMed] [Google Scholar]
- Barker FG, 2nd, Davis RL, Chang SM, Prados MD. Necrosis as a prognostic factor in glioblastoma multiforme. Cancer. 1996;77:1161–1166. doi: 10.1002/(sici)1097-0142(19960315)77:6<1161::aid-cncr24>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorge JD, Chan TO, Antczak M, Kung HJ, Fujita DJ. Activated type I phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc Natl Acad Sci USA. 1990;87:3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1α, HIF-2α, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–7355. [PubMed] [Google Scholar]
- Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: A new world of angiogenesis research. Am J Pathol. 2001;158:789–796. doi: 10.1016/S0002-9440(10)64025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- Brodsky S, Chen J, Lee A, Akassoglou K, Norman J, Goligorsky MS. Plasmin-dependent and -independent effects of plasminogen activators and inhibitor-1 on ex vivo angiogenesis. Am J Physiol Heart Circ Physiol. 2001;281:H1784–H1792. doi: 10.1152/ajpheart.2001.281.4.H1784. [DOI] [PubMed] [Google Scholar]
- Broholm H, Laursen H. Vascular endothelial growth factor (VEGF) receptor neuropilin-1’s distribution in astrocytic tumors. APMIS. 2004;112:257–263. doi: 10.1111/j.1600-0463.2004.apm11204-0505.x. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Cai YC, Barnard G, Hiestand L, Woda B, Colby J, Banner B. Florid angiogenesis in mucosa surrounding an ileal carcinoid tumor expressing transforming growth factor-alpha. Am J Surg Pathol. 1997;21:1373–1377. doi: 10.1097/00000478-199711000-00013. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero P, Okamoto K, Coumailleau P, O’Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol Cell Biol. 2000;20:402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Choi E, Yeo EJ, Lee JH, Kim MS, Park JW. A new HIF-1 alpha variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J Cell Sci. 2001;114:4051–4061. doi: 10.1242/jcs.114.22.4051. [DOI] [PubMed] [Google Scholar]
- Chun YS, Choi E, Kim TY, Kim MS, Park JW. A dominant-negative isoform lacking exons 11 and 12 of the human hypoxia-inducible factor-1alpha gene. Biochem J. 2002;362:71–79. doi: 10.1042/0264-6021:3620071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Lee KH, Choi E, Bae SY, Yeo EJ, Huang LE, Kim MS, Park JW. Phorbol ester stimulates the nonhypoxic induction of a novel hypoxia-inducible factor 1alpha isoform: Implications for tumor promotion. Cancer Res. 2003;63:8700–8707. [PubMed] [Google Scholar]
- Clarke K, Smith K, Gullick WJ, Harris AL. Mutant epidermal growth factor receptor enhances induction of vascular endothelial growth factor by hypoxia and insulin-like growth factor-1 via a PI3 kinase dependent pathway. Br J Cancer. 2001;84:1322–1329. doi: 10.1054/bjoc.2001.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews ST. Control of cell lineage-specific development and transcription by bHLH-PAS proteins. Genes Dev. 1998;12:607–620. doi: 10.1101/gad.12.5.607. [DOI] [PubMed] [Google Scholar]
- Cruz A, Parnot C, Ribatti D, Corvol P, Gasc JM. Endothelin-1, a regulator of angiogenesis in the chick chorioallantoic membrane. J Vasc Res. 2001;38:536–545. doi: 10.1159/000051089. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–1146. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–1908. [PubMed] [Google Scholar]
- Egidy G, Eberl LP, Valdenaire O, Irmler M, Majdi R, Diserens AC, Fontana A, Janzer RC, Pinet F, Juillerat-Jeanneret L. The endothelin system in human glioblastoma. Lab Invest. 2000;80:1681–1689. doi: 10.1038/labinvest.3780178. [DOI] [PubMed] [Google Scholar]
- El-Obeid A, Bongcam-Rudloff E, Sorby M, Ostman A, Nister M, Westermark B. Cell scattering and migration induced by autocrine transforming growth factor alpha in human glioma cells in vitro. Cancer Res. 1997;57:5598–5604. [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- Freedman SJ, Sun ZY, Kung AL, France DS, Wagner G, Eck MJ. Structural basis for negative regulation of hypoxia-inducible factor-1alpha by CITED2. Nat Struct Biol. 2003;10:504–512. doi: 10.1038/nsb936. [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Liu E, Sinha S, Cook S, Milstone DS, MacRae CA, Mariotti M, Kuhlencordt PJ, Force T, Rosenzweig A, St-Arnaud R, Dedhar S, Gerszten RE. Integrin-linked kinase regulates endothelial cell survival and vascular development. Mol Cell Biol. 2004;24:8134–8144. doi: 10.1128/MCB.24.18.8134-8144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garayoa M, Martinez A, Lee S, Pio R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, Gassmann M, Cuttitta F. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: A possible promotion mechanism of carcinogenesis. Mol Endocrinol. 2000;14:848–862. doi: 10.1210/mend.14.6.0473. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- Gerosa MA, Talarico D, Fognani C, Raimondi E, Colombatti M, Tridente G, De Carli L, Della Valle G. Overexpression of N-ras oncogene and epidermal growth factor receptor gene in human glioblastomas. J Natl Cancer Inst. 1989;81:63–67. doi: 10.1093/jnci/81.1.63. [DOI] [PubMed] [Google Scholar]
- Gothie E, Richard DE, Berra E, Pages G, Pouyssegur J. Identification of alternative spliced variants of human hypoxia-inducible factor-1alpha. J Biol Chem. 2000;275:6922–6927. doi: 10.1074/jbc.275.10.6922. [DOI] [PubMed] [Google Scholar]
- Grant MB, Caballero S, Bush DM, Spoerri PE. Fibronectin fragments modulate human retinal capillary cell proliferation and migration. Diabetes. 1998;47:1335–1340. doi: 10.2337/diab.47.8.1335. [DOI] [PubMed] [Google Scholar]
- Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DA, Nakamura E, Lorimer IA, Lee S. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL(−/−) renal cell carcinoma cells. J Biol Chem. 2003;278:44966–44974. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: Suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun. 2001;287:808–813. doi: 10.1006/bbrc.2001.5659. [DOI] [PubMed] [Google Scholar]
- Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, Reese ED, Herbstreith MH, Laping NJ, Friedman HS, Bigner DD, Wang XF, Rich JN. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther. 2004;3:737–745. [PubMed] [Google Scholar]
- Hjortland GO, Lillehammer T, Somme S, Wang J, Halvorsen T, Juell S, Hirschberg H, Fodstad O, Engebraaten O. Plasminogen activator inhibitor-1 increases the expression of VEGF in human glioma cells. Exp Cell Res. 2004;294:130–139. doi: 10.1016/j.yexcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- Hu P, Margolis B, Skolnik EY, Lammers R, Ullrich A, Schlessinger J. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol Cell Biol. 1992;12:981–990. doi: 10.1128/mcb.12.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SB, Brat DJ, Olson JJ, Von Deimling A, Zhou W, Van Meir EG. Alterations in molecular pathways of diffusely infiltrating glial neoplasms: Application to tumor classification and anti-tumor therapy. Int J Oncol. 2003;23:857–869. [PubMed] [Google Scholar]
- Hur E, Chang KY, Lee E, Lee SK, Park H. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not the stabilization or DNA binding ability of hypoxia-inducible factor-1alpha. Mol Pharmacol. 2001;59:1216–1224. doi: 10.1124/mol.59.5.1216. [DOI] [PubMed] [Google Scholar]
- Iimuro S, Shindo T, Moriyama N, Amaki T, Niu P, Takeda N, Iwata H, Zhang Y, Ebihara A, Nagai R. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ Res. 2004;95:415–423. doi: 10.1161/01.RES.0000138018.61065.d1. [DOI] [PubMed] [Google Scholar]
- Isogai C, Laug WE, Shimada H, Declerck PJ, Stins MF, Durden DL, Erdreich-Epstein A, DeClerck YA. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001;61:5587–5594. [PubMed] [Google Scholar]
- Iwasaki H, Hirata Y, Iwashina M, Sato K, Marumo F. Specific binding sites for proadrenomedullin N-terminal 20 peptide (PAMP) in the rat. Endocrinology. 1996;137:3045–3050. doi: 10.1210/endo.137.7.8770930. [DOI] [PubMed] [Google Scholar]
- Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–686. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Jougasaki M, Burnett JC., Jr Adrenomedullin: Potential in physiology and pathophysiology. Life Sci. 2000;66:855–872. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Kaur B, Tan C, Brat DJ, Post DE, Van Meir EG. Genetic and hypoxic regulation of angiogenesis in glioma. J Neurooncol. 2004;70:229–243. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Samoylenko A, Roth U, Jungermann K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood. 2003;101:907–914. doi: 10.1182/blood-2002-06-1693. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim JH, Ryu YS, Jung SH, Nah JJ, Koh GY. Characterization and expression of a novel alternatively spliced human angiopoietin-2. J Biol Chem. 2000;275:18550–18556. doi: 10.1074/jbc.M910084199. [DOI] [PubMed] [Google Scholar]
- Kim W, Moon SO, Sung MJ, Kim SH, Lee S, So JN, Park SK. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J. 2003;17:1937–1939. doi: 10.1096/fj.02-1209fje. [DOI] [PubMed] [Google Scholar]
- Kitamuro T, Takahashi K, Nakayama M, Murakami O, Hida W, Shirato K, Shibahara S. Induction of adrenomedullin during hypoxia in cultured human glioblastoma cells. J Neurochem. 2000;75:1826–1833. doi: 10.1046/j.1471-4159.2000.0751826.x. [DOI] [PubMed] [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Marti HH, Plate KH. Coexpression of erythropoietin and vascular endothelial growth factor in nervous system tumors associated with von Hippel-Lindau tumor suppressor gene loss of function. Blood. 1998;92:3388–3393. [PubMed] [Google Scholar]
- Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- Kroncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: Cytotoxicity versus cytoprotection—how, why, when, and where? Nitric Oxide. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Kurimoto M, Endo S, Arai K, Horie Y, Nogami K, Takaku A. TM-1 cells from an established human malignant glioma cell line produce PDGF, TGF-alpha, and TGF-beta which cooperatively play a stimulatory role for an autocrine growth promotion. J Neurooncol. 1994;22:33–44. doi: 10.1007/BF01058353. [DOI] [PubMed] [Google Scholar]
- Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- Lando D, Pongratz I, Poellinger L, Whitelaw ML. A redox mechanism controls differential DNA binding activities of hypoxia-inducible factor (HIF) 1alpha and the HIF-like factor. J Biol Chem. 2000;275:4618–4627. doi: 10.1074/jbc.275.7.4618. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002a;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002b;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Park JW, Chun YS. Non-hypoxic transcriptional activation of the aryl hydrocarbon receptor nuclear translocator in concert with a novel hypoxia-inducible factor-1alpha isoform. Nucleic Acids Res. 2004;32:5499–5511. doi: 10.1093/nar/gkh880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitlein J, Aulwurm S, Waltereit R, Naumann U, Wagenknecht B, Garten W, Weller M, Platten M. Processing of immunosuppressive pro-TGF-beta 1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases. J Immunol. 2001;166:7238–7243. doi: 10.4049/jimmunol.166.12.7238. [DOI] [PubMed] [Google Scholar]
- Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang DY, Hyun JS, Champion HC, Abdel-Mageed AB, Hellstrom WJ. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus caver-nosum. J Androl. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- Li JM, Wu XL, Zhu QJ, Wang QJ. [Endothelin-1 expression and quantitative analysis in astrocytomas] [Chinese] Ai Zheng. 2002a;21:1109–1111. [PubMed] [Google Scholar]
- Li Z, Shimada Y, Uchida S, Maeda M, Kawabe A, Mori A, Itami A, Kano M, Watanabe G, Imamura M. TGF-alpha as well as VEGF, PD-ECGF and bFGF contribute to angiogenesis of esophageal squamous cell carcinoma. Int J Oncol. 2000b;17:453–460. doi: 10.3892/ijo.17.3.453. [DOI] [PubMed] [Google Scholar]
- Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557–1570. doi: 10.1016/S0002-9440(10)63413-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, Tanaka H, Cao Y, Berkenstam A, Poellinger L. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414:550–554. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- Martinez A, Zudaire E, Portal-Nunez S, Guedez L, Libutti SK, Stetler-Stevenson WG, Cuttitta F. Proadrenomedullin NH2-terminal 20 peptide is a potent angiogenic factor, and its inhibition results in reduction of tumor growth. Cancer Res. 2004;64:6489–6494. doi: 10.1158/0008-5472.CAN-04-0103. [DOI] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard MA, Ohh M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am J Nephrol. 2004;24:1–13. doi: 10.1159/000075346. [DOI] [PubMed] [Google Scholar]
- Maynard MA, Qi H, Chung J, Lee EH, Kondo Y, Hara S, Conaway RC, Conaway JW, Ohh M. Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem. 2003;278:11032–11040. doi: 10.1074/jbc.M208681200. [DOI] [PubMed] [Google Scholar]
- McMahon GA, Dignam JD, Gentry LE. Structural characterization of the latent complex between transforming growth factor beta 1 and beta 1-latency-associated peptide. Biochem J. 1996;313 (Pt. 1):343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, Forstreuter F, Mehdorn HM, Held-Feindt J. Functional significance of vascular endothelial growth factor receptor expression on human glioma cells. J Neurooncol. 2004;67:9–18. doi: 10.1023/b:neon.0000021737.89357.cc. [DOI] [PubMed] [Google Scholar]
- Metzen E, Stiehl DP, Doege K, Marxsen JH, Hellwig-Burgel T, Jelkmann W. Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: Identification of a functional hypoxia-responsive element. Biochem J. 2004 doi: 10.1042/BJ20041736. published online November 24 ( http://www.biochemj.org). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- Millauer B, Shawver LK, Plate KH, Risau W, Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- Mori T, Abe T, Wakabayashi Y, Hikawa T, Matsuo K, Yamada Y, Kuwano M, Hori S. Up-regulation of urokinase-type plasminogen activator and its receptor correlates with enhanced invasion activity of human glioma cells mediated by transforming growth factor-alpha or basic fibroblast growth factor. J Neurooncol. 2000;46:115–123. doi: 10.1023/a:1006339717748. [DOI] [PubMed] [Google Scholar]
- Mottonen J, Strand A, Symersky J, Sweet RM, Danley DE, Geoghegan KF, Gerard RD, Goldsmith EJ. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992;355:270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- Muracciole X, Romain S, Dufour H, Palmari J, Chinot O, Ouafik L, Grisoli F, Branger DF, Martin PM. PAI-1 and EGFR expression in adult glioma tumors: Toward a molecular prognostic classification. Int J Radiat Oncol Biol Phys. 2002;52:592–598. doi: 10.1016/s0360-3016(01)02699-2. [DOI] [PubMed] [Google Scholar]
- Murga M, Fernandez-Capetillo O, Tosato G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 2004 doi: 10.1182/blood-2004-07-2598. published online November 2 ( http://www.bloodjournal.org). [DOI] [PubMed] [Google Scholar]
- Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, Bowtell DD, Ronai Z. Siah2 regulates stability of prolylhydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–128. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476–485. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nister M, Libermann TA, Betsholtz C, Pettersson M, Claesson-Welsh L, Heldin CH, Schlessinger J, Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48:3910–3918. [PubMed] [Google Scholar]
- Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibro-genesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Obara S, Nakata M, Takeshima H, Katagiri H, Asano T, Oka Y, Maruyama I, Kuratsu J. Integrin-linked kinase (ILK) regulation of the cell viability in PTEN mutant glioblastoma and in vitro inhibition by the specific COX-2 inhibitor NS-398. Cancer Lett. 2004;208:115–122. doi: 10.1016/j.canlet.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Oehler MK, Hague S, Rees MC, Bicknell R. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene. 2002;21:2815–2821. doi: 10.1038/sj.onc.1205374. [DOI] [PubMed] [Google Scholar]
- Onesto C, Berra E, Grepin R, Pages G. Poly(A)-binding protein-interacting protein 2, a strong regulator of vascular endothelial growth factor mRNA. J Biol Chem. 2004;279:34217–34226. doi: 10.1074/jbc.M400219200. [DOI] [PubMed] [Google Scholar]
- Osada H, Tokunaga T, Nishi M, Hatanaka H, Abe Y, Tsugu A, Kijima H, Yamazaki H, Ueyama Y, Nakamura M. Overexpression of the neuropilin 1 (NRP1) gene correlated with poor prognosis in human glioma. Anticancer Res. 2004;24:547–552. [PubMed] [Google Scholar]
- Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- Ouafik L, Sauze S, Boudouresque F, Chinot O, Delfino C, Fina F, Vuaroqueaux V, Dussert C, Palmari J, Dufour H, Grisoli F, Casellas P, Brunner N, Martin PM. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am J Pathol. 2002;160:1279–1292. doi: 10.1016/S0002-9440(10)62555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274:L212–219. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- Paul SA, Simons JW, Mabjeesh NJ. HIF at the crossroads between ischemia and carcinogenesis. J Cell Physiol. 2004;200:20–30. doi: 10.1002/jcp.10479. [DOI] [PubMed] [Google Scholar]
- Ploplis VA, Balsara R, Sandoval-Cooper MJ, Yin ZJ, Batten J, Modi N, Gadoua D, Donahue D, Martin JA, Castellino FJ. Enhanced in vitro proliferation of aortic endothelial cells from plasminogen activator inhibitor-1-deficient mice. J Biol Chem. 2004;279:6143–6151. doi: 10.1074/jbc.M307297200. [DOI] [PubMed] [Google Scholar]
- Post DE, Devi NS, Li Z, Brat DJ, Kaur B, Nicholson A, Olson JJ, Zhang Z, Van Meir EG. Cancer therapy with a replicating oncolytic adenovirus targeting the hypoxic microenvironment of tumors. Clin Cancer Res. 2004;10:8603–8612. doi: 10.1158/1078-0432.CCR-04-1432. [DOI] [PubMed] [Google Scholar]
- Rakic JM, Maillard C, Jost M, Bajou K, Masson V, Devy L, Lambert V, Foidart JM, Noel A. Role of plasminogen activator-plasmin system in tumor angiogenesis. Cell Mol Life Sci. 2003;60:463–473. doi: 10.1007/s000180300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a proangiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Ribatti D, Guidolin D, Conconi MT, Nico B, Baiguera S, Parnigotto PP, Vacca A, Nussdorfer GG. Vinblastine inhibits the angiogenic response induced by adrenomedullin in vitro and in vivo. Oncogene. 2003;22:6458–6461. doi: 10.1038/sj.onc.1206789. [DOI] [PubMed] [Google Scholar]
- Rich JN, Zhang M, Datto MB, Bigner DD, Wang XF. Transforming growth factor-beta-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J Biol Chem. 1999;274:35053–35058. doi: 10.1074/jbc.274.49.35053. [DOI] [PubMed] [Google Scholar]
- Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–1413. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- Rubenstein M, Glick R, Lichtor T, Mirochnik Y, Chou P, Guinan P. Treatment of the T98G glioblastoma cell line with antisense oligonucleotides directed toward mRNA encoding transforming growth factor-alpha and the epidermal growth factor receptor. Med Oncol. 2001;18:121–130. doi: 10.1385/MO:18:2:121. [DOI] [PubMed] [Google Scholar]
- Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati M, Cervoni L, Artico M, Caruso R, Gagliardi FM. Long-term survival in patients with supratentorial glioblastoma. J Neurooncol. 1998;36:61–64. doi: 10.1023/a:1017926603341. [DOI] [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- Schaffer L, Scheid A, Spielmann P, Breymann C, Zimmermann R, Meuli M, Gassmann M, Marti HH, Wenger RH. Oxygen-regulated expression of TGF-beta 3, a growth factor involved in trophoblast differentiation. Placenta. 2003;24:941–950. doi: 10.1016/s0143-4004(03)00166-8. [DOI] [PubMed] [Google Scholar]
- Scheid A, Wenger RH, Christina H, Camenisch I, Ferenc A, Stauffer UG, Gassmann M, Meuli M. Hypoxia-regulated gene expression in fetal wound regeneration and adult wound repair. Pediatr Surg Int. 2000;16:232–236. doi: 10.1007/s003830050735. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel U, Moots PL, Rosenblum MK, Thaler HT, Furneaux HM. Expression of transforming growth factor alpha in human gliomas. Oncogene. 1990;5:1839–1842. [PubMed] [Google Scholar]
- Schreiber AB, Winkler ME, Derynck R. Transforming growth factor-alpha: A more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Schuster SJ, Koury ST, Bohrer M, Salceda S, Caro J. Cellular sites of extrarenal and renal erythropoietin production in anaemic rats. Br J Haematol. 1992;81:153–159. doi: 10.1111/j.1365-2141.1992.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Seeler JS, Dejean A. Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol. 2003;4:690–699. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. O2-regulated gene expression: Transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D, Cairncross JG, Forsyth PA. Long-term survivors of glioblastoma: Statistical aberration or important unrecognized molecular subtype? Cancer J. 2003;9:214–221. doi: 10.1097/00130404-200305000-00009. [DOI] [PubMed] [Google Scholar]
- Shao R, Zhang FP, Tian F, Anders Friberg P, Wang X, Sjoland H, Billig H. Increase of SUMO-1 expression in response to hypoxia: Direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004;569:293–300. doi: 10.1016/j.febslet.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- Siren AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- Sone M, Takahashi K, Totsune K, Murakami O, Arihara Z, Satoh F, Mouri T, Shibahara S. Expression of endothelin-1 and endothelin receptors in cultured human glioblastoma cells. J Cardiovasc Pharmacol. 2000;36:S390–392. doi: 10.1097/00005344-200036051-00113. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1alpha in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisiteroleofangiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Tait CR, Jones PF. Angiopoietins in tumours: The angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Totsune K, Kitamuro T, Sone M, Murakami O, Shibahara S. Three vasoactive peptides, endothelin-1, adrenomedullin and urotensin-II, in human tumour cell lines of different origin: Expression and effects on proliferation. Clin Sci (Lond) 2002;103 (suppl. 48):35S–38S. doi: 10.1042/CS103S035S. [DOI] [PubMed] [Google Scholar]
- Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, Nose T. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res. 1996;56:2185–2190. [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Res. 2005;65:605–612. [PubMed] [Google Scholar]
- Tang P, Jasser SA, Sung JC, Shi Y, Steck PA, Yung WK. Transforming growth factor-alpha antisense vectors can inhibit glioma cell growth. J Neurooncol. 1999;43:127–135. doi: 10.1023/a:1006272019933. [DOI] [PubMed] [Google Scholar]
- Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23:3937–3944. [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman JR, Zhao Y, Hewitson KS, Tian YM, Pugh CW, Ratcliffe PJ, Mole DR. Determination and comparison of specific activity of the HIF-prolyl hydroxylases. FEBS Lett. 2004;576:145–150. doi: 10.1016/j.febslet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, Huang T, Papadopoulos N, Maisonpierre PC, Davis S, Yancopoulos GD. Angiopoietins 3 and 4: Diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meir EG. Cytokines and tumors of the central nervous system. Glia. 1995;15:264–288. doi: 10.1002/glia.440150308. [DOI] [PubMed] [Google Scholar]
- Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fill-more HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor–mediated signaling in gliomas. Neuro-Oncol. 2004;6:188–199. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waha A, Baumann A, Wolf HK, Fimmers R, Neumann J, Kindermann D, Astrahantseff K, Blumcke I, von Deimling A, Schlegel U. Lack of prognostic relevance of alterations in the epidermal growth factor receptor-transforming growth factor-alpha pathway in human astrocytic gliomas. J Neurosurg. 1996;85:634–641. doi: 10.3171/jns.1996.85.4.0634. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, Hermann DM. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2004a doi: 10.1093/brain/awh325. published online October 27 ( http://brain.oupjournals.org). [DOI] [PubMed] [Google Scholar]
- Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave–stimulated osteo-blasts. J Biol Chem. 2004b;279:10331–10337. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647–657. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Wick W, Platten M, Weller M. Glioma cell invasion: Regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- Wieser R. The transforming growth factor-beta signaling pathway in tumorigenesis. Curr Opin Oncol. 2001;13:70–77. doi: 10.1097/00001622-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu YJ, Yu Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 2004;64:6119–6126. doi: 10.1158/0008-5472.CAN-04-1054. [DOI] [PubMed] [Google Scholar]
- Yamada N, Kato M, Yamashita H, Nister M, Miyazono K, Heldin CH, Funa K. Enhanced expression of transforming growth factor-beta and its type-I and type-II receptors in human glioblastoma. Int J Cancer. 1995;62:386–392. doi: 10.1002/ijc.2910620405. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Bell HS, Shinoda J, Holmes MC, Wharton SB, Whittle IR. Glioma tumourgenicity is decreased by iNOS knockout: Experimental studies using the C6 striatal implantation glioma model. Br J Neurosurg. 2002;16:567–572. [PubMed] [Google Scholar]
- Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- Yin JH, Yang DI, Ku G, Hsu CY. iNOS expression inhibits hypoxia-inducible factor-1 activity. Biochem Biophys Res Commun. 2000;279:30–34. doi: 10.1006/bbrc.2000.3896. [DOI] [PubMed] [Google Scholar]
- Yoshida D, Takahashi H, Teramoto A. Inhibition of glioma angiogenesis and invasion by SI-27, an anti-matrix metalloproteinase agent in a rat brain tumor model. Neurosurgery. 2004;54:1213–1220. doi: 10.1227/01.neu.0000119237.46690.c6. [DOI] [PubMed] [Google Scholar]
- Yung WK, Zhang X, Steck PA, Hung MC. Differential amplification of the TGF-alpha gene in human gliomas. Cancer Commun. 1990;2:201–205. doi: 10.3727/095535490820874416. [DOI] [PubMed] [Google Scholar]
- Zadeh G, Guha A. Neoangiogenesis in human astrocytomas: Expression and functional role of angiopoietins and their cognate receptors. Front Biosci. 2003;8:e128–e137. doi: 10.2741/964. [DOI] [PubMed] [Google Scholar]
- Zadeh G, Koushan K, Pillo L, Shannon P, Guha A. Role of Ang1 and its interaction with VEGF-A in astrocytomas. J Neuropathol Exp Neurol. 2004a;63:978–989. doi: 10.1093/jnen/63.9.978. [DOI] [PubMed] [Google Scholar]
- Zadeh G, Qian B, Okhowat A, Sabha N, Kontos CD, Guha A. Targeting the tie2/tek receptor in astrocytomas. Am J Pathol. 2004b;164:467–476. doi: 10.1016/S0002-9440(10)63137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Hooper A, Friedlander DR, Chan W, Holash J, Wiegand SJ, Yancopoulos GD, Grumet M. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159:391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: Association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2003;101:2253–2260. doi: 10.1182/blood-2002-02-0629. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wu Y, Chau CH, Ann DK, Bertolami CN, Le AD. Crosstalk of hypoxia-mediated signaling pathways in upregulating plasminogen activator inhibitor-1 expression in keloid fibroblasts. J Cell Physiol. 2004;199:89–97. doi: 10.1002/jcp.10452. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hague S, Manek S, Zhang L, Bicknell R, Rees MC. PCR display identifies tamoxifen induction of the novel angiogenic factor adrenomedullin by a non estrogenic mechanism in the human endometrium. Oncogene. 1998;16:409–415. doi: 10.1038/sj.onc.1201768. [DOI] [PubMed] [Google Scholar]
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- Zhou J, Schmid T, Frank R, Brune B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- Ziegler SF, Bird TA, Schneringer JA, Schooley KA, Baum PR. Molecular cloning and characterization of a novel receptor protein tyrosine kinase from human placenta. Oncogene. 1993;8:663–670. [PubMed] [Google Scholar]
- Ziel KA, Campbell CC, Wilson GL, Gillespie MN. Ref-1/Ape is critical for formation of the hypoxia-inducible transcriptional complex on the hypoxic response element of the rat pulmonary artery endothelial cell VEGF gene. FASEB J. 2004;18:986–988. doi: 10.1096/fj.03-1160fje. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Fischer JA, Frei K, Fischer AH, Reinscheid RK, Muff R. Identification of adrenomedullin receptors in cultured rat astrocytes and in neuroblastoma x glioma hybrid cells (NG108-15) Brain Res. 1996;724:238–245. doi: 10.1016/0006-8993(96)00337-x. [DOI] [PubMed] [Google Scholar]
- Zudaire E, Martinez A, Cuttitta F. Adrenomedullin and cancer. Regul Pept. 2003;112:175–183. doi: 10.1016/s0167-0115(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]