Abstract
Long-term survivors of glioblastoma multiforme, the most common form of primary intracranial malignancy in adults, are extremely rare. Experimental animal models that more closely resemble human disease are essential for the identification of effective novel therapies. We report here an extensive analysis of the 4C8 glioma model to assess its suitability for evaluating novel type 1 herpes simplex virus (HSV-1) therapies of malignant glioma. We first determined that expression of major histocompatibility complex I and II and of αvβ3 in the 4C8 model was comparable to that seen in human glioma cells. Next, using a panel of Δγ134.5 HSVs, we demonstrated that, in vitro, 4C8 cells were as sensitive as human glioma cells to both infection and lysis and that the 4C8 cells supported the production of foreign gene products. Replication competence of HSV was demonstrated in vitro. Finally, 4C8 intracranial gliomas were established in immunologically competent syngeneic B6D2F1 mice, treated by intratumoral injection of selected engineered HSVs, including the interleukin-12-expressing virus, M002. Survival data from these studies demonstrated that 4C8 cells in vivo are sensitive to both direct oncolysis and HSV-mediated interleukin-12 expression. Fluorescence-activated cell sorting analyses of immune-related infiltrating cells supported the concept that survival was prolonged in part because of antitumor actions of these cells. We conclude that the 4C8/B6D2F1 syngeneic glioma model is suitable for preclinical evaluation of HSV-based therapies and that M002 is a superior virus for the treatment of murine glioma in this model.
Keywords: glioma, herpes simplex virus, gene therapy, cytokine, mouse models
Various animal models of malignant brain tumors have been used to evaluate novel treatment strategies (reviewed by Pilkington and Lantos [1990] and Barth [1998]). Most of the models that have been used in the past employed transplantable glial tumors initially induced by a variety of carcinogenic agents and then implanted serially into syngeneic hosts. Unfortunately, many of these tumors do not reflect the actual oncogenic changes that characterize human gliomas and thus are unlikely to be accurate models of the human tumor. Spontaneously arising astrocytomas in the VMDk mouse strain have been shown to have a variable rate of producing intracranial tumors, some with long latency before tumors developed. Two cell lines derived from VMDk astrocytomas have been used more successfully by investigators to explore novel therapies (Ashley et al., 1998; Chambers et al., 1995; Friese et al., 2003). Recently, various permutations of transgenic mice have been generated, and malignant gliomas arising as a consequence of transgene expression have been isolated and partially characterized. One of these is the MOCH-1 malignant glioma that arose in a mouse transgenic for the neu oncogene driven by the myelin basic protein promoter (Dyer and Philibotte, 1995). The neu oncogene encodes a protein that resembles the human erbB-2 receptor and was first identified in a rat glioma. The 4C8 cell line was cloned from the MOCH-1 mouse tumor and grows as either astrocyte-like or oligodendroglia-like cells in tissue culture, the phenotype depending on culture conditions. In the presence of >4% serum, it assumes the phenotype of an astrocytoma and develops into malignant gliomas when transplanted into brains of syngeneic B6D2F1 (C57BL/6 × DBA/2 F1)3 mice. The 4C8 cells grow with invasion of normal brain parenchyma and develop into a highly vascularized tumor with areas of necrosis and pseudopallisading, which are characteristic histopathological features of human glioblastoma multiforme (Dyer and Philibotte, 1995). This tumor therefore appears to be an excellent one to use to study therapeutic effects of different agents in an orthotopic, immunocompetent mouse model of glioma. We subsequently refer to this model as the 4C8/B6D2F1 model and have restricted our studies to the astrocytoma phenotype of 4C8.
We and others have been evaluating the antitumor efficacy of numerous non-neurovirulent, genetically engineered type 1 herpes simplex viruses (HSV-1), all of which lack the neurovirulence gene, γ134.5 (Markert et al., 2000a; Mineta et al., 1995). These mutant viruses are unable to replicate in nondividing cells such as neurons and quiescent glia. However, they replicate in proliferating tumor cells and kill them effectively, presumably through oncolysis. More recently, several Δγ134.5 HSVs have been genetically engineered to express different immune-modulating cytokines, and we have previously demonstrated that this combination may improve the antitumor effect over direct oncolysis alone (Andreansky et al., 1998; Parker et al., 2000). This strategy seeks to induce an antitumor immune response by HSV-mediated expression of proinflammatory cytokines in the infected tumor cells. In undertaking preclinical studies with the intention of advancing novel HSV therapies to human trials, it is critical to utilize an animal model in which both risk of toxicity and efficacy can be evaluated. While several murine models of intracranial human glioma exist in nude or severe combined immunodeficient (SCID) mice, such models are not useful for examining the contributing effects of host immune response on the antitumor efficacy of HSV, or the influences of HSV-mediated cytokine gene therapy. The objective of these studies was to determine if the 4C8/B6D2F1 model is a more relevant model for assessment of HSV-based antiglioma therapies. Ideally, an appropriate experimental murine model for glioma therapy that incorporates these engineered HSVs would express levels of major histocompatibility complex (MHC) class I and II antigens at the cell surface that are similar to levels seen in human tumors, which may allow an enhancement of the antitumor responses elicited by the engineered HSVs. In addition, inappropriate αv and β3 integrin expression would be expected, as has been previously shown with most human glioma cells. The αv and β3 integrins are known to be involved with cell migration, cell survival from apoptosis, and angiogenesis. MHC I and MHC II antigen expression by 4C8 cells was examined by fluorescence-activated cell sorting (FACS) analysis, while αvβ3 integrin expression was determined by using fluorochrome-conjugated antibodies. A small proportion of 4C8 tumor cells did express MHC class I, with minimal class II expression also observed. We also determined that a high proportion of 4C8 cells do express αvβ3 integrins at levels comparable to those of human gliomas.
Different mouse strains show variable sensitivity to HSV-1 and HSV-2, largely because of differences in expression of the receptor that HSV uses to enter the cell, namely, nectin-1 (Cocchi et al., 1998). For example, the C57BL/6 strain is very resistant, but the DBA/2 strain is quite sensitive to HSV infection (Lopez, 1981). The strain of mice from which the MOCH-1 tumor arose, B6D2F1, is sensitive to HSV (G.Y. Gillespie, unpublished data). However, little was known about HSV growth and foreign gene expression in this cell line. We determined the in vitro and in vivo sensitivity of the 4C8 cell line to infection, foreign gene production, and tumor cell killing (oncolysis) using a panel of Δγ134.5 HSVs. In vitro, 4C8 cells were as sensitive as human glioma cells to infection by these genetically engineered HSVs. In addition, the 4C8 cells supported the production of foreign gene products from all of the engineered viruses. Finally, an interleukin (IL)-12-expressing HSV, M002, was more efficacious than its parent virus in prolonging survival of mice harboring intracranial 4C8 gliomas.
These properties, together with an in vivo analysis of the therapeutic effectiveness of Δγ134.5 HSVs, suggest that the 4C8/B6D2F1 model is a useful murine model for experimental preclinical studies of HSV-based brain tumor therapies.
Materials and Methods
Cell Lines and Viruses
The 4C8 murine glioma cell line was a gift. Cells were maintained in Corning tissue culture plasticware (Corning, Inc., Corning, N.Y.) in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 (+7% fetal bovine serum and l-glutamine). Cells were harvested with 0.05% trypsin/0.53 mM EDTA. The GL261 mouse glioma of C57BL/6 origin has been previously described (Andreansky et al., 1998). Viruses employed in these studies are described in Table 1. R3659 is the parent virus for most of the viruses described and has the thymidine kinase gene inserted into deleted regions of both γ134.5 loci, with a deletion in the native thymidine kinase locus (Lagunoff and Roizman, 1994). R4009 has a stop codon in both loci of the γ134.5 gene, preventing normal expression of this gene. R8306, R8308, R8314, R8316, R8320, M002, M004, and M012 all have their respective foreign genes inserted into deleted regions of both γ134.5 loci under the Egr-1 promoter as we have previously described (Andreansky et al., 1998; Parker et al., 2000). Tet-onf luciferase is a similar construct, with the luciferase expressed by the Tet-on promoter (Clontech, Palo Alto, Calif.). R849 is a similar construct that expresses the lacZ gene from the native γ134.5 promoter. The granulocyte-macrophage colony-stimulating factor (GM-CSF) insert in M004 was cloned from hGM-AUGUA plasmid (Rajagopalan et al., 1995), which was a gift. The novH (human nov) plasmid has been described previously (Martinerie et al., 1994). The IL-2 and IL-5 genes and the cytosine deaminase gene from E. coli were each obtained from the American Type Culture Collection (Manassas, Va.). All of the viruses described above were constructed in the laboratories of the authors. G207 (MediGene, Inc., San Diego, Calif.) is deleted for 1000 bp in both loci of the γ134.5 gene and also harbors an insertion of CMV IE gene promoter–driven lacZ gene into UL39.
Table 1.
Comparative virulence of genetically engineered HSV for 4C8 or GL261 murine glioma cell lines
| PFU/TD50 | |||||
|---|---|---|---|---|---|
| 4C8 | GL261 | ||||
| Virus Designation | Reference | Foreign Gene Insert | (mean ± SD)* | Human Glioma Lines (range; mean)† | |
| R4009 | Chambers et al., 1995 | None | 0.1 ± 0.08 | 322 ± 34 | 1.4–13.6; 5.06‡ |
| R3659 | Lagunoff and Roizman, 1994 | None | 1.3 ± 1.22 | >1000 | 1.9–14.4; 8.15§ |
| R8306 | Andreansky et al., 1998 | IL-4 | 1.3 ± 0.92 | 10.9 ± 6.7 | ND |
| R8308 | Andreansky et al., 1998 | IL-10 | 1.7 ± 1.38 | 63.4 ± 27.0 | ND |
| R8320 | # | Nov | 5.1 ± 5.71 | ND | ND |
| M012 | # | Cytosine deaminase | 2.5 ± 1.51 | ND | ND |
| R8314 | # | IL-2 | 0.8 ± 0.11 | >1000 | ND |
| R8316 | # | IL-5 | 0.4 ± 0.09 | >1000 | ND |
| G207 | Mineta et al., 1995 | lacZ | 0.1 ± 0.08 | >1,000 | ND |
| M002 | Parker et al., 2000 | IL-12 | 8.2 ± 6.08 | >1000 | 1.1–7.8; 4.45§ |
| R849 | Andreansky et al., 1997 | lacZ | 0.3 ± 0.06 | ND | 2.43–4.0; 3.21‡ |
| M004 | # | GM-CSF | 8.9 ± 5.46 | >1000 | ND |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; HSV, herpes simplex virus; IL, interleukin; ND, not determined; PFU/TD50, the number of plaque-forming units required to kill 50% of cells at risk within a given period.
PFU/TD50 is for a 72-h incubation period. Averages ± standard deviations are based on at least 3 separate assays on different dates.
Previously reported PFU/TD50 values for 10 different human glioma cell lines are provided for comparison (Andreansky et al. 1997; Parker et al., 2000).
G. Y. Gillespie, unpublished data.
Direct In Vitro Oncolytic Sensitivity of 4C8 Cells
The 4C8 cells were plated in 96-well Corning microplates (Corning, Inc.) at 5 × 103 cells per well and incubated overnight. Dilutions of each HSV listed in Table 1 (R3659, R4009, R8306, R8308, R8314, R8316, M012, R8320, G207, M002, M004, and R849) ranging in half-log dilutions from 0.01 plaque-forming unit (PFU) per cell to 10 PFU/cell were added after 24 h in four to six replicate columns and allowed to incubate 72 h. During the last 4 h of incubation, 40 μl of alamarBlue (Trek Diagnostic Systems, Inc., West Lake, Ohio) was added to each well, and the plate was reincubated. The conversion of the blue dye to a pink color by mitochondrial oxidation was taken as a direct sign of cell viability and was measured in a microplate spectrophotometer (optical density [OD], 562–590 nm). The mean OD values for each virus dilution were used to construct a dose-response plot, and a regression analysis for the linear portion of the sigmoidal plot was used to estimate a toxic dose (TD), the PFU/TD50, defined as the number of plaque-forming units of virus needed to kill 50% of the cells at risk. The lower the PFU/TD50, the more sensitive the cells were to that particular virus. In some instances the cells were so insensitive to the virus that the estimated PFU/TD50 was in excess of the maximum number of PFUs per cell used in the assay. Results from three or more studies with each virus performed at different times were averaged, and the standard deviation was determined. In vitro HSV replication was determined by infecting subconfluent monolayers of 4C8 cells in 24-well plates with 0.1 or 1.0 PFU/cell followed by incubation for up to 96 h. At 24-h intervals, four to eight wells were harvested by scraping and freeze-thawing the cells, and 100 μl of serial 10-fold dilutions of each of the lysates was plated on replicate monolayers of Vero cells (available from American Type Culture Collection) in six-well plates. Plates were harvested at 36 h, the numbers of plaques were determined and corrected for dilution, and the results were expressed as plaque-forming units per milliliter. Means and standard deviations of four to eight samples were calculated and plotted on a log scale versus the time after infection.
Determination of Expression of Foreign Gene Product
The 4C8 cells were plated in 24-well plates at 5 × 105 cells/well and incubated overnight. Each virus (R3659, R8306, M002, M004, R849, and Tet-onf luciferase [Clontech]) was diluted to 104 (multiplicity of infection [MOI] = 0.1; MOI of 1 PFU/10 cells) and 105 (MOI = 1) PFU/ml, and 1 ml was added at each dilution to triplicate or quadruplicate wells. Three replicate plates were made, and one plate was harvested at 24, 48, and 72 h. Supernates were harvested, clarified by centrifugation, and frozen. Cells were lysed in radioimmunoprecipitation assay buffer and centrifuged, the supernate was removed, and the cells were frozen. Supernates were used as samples in commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, Minn.). If the supernates were determined to be negative for cytokine presence, then cell lysates were analyzed. Lysates (10 μl) from cells infected with the virus Tet-onf luciferase were analyzed by using 10 μl of Luciferase Assay Substrate (Promega, Madison, Wis.), and the light generation of the reaction was measured in the RLU luminometer (Promega). Negative controls consisted of cell lysates from 4C8 cells infected with R3659 virus. Lysates (50 μl) from cells infected with R849 (lacZ insert) were plated in three replicate wells in a 96-well microplate, mixed with 50 μl of Dulbecco’s phosphate-buffered saline, and incubated (37°C, 24 h) with 50 μl of o-nitrophenyl-d-galactopyranoside. Color development was detected by using a microplate spectrophotometer at 405 nm. The intensity of the yellow color was used as an estimate of lacZ produced as compared with the color change induced by addition of 50 μl of β-galactosidase at 5 ng/ml (Sigma Chemical Co., St. Louis, Mo.).
Immunofluorescence Studies for αvβ3 and MHC Class I and II Molecules
The 4C8 cells (2 × 105) were treated separately with a panel of antimouse monoclonal antibodies (Pharmingen, San Diego, Calif.) for αv (H9.2BF), β3 (2C9.G2), H-2Dd (34-2-12), H-2Db, H-2Kb (AF6-88.5), H-2Kd, and (SF1-1.1). Mouse antimouse H-2Kb–fluorescein isothiocyanate (FITC) (#060101D) and mouse antimouse H-2Db–phycoerythrin (PE) (#06115A) were used together. Mouse antimouse H-2Kd–FITC (#553585) and mouse antimouse H-2Dd–PE (#06135A) were used together. Mouse antimouse I-Ab–FITC (#06044D) and mouse an-timouse I-Ad–PE (#06035A) were used together. Hamster antimouse CD51 (αv) (#0151D) and hamster antimouse CD61 (β3) (#01861D) IgGs were used separately or together followed by a mouse antihamster IgG cocktail-PE (#12355B) to detect hamster IgG. Appropriate fluorochrome-conjugated secondary antibodies were used. A Becton-Dickinson (Mountain View, Calif.) FACStar flow cytometer was used to quantify antibody binding to cell surface antigens.
Intracranial Tumor Studies
Mouse studies were conducted with the approval of the University of Alabama at Birmingham Institutional Animal Care and Use Committee (APN#031203989), which reviewed all protocols. Initial studies with graded numbers of 4C8 cells injected intracranially confirmed a previous report of the invasive tumor growth (Weiner et al., 1999). For examination of tumor growth, mice were killed at specified time points, and brains were removed intact and fixed in 10% paraformaldehyde. Serial 2-mm coronal sections of the brain with one section through the initial injection site were embedded in paraffin, and 8- to 12-μm sections were prepared and stained with hematoxylin and eosin. For therapy studies, 4C8 cells, harvested from tissue culture, were injected intracerebrally (right caudate nucleus) in 5 μl (5 × 105 cells) of serum-free DMEM/F12 with 5% methyl cellulose by using a stereotaxic frame as previously described (Chambers et al., 1995). Seven to 21 days later, mice were randomized into groups of nine to 11 mice, and 10 μl of virus (R3659, M002) at 0.2 or 1 × 109 PFU/ml or sterile excipient was then injected to the same stereotaxic coordinates. Mice were either killed at designated intervals or monitored for survival. Moribund mice were killed, and the date of death was recorded as previously described (Chambers et al., 1995). Plots of Kaplan-Meier survival estimates were constructed, and the significance of difference among the experimental and control groups was assessed by log-rank (Mantel-Cox) pairwise comparisons. Each study contained a control group to assess tumor take that was treated by intracranial injection of sterile saline for injection.
FACS Analysis of Tumor-Infiltrating Immune-Related Cells
To determine the nature of the immune response elicited by HSV treatment of 4C8 gliomas in the brains of mice, we harvested two to three brains from each of the four treatment groups defined above and prepared single cell homogenates. Briefly, two to three mice were killed at each of the specified time points after intratumoral injection of saline, R3659, or M002; each brain was removed; and the cerebrum was dissected free of meninges, olfactory bulbs, cerebellum, and brain stem. Each brain was minced and finely homogenized by using a chopping motion with a razor blade. Homogenate was resuspended in ice-cold DMEM/F12, and a single cell suspension was obtained by filtration through 170-μm nylon mesh (Sefar America, Inc., Kansas City, Mo.) into sterile 50-ml conical tubes. The single cell suspension was washed once in ice-cold DMEM/F12 and resuspended to a standard volume of 10 ml. Aliquots (100 μl each) were dispensed into microfuge tubes on ice containing 900 μl of ice-cold Dulbecco’s phosphate-buffered saline with 5% fetal bovine serum and 2 mM NaNO3 (FACS buffer). The cells were pelleted by brief centrifugation in a microfuge, and supernate was discarded. The pellets were resuspended in 10 μl of one of the following fluorochrome-conjugated antibodies: FITC-anti-CD4 (clone GK1.5, Pharmingen), PE-anti-CD8 (clone 53-6.7, Pharmingen), PE-anti-NK (anti-CD49b, clone DX-5, Pharmacia [Uppsala, Sweden]). After incubation (60 min, 4ºC), red cells were lysed by using PharM Lyse buffer (BD Biosciences, Pharmingen) according to the manufacturer’s protocol. Remaining cells were washed three times with ice-cold FACS buffer and resuspended in ice-cold 1% freshly made paraformaldehyde. Labeled and fixed cells were stored at 4°C until analysis by using a BD FACStar flow cytometer (BD Biosciences) in the AIDS Flow Cytometry Facility. For quality control, a portion of the saline-treated brain homogenate was mixed with a portion of spleen homogenate and processed as described above. The side-scatter:forward-angle light-scatter profiles of the splenocytes mixed with brain homogenate were used to set gates for each immune-related cell type. The data were reviewed by using FCS Express Version 2 (DeNovo Software, Thornhill, Ont., Canada), and results were expressed as percentage of gated cells for each cell type identified by the antibodies. Mean values were calculated for the mice at each time point for each treatment group, and where three mice constituted a single assay point, the standard deviations were calculated. For virus recovery studies, samples of brain homogenate from each mouse injected with R3659 or M002 HSV were titered on Vero cells as described above to determine persistence of the viruses.
Results
Sensitivity of 4C8 Glioma Cells To Δγ134.5 HSV Oncolysis In Vitro
The Δγ134.5 HSVs that were employed in these studies are described in Table 1. Most of these have been previously reported and have been shown to infect and kill numerous human glial tumor cell lines as assessed by the alamarBlue (Trek Diagnostic Systems, Inc.) assay (Andreansky et al., 1997, 1998; Parker et al., 2000). In studies that were performed at least three times, the alamarBlue (Trek Diagnostic Systems, Inc.) assay was used to estimate the sensitivity of 4C8 mouse glioma cells to infection and oncolysis by these engineered HSVs. In comparison, 4C8 glioma cells were as sensitive as human glial tumor cells to these mutant HSVs (Andreansky et al., 1997). In contrast, the GL261 mouse glioma cell line was highly resistant to oncolysis by this same HSV panel.
Many of these Δγ134.5 HSVs have been genetically engineered to express different cytokines, and we sought to determine the extent to which infected 4C8 cells would support production of the encoded foreign proteins. For most of these foreign proteins, semiquantitative ELISA or luminometry assays are commercially available. We infected 4C8 cells at two different MOIs with each of three mutant HSVs and harvested either supernates or cell lysates for quantitative analyses. The ELISA data confirmed the ability of 4C8 cells infected with the engineered HSVs to support expression of foreign gene products specific for each virus (Table 2). Intriguingly, with the exception of the IL-4 virus, higher levels of foreign gene expression were observed when a lower MOI was used. Additionally, a low level of GM-CSF was produced by 4C8 cells infected with R3659, implying an innate capacity for GM-CSF expression by these cells. This finding is consistent with previously described GM-CSF expression by human glioma cells (Frei et al., 1992; Mueller et al., 1999; Yamanaka et al., 1994). We next examined the capacity of 4C8 cells infected with 0.1 or 1.0 MOI of HSV R849 encoding the lacZ gene to produce the bacterial enzyme β-galactosidase, as evidenced by conversion of the o-nitrophenyl-d-galactopyranoside substrate over a 24-h period. We observed levels of foreign gene expression, based on the extent of enzymatic action, for both MOIs (OD405nm = 1.44 and 0.98, respectively). These levels of conversion were comparable to that of a “positive control” sample (OD405nm = 1.10) that contained the enzyme β-galactosidase (0.25 pg).
Table 2.
Cytokine production in supernates of 4C8 cells inoculated with genetically engineered HSV
| Concentration (pg/ml) per 105cells/day | ||||||
|---|---|---|---|---|---|---|
| Virus Designation | Cytokine | ELISA Sensitivity (pg/ml) | MOI (PFU/cell) | 24 h | 48 h | 72 h |
| M002 | mIL-12 | 2.5 | 1.0 | 2,048 | 1,999 | 2,080 |
| 0.1 | 1,059 | 2,383 | 2,831 | |||
| R3659* | None | 1.0 | 0 | 0 | 0 | |
| 0.1 | 0 | 0 | 0 | |||
| R8306 | mIL-4 | 2.0 | 1.0 | 8,364 | 8,783 | 10,269 |
| 0.1 | 6,488 | 7,225 | 8,220 | |||
| R3659 | None | 1.0 | <2 | <2 | 2.6 | |
| 0.1 | 2.0 | <2 | 0 | |||
| M004 | GM-CSF | 1.0 | 1.0 | 6,621 | 14,628 | 17,905 |
| 0.1 | 5,421 | 19,518 | 40,316 | |||
| R3659 | None | 1.0 | 0 | 0 | 1.3 | |
| 0.1 | 58.7 | 181.3 | 237.4 | |||
Abbreviations: ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte-macrophage colony-stimulating factor; HSV, herpes simplex virus; mIL, mouse interleukin; MOI, multiplicity of infection; PFU, plaque-forming units.
R3659 is the control parent virus that does not contain any genes for foreign protein expression.
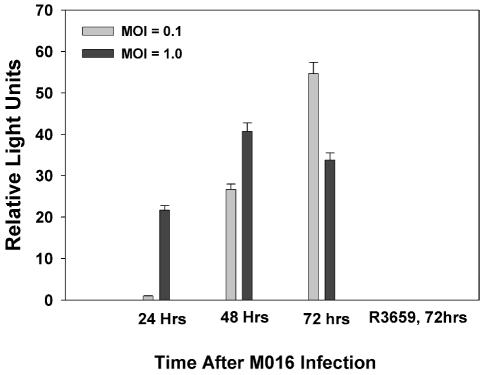
While all other studies were performed with cell supernates, it appears that 4C8 cells infected with a Δγ134.5 HSV encoding the luciferase gene are able to produce a small amount of luciferase, but they are unable to release the enzyme. Harvested supernates did not possess any luciferase activity, but when cell lysates were examined, there was evidence of some luciferase production (Fig. 1). This virus has since been used in studies to follow HSV distribution and persistence in murine tumors using a Xenogen IVIS bioluminescence system (Xenogen Biosciences, Cranbury, N.J.) (data not shown).
Fig. 1.
Luciferase production in infected cells. 4C8 cells were infected with R3659 (control) or M016 luciferase-expressing Δγ134.5 HSV by using two different MOIs (0.1 and 1.0 PFU/cell), and supernates were harvested at 24, 48, or 72 h for determination of secreted luciferase by using a luminometer-based bioluminescence assay as described in the Materials and Methods section. Values shown represent the means of relative bioluminescence readings for three to four individual wells at each condition. Error bars represent standard errors of the mean.
In all of these studies, we noted that at the higher MOI, foreign gene production was greater at the early time points but decayed over time, presumably as infected cells were killed. At the lower MOI, the level of foreign gene production increased steadily, sometimes greatly exceeding the levels seen earlier at the higher MOI of virus. In this latter instance, it is reasonable to assume that only a smaller percentage of cells were initially infected and the amount of foreign gene product increased with time as the infection spread throughout the monolayer.
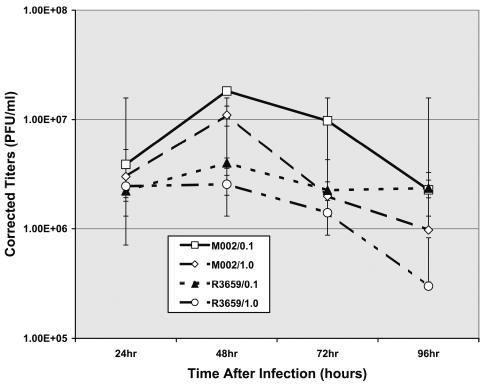
We determined the in vitro replication rates of both R3659 and M002 HSV in 4C8 glioma cells after infection at 0.1 or 1.0 PFU/cell. Virus yield, determined at 24-h intervals, was very similar for both viruses, with a peak production recovered at 48 h after infection (Fig. 2).
Fig. 2.
Replication rates of R3659 and M002 HSV in 4C8 cells. Replication rates were compared in 4C8 mouse glioma cells growing in vitro by infecting cells at 0.1 or 1.0 PFU/cell and harvesting infected cells at 24, 48, 72, or 96 h to recover infectious virus. Mean virus yields in four to eight replicates at each time point were determined by titration on Vero cells and standard deviations determined.
Cell Surface Antigen Expression
We sought to determine the extent to which a population of 4C8 glioma cells expressed MHC class I and II antigens by using FACS analyses. Since these cells were derived from a tumor arising in an F1 hybrid mouse, we employed antibodies that were specific for the haplotypes expressed by the two parental strains, C57BL/6 (H-2b) and DBA/2 (H-2d). Both the K and D antigens of the H-2 complex were identified. In addition, we used antibodies specific for the haplotypes for the Ia (MHC II) complex. FACS analysis showed that a modest number of 4C8 cells possessed components of MHC class I molecules (Table 3). There was more expression of the class I molecule at the H-2D end of the complex in both haplotypes. The 4C8 cells were positive for both H-2b and H-2d haplotypes, as would have been predicted. Moreover, a smaller percentage of 4C8 cells expressed FACS-detectable levels of Ia, the MHC class II molecules. As seen in the class I molecules, the class II haplotypes were also not equally expressed. FACS analysis indicated a majority of the IAd haplotype present, with a smaller amount of the IAb haplotype seen.
Table 3.
FACS analyses of 4C8 cells using fluorescent antibodies specific for MHC class I (H-2) and II (Ia) for haplotypes b and d (% gated cells)
| H-2b | H-2d | IA | ||||
|---|---|---|---|---|---|---|
| Source of Cells | Kb | Db | Kd | Dd | b | d |
| B6D2F1 splenocytes | 54.2 | 54.4 | 59.3 | 56.8 | 15.7 | 24.4 |
| 4C8 cells | 5.7 | 30 | 1 | 9.9 | 1.3 | 9.4 |
Abbreviations: FACS, fluorescence-activated cell sorting; MHC, major histocom-patibility complex.
Since human gliomas have been reported to express the αvβ3 integrins, we also determined the expression of these two integrins independently (Table 4). The majority of 4C8 cells were positive for either αv or β3; these populations clearly overlapped, and almost 99% of cells expressed at least one of the integrins when the two antibodies were examined in combination.
Table 4.
Expression of integrin αv or β3 chains by 4C8 murine glioma cells
| Antibody Specific for | % Gated Cells |
|---|---|
| αv | 83.4 |
| β3 | 76.1 |
| αv or β3 | 99.0 |
FACS analysis of 4C8 cells reacted with monoclonal hamster antibodies to murine αv integrin (CD51) or β3 integrin (CD61) as described in the Methods section.
Intracranial Tumor Studies
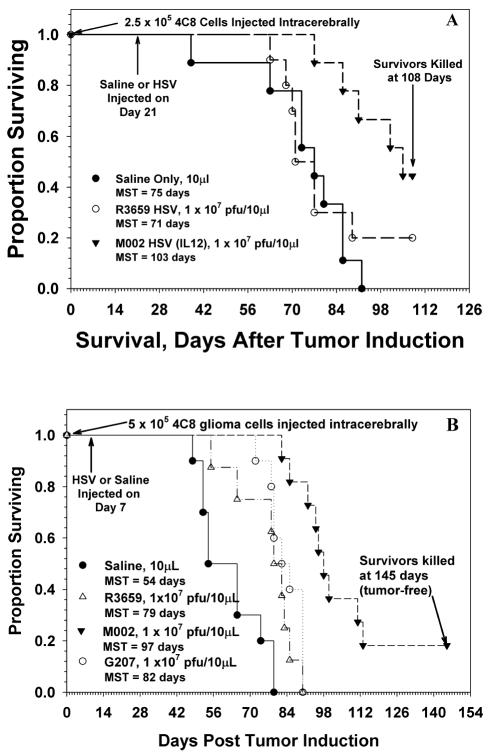
Injection of varying doses of 4C8 cells intracerebrally in B6D2F1 mice yielded dose-dependent survival, with a median survival of 62 days for an injected dose of 1 × 105 cells (data not shown). These tumors initially begin as small, locally invasive lesions with increasing vascularity and eventually become large, space-occupying masses that kill their hosts by increasing intracranial pressure (Fig. 3). We have treated mice beginning at seven days after tumor induction, when the tumors are small and locally invasive (Fig. 3A), or later at 21 days (Fig. 3C), when the tumors are significantly larger with evidence of local and distant spread in the brain. In this latter situation, the in vivo sensitivity of 4C8 cells to HSV therapy with IL-12-expressing virus was compared with saline only or therapy using a backbone Δγ134.5 HSV that does not express foreign proteins, R3659 (Fig. 4A). Mice that received only saline as a therapy had a median survival of 75 days (95% confidence interval [CI], 63–86 days), which was not significantly different from that seen with the R3659 HSV (median survival, 71 days; 95% CI, 68 to >108 days). There were no long-term survivors due to the saline therapy, but 20% of the animals treated with R3659 HSV survived to the sacrifice point (108 days) and were determined histologically to be tumor free. Moreover, we determined that the median and overall survival of 4C8-tumor-bearing mice was increased when the mice were treated with M002, the Δγ134.5 HSV virus that expresses IL-12. Mice treated with the IL-12-secreting HSV (M002) lived significantly longer than saline- or R3659-HSV-treated mice (P = 0.0004 and P = 0.0294, respectively), with a median survival time of >108 days. The number of long-term survivors was increased in M002-treated animals compared with the saline or R3659-HSV-treated animals.
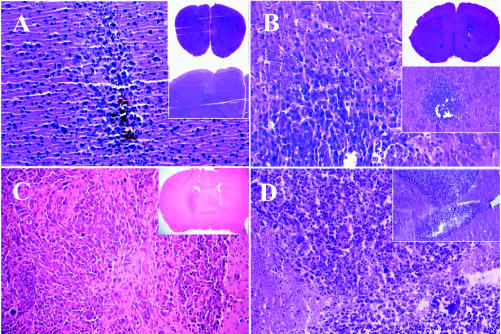
Fig. 3.
Intracranial tumors at 7, 10, 21, and 53 days after tumor induction. Tumors induced by injecting 1 × 105 4C8 cells in 5 μl into the right caudate nucleus of B6D2F1 mice grow slowly, and the injected mice had a median survival of 62 days. At 7 days (panel A) and 10 days (panel B) histologic examination reveals microscopic, moderately invasive tumors (magnification 200×) that infiltrate locally and distantly by day 21 (panel C) and eventually grow to large, space-occupying lesions (panel D, day 53) with evidence of necrosis, multinucleated giant cells, distinctive nuclear pleomorphism, and occasional mitotic cells. Insets in each panel show lower magnifications of each tumor.
Fig. 4.
Survival of mice after intracranial injection of 4C8 glioma cells. A. Gliomas were induced in B6D2F1 mice by intracerebral injection of 2.5 × 105 4C8 followed 21 days later with injection of 10 μl of saline or 1 × 107 PFU of either R3659 (control) or M002 (IL-12). Nine to 11 mice constituted each group. Median survival time (MST) for each treatment group is shown. B. Gliomas were induced in B6D2F1 hybrid mice by intracerebral injection of 5 × 105 4C8 cells in 5 μl of vehicle. Seven days later mice were reoperated, and tumors of nine to 11 mice in each group were injected with 10 μl of saline or one of the virus dilutions. Mice receiving only saline as a therapy had a median survival of 54 days. Those receiving control virus with no interleukin insert (R3659) or the clinical candidate HSV (G207) had median survivals (79 and 82 days, respectively) significantly longer (P = 0.0004 and 0.0092, respectively) than saline-treated mice. Mice treated with the IL-12-secreting HSV (M002) lived significantly longer than mice treated with either saline or non-cytokine-expressing R3659 or G207 HSV (P = 0.00002, 0.0003, and 0.00007, respectively).
We next compared the efficacy of M002 HSV at 1 × 107 PFU with the same dose of R3659 or G207 HSV in this model system, in which the mice were treated seven days after tumor induction, and followed the mice for a much longer period (Fig. 4B). This latter virus has been used clinically in patients (Markert et al., 2000b). In this study, all three viruses significantly prolonged survival of mice injected with R3659 (P = 0.0092), M002 (P = 0.000002), or G207 (P = 0.0004) over mice that received saline intratumorally (median survival, 54 days; 95% CI, 54–65 days). Those that were treated with M002 HSV survived significantly longer (median survival, 97 days; 95% CI, 92–113 days) than those that received R3659 (median survival, 79 days; 95% CI, 65–85 days; P = 0.00007) or G207 (median survival, 82 days; 95% CI, 78–85 days; P = 0.0003), the clinically approved HSV. The survivals for mice that received either R3659 or G207 were not significantly different (P = 0.2133) from each other. M002-HSV-treated mice that were killed at 145 days were determined to be tumor free by histological examination. In both of these studies, nine to 11 mice constituted each group.
FACS Analysis of Tumor-Infiltrating Immune-Related Cells
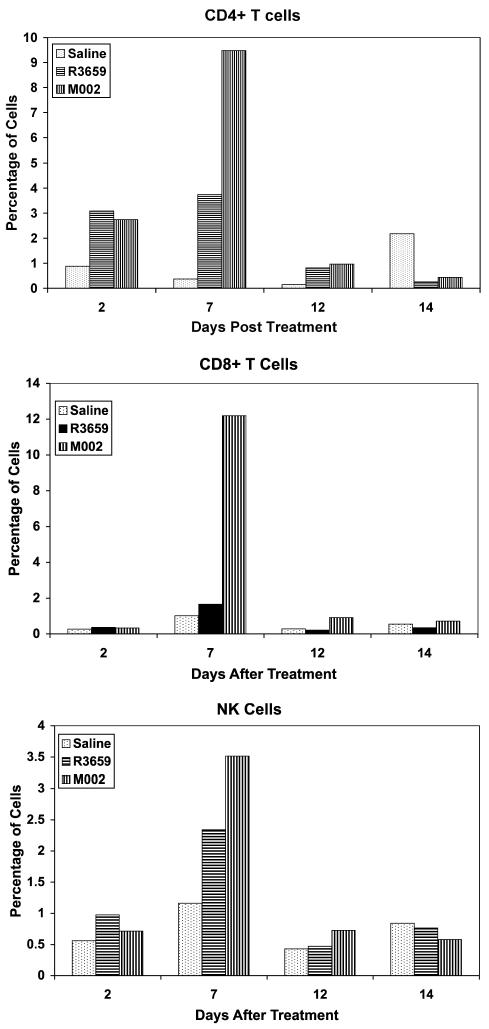
We next sought to determine the relative proportions of CD4+ or CD8+ T cells or NK cells that would infiltrate a 4C8 tumor in response to virus treatment and to determine the kinetics of this response. These studies were initiated in mice bearing 21-day-old tumors to provide a larger tumor mass and to permit adequate time for any local inflammatory response to the initial tumor cell injection to subside. We repeated this study in three separate experiments using four to six time points each between two and 15 days after tumor therapy. The results were qualitatively similar, and the data for one of the experiments that was representative of this pattern are shown in Fig. 5. In all three experiments, there was an increase in CD4+ T, CD8+ T, and NK cells for the tumors injected with either R3659 or M002, but not for the saline-injected tumors. However, the tumors injected with M002 showed the greatest increases in these three cell types, with a peak appearance between six and eight days after virus injection. In two of the three studies, the percentages of CD8+ T cells exceeded those of the CD4+ T cells, similar to the differences seen in Fig. 5.
Fig. 5.
CD4+ T cells, CD8+ T cells, and NK cells infiltrating a 4C8 tumor. B6D2F1 mice were induced with 4C8 gliomas that were allowed to grow for 21 days and were then treated intratumorally by injection of 10 μl of either saline, 1 × 107 PFU R3659, or 1 × 107 PFU M002. Two mice from each group were killed at 2, 7, 12, and 14 days after therapy, and the cerebellums were homogenized and filtered through 170-μm pore Nytex (Tetko, Inc., Elmsford, N.Y.) gauze mesh. Aliquots of each were stained with FITC- or PE-conjugated monoclonal antibodies to CD4 T cells, CD8 T cells, or NK cells (GK1.5, 53-6.7, or anti-CD49b, clone DX-5, respectively [Pharmacia]) and subjected to FACS analyses. Data are expressed as percent of gated cells by using side-scatter and forward-angle light scatter indices from similarly treated B6D2F1 spleen cells to define the gate.
In Situ Viral Replication in 4C8 Gliomas
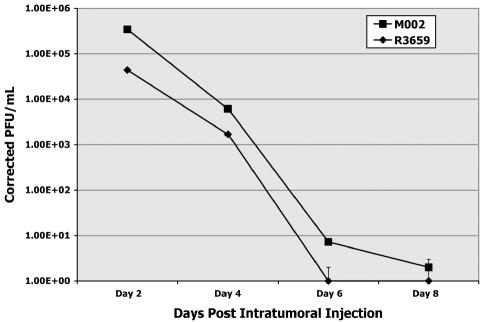
Finally, we quantified the persistence of the R3659 and M002 HSV in 4C8 gliomas growing in the brains of B6D2F1 mice. Virus yields were determined by plaque-titering on Vero cells from brain tumor homogenates prepared 2, 4, 6, and 8 days after virus injection (1 × 107 PFU) into 21-day-old 4C8 gliomas (Fig. 6). Two to three animals were used for each time point. There was a steady and dramatic decline in recovered virus for both, with a 10-fold difference between R3659 and M002 at the first time point (day 2). By day 6, no detectable R3659 HSV was recovered. M002 persisted at very low but detectable levels until day 8.
Fig. 6.
Replication of M002 and R3659 HSV in intracranial 4C8 gliomas, determined at specified time points after intratumoral injection as performed for the efficacy studies. Brains of two to three mice treated with either virus were removed, and the cerebrum of each was dissected free of cerebellum, brain stem, and olfactory bulbs. The cerebrum (both hemispheres) of each mouse was minced separately and homogenized, and serially diluted aliquots were assayed on confluent monolayers of Vero cells to quantify plaque-forming units. These titers were corrected for dilution and averaged for each time point.
Discussion
Despite a plethora of novel and continually evolving therapies, the prognosis for a patient diagnosed with glioblastoma multiforme has remained virtually unchanged for the past six decades. A critical factor in the preclinical evaluation of many of these novel therapies is the requirement that both toxicity and efficacy be established in an appropriately relevant animal model. Ideally, such an animal model to evaluate new glioma therapies would share not only histological features and clinical behavior but also the important molecular characteristics expressed by its human counterpart (Dyer et al., 1995; Weiner et al., 1999). While there will likely not be a consensus on those characteristics that are considered ideal for any animal model of malignant glioma, it is important to continue to evaluate new models as they evolve in the hopes of identifying one or more that approach its human counterpart. 4C8 is a murine glioma cell line that was established from a spontaneous glial neoplasm arising in a transgenic mouse, and the 4C8 cell line resembles a malignant human glioma histologically. When implanted orthotopically in the brains of immunologically competent mice, this cell line induces glioma-like tumors that are uniformly fatal within a reasonable time frame that is consistent with evaluation of therapeutic endeavors (Weiner et al., 1999). We sought to further characterize this cell line to determine if it could be used to evaluate mutant HSV vector therapies preclinically. It should be pointed out that these comparisons between 4C8 and human glioma cells were not chosen to be comprehensive in nature but were highly selective with regard to susceptibility to infection by cytokine-expressing Δγ134.5 HSVs and with regard to expression of class I and II MHC antigens and other selected molecules that might be of consequence in development of an immune response.
The therapeutic use of the HSV-1 viruses that have been genetically engineered to delete both copies of γ134.5, the so-called neurovirulence gene, is currently being intensively explored, both in numerous preclinical studies and in phase 1 clinical trials (Chambers et al., 1995; Markert et al., 1993, 2000a, Markert et al., b). The preclinical studies have focused on establishing intracerebral tumors in immunocompromised (SCID or nude) mice by implanting human glioma cell lines for testing both toxicity and efficacy. Human gliomas, including both established cell lines and tumor explants, have uniformly exhibited susceptibility to HSV-1 infection (Markert et al., 2000a; Martuza et al., 1991).
In a limited number of studies, murine tumors syngeneic with different mouse strains have been employed to evaluate the role of the immune system in this therapeutic approach. Unfortunately, murine gliomas arising in strains of mice that exhibit sensitivity to HSV infection equivalent to that seen in human gliomas are very uncommon (Lopez, 1981). We sought to determine if the 4C8 mouse glioma that is syngeneic with B6D2F1 hybrid mice might be comparable to human gliomas in terms of susceptibility to genetically engineered Δγ134.5 HSV.
We have demonstrated that 4C8 is sensitive to oncolysis by Δγ134.5 HSV by showing that any of a large panel of Δγ134.5 HSVs that we have produced can infect and kill these cells effectively at low MOI. In contrast to the highly variable sensitivity of the GL261 glioma of C57BL/6 mice to these same viruses, 4C8 cells were uniformly susceptible to infection and to direct oncolysis in vitro. This suggests that this cell line could be productively employed in a valid animal model for testing new HSV therapies.
Many of the Δγ134.5 HSV constructs we have made contain inserts of foreign (nonviral) genes that encode other genes for immune-modulating molecules or suicide enzymes. It was necessary to determine the ability of 4C8 cells to support the production and secretion of these foreign gene products in vitro. Our data showed that 4C8 cells sustain production of the cytokines specific for each virus. The levels of most of the cytokines produced were thought to be sufficient to be able to result in a physiological response in vivo. After 72 h of incubation, all supernates except the supernate of cells infected with M002 (virus with insert for mouse interleukin-12) contained several nanograms of cytokines per milliliter (Table 2). Interestingly, we observed in almost all instances that lower MOI (0.1 PFU/cell) yielded higher amounts of foreign gene products at 72 h. We speculate that, at the higher MOI of 1 PFU/cell, all cells were infected early and were lysed by the virus before significant amounts of foreign gene product could accumulate in the supernates. However, the lower MOI permitted some cells to escape initial infection but to be infected subsequently, yielding a higher secreted yield of foreign gene product. Replication kinetics of both the R3659 and M002 HSVs in 4C8 cells growing in vitro confirms the notion that in this closed system at lower MOI, virus yields, although lower, persist for a longer time period because fewer cells are infected initially, and this leaves more uninfected cells to support the continued spread of the infection.
Taken together, these in vitro studies of oncolysis and foreign gene expression support our hypothesis that HSV viral therapy of tumors could potentially be more effective if two mechanisms were employed, namely, (1) direct oncolysis of infected cells and (2) secretion of cytokines that would attract cells of the immune system in a paracrine fashion to destroy surviving tumor cells.
Given that the induction of an immune response to the tumor itself is key to this combined modality approach to glioma therapy, it was necessary to define the MHC expression profile of 4C8 tumor cells. Clearly, the presence of major histocompatibility molecules would govern the role of this glioma cell line in eliciting an immune response in concert with specific immune-modulating therapies. We sought the presence of class I (H-2) and class II (Ia) MHC molecules using fluorochrome-conjugated monoclonal antibodies directed to either d or b haplotypes, basing our search on the report that 4C8 cells were from an F1 hybrid (B6D2F1) mouse. B6 mice possess the H-2Kb, H-2Db (MHC class I), and IAb (MHC class II) haplotypes. DBA/2 (D2) mice possess the H-2Kd, H-2Dd, and IAd haplotypes. Our analyses revealed a modest number of MHC class I components, but not the entire complex. There was greater expression of epitopes encoded by the H-2D end of the MHC than by those of the H-2K end. For MHC class I molecules, the expression was approximately equal for both haplotypes, which was expected. However, this was not the case with class II MHC expression. There was unequal expression in two haplotypes, and fewer cells possessed class II markers. This result of higher class I expression than class II is paralleled by results from recent studies in human gliomas. Human glioma cells have relatively low but constant levels of expression of class I molecules, although expression of β2-microglobulin of MHC class I is upregulated in human glioblastoma cells. Similar to 4C8 cells, MHC class II molecules are not highly expressed on human glioblastoma cells (Parney et al., 2000). This difference in expression could be due to a change that 4C8 cells undergo as they move from in situ to cell culture. In most instances, long-term culture of human glioma cells results in loss of MHC class II expression, and that may be what has occurred with the 4C8 cells. Notably, these cells were not exposed to either α interferon or γ-interferon, which can upregulate expression of MHC class I or class II molecules, respectively.
In addition to MHC antigens on the cell surface, we determined that the majority of 4C8 cells possess the integrin chains αv and β3. The integrin αvβ3, along with αvβ5, is known to play a role in the angiogenic process of tumors. Antibodies to murine β5 were not available for us to assess expression of murine β5. Almost all human glioma cells possess either of these two integrins, and the possibility of inducing apoptosis via binding a cyclic peptide, RGDfV, to this integrin is being explored as a possible nontoxic therapy (Chatterjee et al., 2000). Preliminary data would suggest 4C8 cells are also sensitive to the apoptosis-inducing effects of cyclic RGDfV peptides (G.Y. Gillespie, unpublished observations). The presence of αvβ3 on 4C8 cells makes it an appropriate animal model to further investigate these therapies as well.
With regard to the in vivo sensitivity of 4C8 cells to Δγ134.5 HSV-based therapies, we showed that the median survival of mice treated with just the backbone, parent Δγ134.5 HSV was lengthened in a dose-dependent fashion. The increase in length of survival was significant for the higher dose employed, and the overall effect was somewhat consistent with results we have reported on several occasions using human glioma cell lines transplanted in the brains of SCID or nude mice (Andreansky et al., 1996, 1997; Chambers et al., 1995; Markert et al., 2000a). By comparison, treatment of 4C8 glioma bearers with the IL-12-secreting Δγ134.5 HSV showed a dramatic improvement over treatment with the parent virus, with increases in the median survival and the proportion of long-term survivors (“cures”), both in a dose-dependent fashion. As we originally observed with the IL-12-secreting virus in A/J mice bearing Neuro-2A brain tumors (Parker et al., 2000), there was a significant increase in antitumor effectiveness that exceeded what would have been expected for the viral oncolytic effect alone. Presumably, this increase is related to an antitumor immune response induced by the secreted IL-12. Our FACS analysis of the CD4+ T, CD8+ T, and NK cells infiltrating 4C8 gliomas after injection of HSV suggested that either virus could elicit an inflammatory response, but the M002 HSV expressing IL-12 elicited the strongest CD4, CD8, and NK cell infiltration into these tumors. Although no functional (i.e., cytotoxic) studies were performed with these tumor-infiltrating cells, it is presumed that they did exert some antitumor effect, especially in the mice with smaller tumors, where tumor burden was minimal, because the survival of these mice was prolonged over those that received the R3659 HSV, which did not produce IL-12. However, further studies to explore these effects are currently under way.
Of concern to the future application of γ134.5-deleted HSV for the treatment of gliomas in patients, we observed that the persistence of either R3659 or M002 after infection of 4C8 cells growing in the brains of B6D2F1 mice had a rapid decay, essentially disappearing by six to eight days. It would seem that there should have been ample uninfected tumor cells within these tumors to maintain continued viral replication, yet the virus yields diminished rather rapidly. The nature of this phenomenon is currently the subject of intensive investigation in our labs, with the goal of finding methods to maintain viral persistence in tumors for the optimum oncolytic effect. This loss of infectious virus may have many causes, for example, development of an interferon-mediated antivirus response, physical impediments to virus spread as large areas of necrotic cells are created, development of antiviral innate or adaptive cellular or humoral immunity, generation of reactive oxygen metabolites, or production of nitrogen oxide. These are all potential candidates for antivirus effects within gliomas. Many of these causes have been extensively described (Wakimoto et al., 2003, 2004), and some parameters have even been mathematically modeled (Wu et al., 2004). The low growth rate of the 4C8 glioma cells also poses a human-like barrier, given that human gliomas characteristically have a growth fraction that is much lower (2%–45%) than that seen with in vitro cultured human glioma cell lines. The relevance is that γ134.5-deleted HSVs absolutely require a proliferating cell host to support viral replication.
In summary, we have determined that the 4C8 glioma cell line and its implantation in the brains of syngeneic B6D2F1 mice exhibit many of the desirable characteristics that we seek in a biologically relevant and predictive experimental murine glioma model. This transplantable tumor model affords a relatively inexpensive and highly useful preclinical evaluation tool in that it provides a more “human-like” glioma in a therapeutically sensitive mouse host.
Acknowledgments
We thank Charissa Dyer (E.K. Shriver, New York, N.Y.) for her kind gift of 4C8, James Malter (University of Wisconsin, Madison, Wis.) for the hGM-AUGUA plasmid, and Medigene, Inc., for providing G207. The authors also thank Suzanne Randall and Sharon Samuel for excellent technical assistance and Marion Spell of the University of Alabama at Birmingham, AIDS flow cytometry facility, for performing the FACS analyses.
Footnotes
Studies performed by the authors were initiated and supported under Program Project Grants PO1 AI 24009 (R.J.W.) and CA71933 (R.J.W., G.Y.G.) and training grant NHLBI T34 HL07473 (E.K.H.) from the National Institutes of Health. This work was also supported in part by the State of Alabama and the National Institute for Neurologic Disorders and Stroke Mentored Clinical Scientist Development Award (1K08NSO1942 [J.M.M.]), the American Association for Neurological Surgeons Young Investigator Award (J.M.M.), the American Cancer Society Institutional Awards (J.M.M.), the American Brain Tumor Association (J.M.M.), a Pediatric Brain Tumor Foundation of the United States Fellowship grant (J.N.P.), and a grant from the Brain Tumor Society (J.N.P.).
U.S. Patent 6,764,675, entitled Herpes simplex virus expressing foreign genes and method for treating cancers therewith, was issued on July 10, 2004, to R.J. Whitley, J.M. Markert, G.Y. Gillespie, and J.N. Parker at the University of Alabama.
Abbreviations used are as follows: B6D2F1, C57BL/6 × DBA/2 F1 mouse strain; CI, confidence interval; DMEM, Dulbecco’s Modified Eagle’s Medium; ELISA, enzyme-linked immunosorbent assay; FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; GM-CSF, granulocyte-macrophage colony-stimulating factor; HSV, herpes simplex virus; IL, interleukin; MHC, major histocompatibility complex; MOI, multiplicity of infection; OD, optical density; PE, phycoerythrin; PFU, plaque-forming unit; SCID, severe combined immunodeficient; TD, toxic dose.
References
- Andreansky SS, He B, Gillespie GY, Soroceanu L. Markert, J. Chou, J. Roizman, B. and, Whitley RJ. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313–11318. doi: 10.1073/pnas.93.21.11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreansky S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509. [PubMed] [Google Scholar]
- Andreansky S, He B, van Cott J, McGhee J, Markert JM, Gillespie GY, Roizman B, Whitley RJ. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- Ashley DM, Sampson JH, Archer GE, Hale LP, Bigner DD. Local production of TGF beta1 inhibits cerebral edema, enhances TNF-alpha induced apoptosis and improves survival in a murine glioma model. J Neuroimmunol. 1998;86:46–52. doi: 10.1016/s0165-5728(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Barth RF. Rat brain tumor models in experimental neuro-oncology: The 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gillespie GY, Soroceanu L, Andreansky S, Chatterjee S, Chou J. Roizman, B. and, Whitley RJ. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Matsumura A, Schradermeier J, Gillespie GY. Human malignant glioma therapy using anti-alpha(v)beta3 integrin agents. J Neurooncol. 2000;46:135–144. doi: 10.1023/a:1006444300504. [DOI] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer CA, Philibotte T. A clone of the MOCH-1 glial tumor in culture: Multiple phenotypes expressed under different environmental conditions. J Neuropathol Exp Neurol. 1995;54:852–863. doi: 10.1097/00005072-199511000-00012. [DOI] [PubMed] [Google Scholar]
- Frei K, Piani D, Malipiero UV, Van Meir E, de Tribolet N, Fontana A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992;148:3140–3146. [PubMed] [Google Scholar]
- Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, Buhring HJ, Dichgans J, Rammensee HG, Steinle A, Weller M. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- Lagunoff M, Roizman B. Expression of a herpes simplex virus 1 open reading frame antisense to the gamma134.5 gene and transcribed by an RNA 3′ coterminal with the unspliced latency-associated transcript. J Virol. 1994;68:6021–6028. doi: 10.1128/jvi.68.9.6021-6028.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. Resistance to herpes simplex virus - type 1 (HSV-1) Curr Top Microbiol Immunol. 1981;92:15–24. doi: 10.1007/978-3-642-68069-4_2. [DOI] [PubMed] [Google Scholar]
- Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: A review. Rev Med Virol. 2000a;10:17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hutner WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000b;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Martinerie C, Huff V, Joubert I, Badzioch M, Saunders G, Strong L, Perbal B. Structural analysis of the human nov proto-oncogene and expression in Wilms tumor. Oncogene. 1994;9:2729–2732. [PubMed] [Google Scholar]
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T. Hunter W.D, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999;155:1557–1567. doi: 10.1016/S0002-9440(10)65472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parney IF, Farr-Jones MA, Chang LJ, Petruk KC. Human glioma immunobiology in vitro: Implications for immunogene therapy. Neurosurgery. 2000;46:1169–1177. doi: 10.1097/00006123-200005000-00030. [DOI] [PubMed] [Google Scholar]
- Pilkington, G.J., and Lantos, P.L. (1990) Pathology of experimental brain tumours. In: Thomas, D.G.T.T. (Ed.), Neuro-Oncology: Primary Malignant Brain Tumours London: Edward Arnold Publishers, pp. 51–76.
- Rajagopalan LE, Burkholder J.K, . Turner J, Culp J, Yang NS, Malter JS. Granulocyte-macrophage colony-stimulating factor mRNA stabilization enhances transgenic expression in normal cells and tissues. Blood. 1995;86:2551–2558. [PubMed] [Google Scholar]
- Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner NE, Pyles RB, Chalk CL, Balko MG, Miller MA, Dyer CA, Warnick RE, Parysek LM. A syngeneic mouse glioma model for study of glioblastoma therapy. J Neuropathol Exp Neurol. 1999;58:54–60. doi: 10.1097/00005072-199901000-00007. [DOI] [PubMed] [Google Scholar]
- Wu JT, Kirn DH, Wein LM. Analysis of a three-way race between tumor growth, a replication-competent virus and an immune response. Bull Math Biol. 2004;66:605–625. doi: 10.1016/j.bulm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Tanaka R, Saitoh T, Okoshi S. Cytokine gene expression on glioma cell lines and specimens. J Neurooncol. 1994;21:243–247. doi: 10.1007/BF01063773. [DOI] [PubMed] [Google Scholar]