Abstract
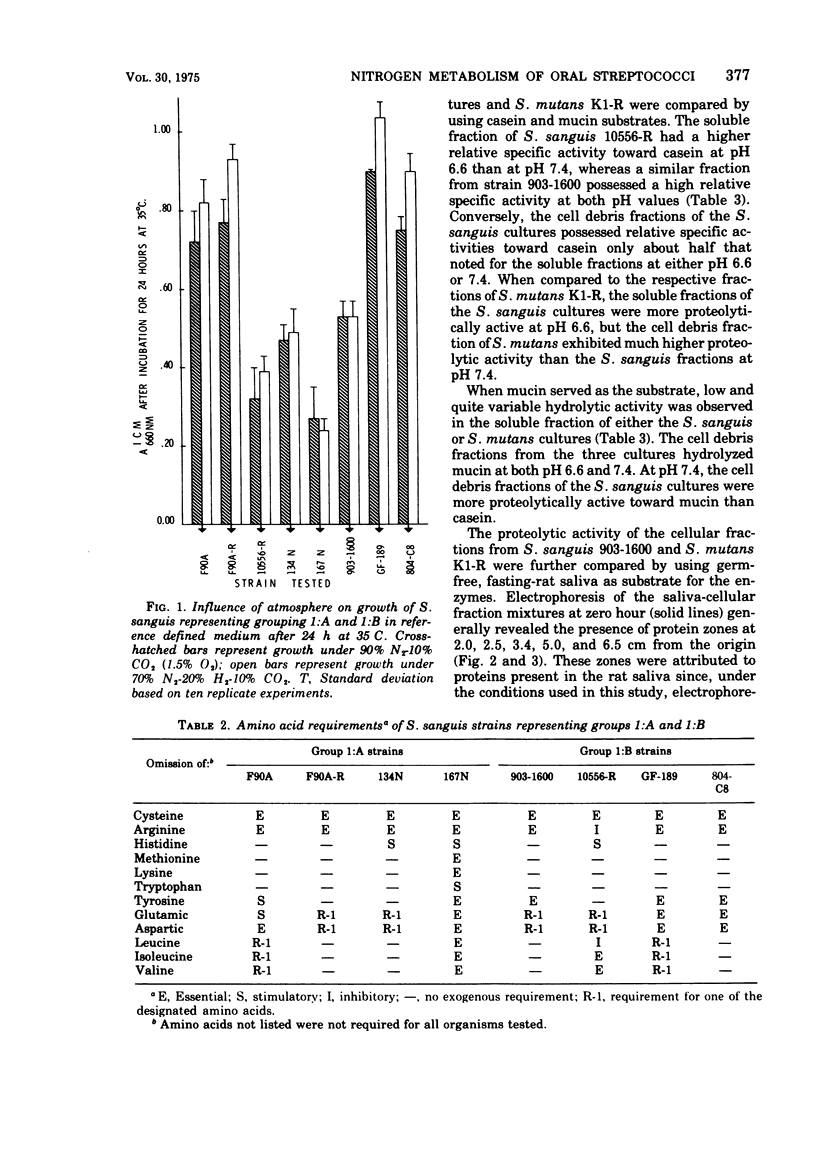
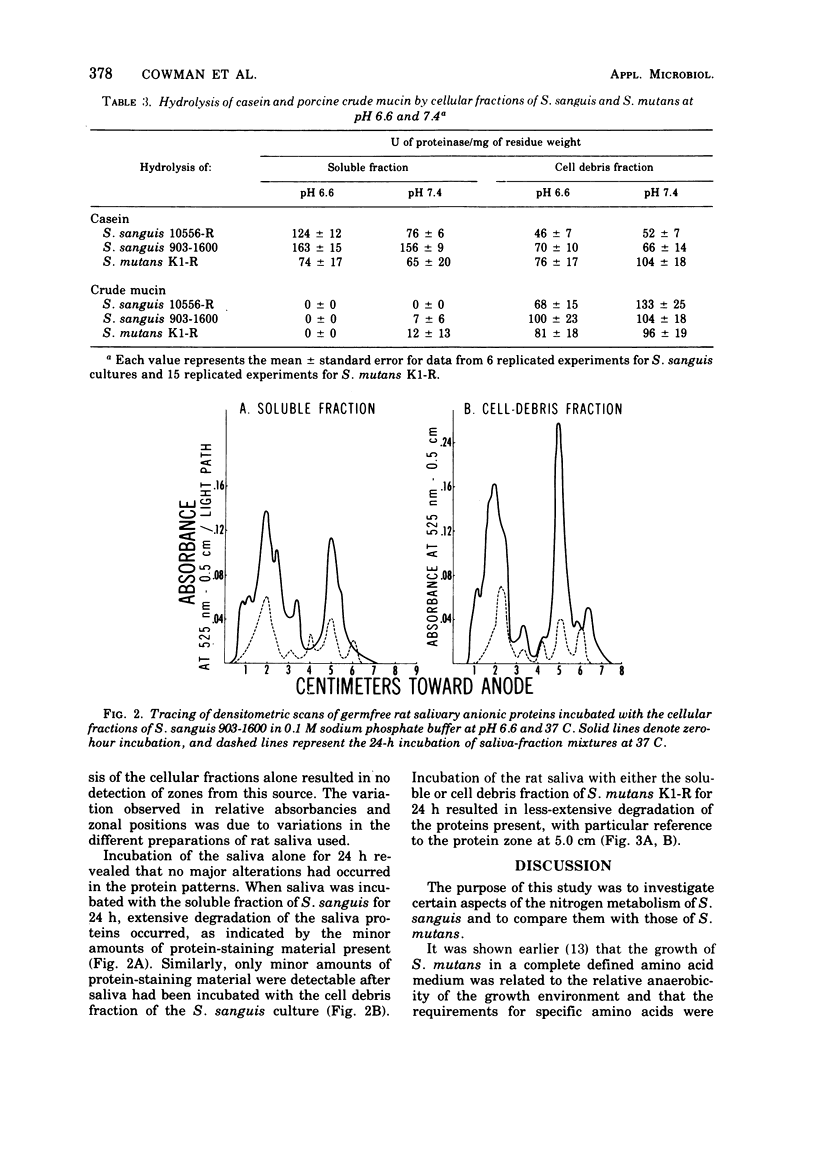
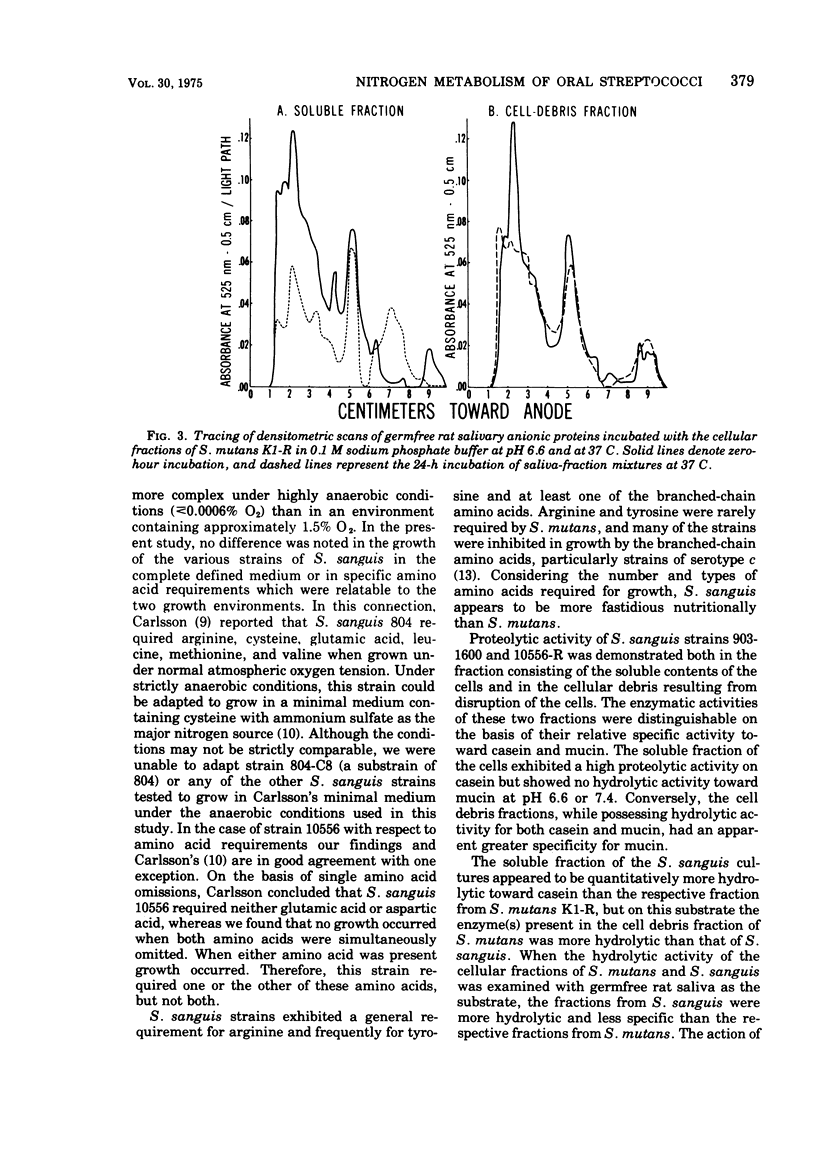
The growth response of Streptococcus sanguis groups 1:A and 1:B in a complete chemically defined medium was not influenced by the oxygen concentration of the growth atmosphere. All of the cultures required cysteine and arginine; tyrosine and branched-chain amino acids were frequently required. Proteolysis of casein, mucin, and the anionic proteins of germfree rat saliva by S. sanguis was demonstrated. Hydrolytic activity toward casein was found in the soluble contents of the cells and in the cellular debris after disruption of the cells, with the soluble fractions exhibiting greater proteolytic activity toward casein. The soluble fractions from S. sanguis did not hydrolyze mucin, but this substrate was hydrolyzed by the cell debris fraction. When the amino acid requirements and proteolytic activity of S. sanguis and S. mutans were compared, these two oral streptococcal species exhibited distinct and characteristic differences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENARDE M. A., FABIAN F. W., ROSEN S., HOPPERT C. A., HUNT H. R. A method for the collection of large quantities of rat saliva. J Dent Res. 1956 Apr;35(2):326–327. doi: 10.1177/00220345560350022801. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Brewer J. H., Allgeier D. L. Safe Self-contained Carbon Dioxide-Hydrogen Anaerobic System. Appl Microbiol. 1966 Nov;14(6):985–988. doi: 10.1128/am.14.6.985-988.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J. Chemically defined medium for growth of Streptococcus sanquis. Caries Res. 1970;4(4):297–304. doi: 10.1159/000259652. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus mutans. Caries Res. 1970;4(4):305–320. doi: 10.1159/000259653. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Nutritional requirements of Streptococcus sanguis. Arch Oral Biol. 1972 Sep;17(9):1327–1332. doi: 10.1016/0003-9969(72)90165-3. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Cowman R. A., Fitzgerald R. J. Effects of oral streptococci on electrophoretic properties of human salivary anionic proteins. J Dent Res. 1975 Mar-Apr;54(2):298–303. [PubMed] [Google Scholar]
- Cowman R. A., Perrella M. M., Fitzgerald R. J. Influence of incubation atmosphere on growth and amino acid requirements of Streptococcus mutans. Appl Microbiol. 1974 Jan;27(1):86–92. doi: 10.1128/am.27.1.86-92.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., JORDAN H. V., STANLEY H. R. Experimental caries and gingival pathologic changes in the gnotobiotic rat. J Dent Res. 1960 Sep-Oct;39:923–935. doi: 10.1177/00220345600390052701. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J. Prevalence of Streptococcus mutans on various tooth surfaces in Negro children. Arch Oral Biol. 1971 Oct;16(10):1237–1240. doi: 10.1016/0003-9969(71)90053-7. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- KRASSE B. THE EFFECT OF THE DIET ON THE IMPLANTATION OF CARIES-INDUCING STREPTOCOCCI IN HAMSTERS. Arch Oral Biol. 1965 Mar-Apr;10:215–221. doi: 10.1016/0003-9969(65)90022-1. [DOI] [PubMed] [Google Scholar]
- Melvaer K. L., Helgeland K., Rölla G. A charged component in purified polysaccharide preparations from Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1974 Jul;19(7):589–595. doi: 10.1016/0003-9969(74)90077-6. [DOI] [PubMed] [Google Scholar]
- Sorrells K. M., Cowman R. A., Swaisgood H. E. Hydrolysis of alpha(s, 1)-Casein B by Streptococcus lactis Membrane Proteinase. J Bacteriol. 1972 Oct;112(1):474–479. doi: 10.1128/jb.112.1.474-479.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON W. T., TOVE S. B., SPECK M. L. EXTRACELLULAR PROTEINASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1964 Jan;87:49–53. doi: 10.1128/jb.87.1.49-53.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



