Abstract
A number of reports have indicated an increasing incidence of primary brain tumors over the past few decades. The purpose of this study was to describe incidence rate trends in a population-based series of newly diagnosed primary nonmalignant and malignant brain and other CNS tumors, contributing five additional years to previously published incidence trends. Data for the years 1985 through 1999 from six collaborating state cancer registries of the Central Brain Tumor Registry of the United States were used to determine incidence trends in the broad age groups 0–19, 20–64, and ⩾65 years, overall and for selected histologies. Multiplicative Poisson regression was used to express trends as average annual percent change (AAPC). Joinpoint regression was used to identify sharp changes in incidence occurring over this period. Overall, incidence increased modestly (AAPC, 1.1; 95% CI, 0.8–1.4). When brain lymphomas were excluded, this increase remained statistically significant. A sharp change in incidence of brain lymphomas from increasing to decreasing over time was identified. Specific histologies that were increasing included anaplastic astrocytomas in individuals aged ⩾65 years, microscopically confirmed gliomas in both adult age groups, and microscopically confirmed glioma, not otherwise specified (NOS), in children. Increases that were not specific to any population subgroup were seen for oligodendrogliomas, ependymomas, meningiomas, and nerve sheath tumors. Decreases were noted for astrocytoma, NOS, nonmicroscopically confirmed gliomas, and pituitary tumors. Improvements in diagnosis and classification are likely reflected in the decreasing trends in unspecified glioma subgroups and the accompanying increasing trends in more specific glioma subgroups. However, increases in meningiomas and nerve sheath tumors deserve further attention.
Keywords: brain tumors, epidemiology, incidence, trends
Reports from the late 1980s and early 1990s indicated that brain tumor incidence was increasing over time in children (Blair and Birch, 1994; Bunin et al., 1996; Gurney et al., 1996; Hjalmers et al., 1999; McKinney et al., 1994; Smith et al., 1998) as well as in the elderly (Ahsan et al., 1995; Greig et al., 1990; Legler et al., 1999; Mao et al., 1991; Polednak, 1991; Polednak and Flannery, 1995; Werner et al., 1995). Study results have included overall increases (Blair and Birch, 1994; Bunin et al., 1996; Greig et al., 1990; Gurney et al., 1996; Mao et al., 1991; McKinney et al., 1994; Polednak, 1991; Werner et al., 1995) as well as histology-specific increases (Blair and Birch, 1994; Bunin et al., 1996; Gurney et al., 1996; Hjalmers et al., 1999; McKinney et al., 1994; Werner et al., 1995). Some authors have attributed the increase in brain tumor incidence to improvements in diagnostic technology (Davis et al., 1991; Desmeules et al., 1992; Legler et al., 1999, Mosso et al., 1992; Radhakrishnan et al., 1995; Smith et al., 1998; Steinberg, 1986; Steinberg et al., 1985). However, whether the entire increase in brain tumor incidence can be attributed to these changes is still debated (Desmeules et al., 1992; Forman, 1999; Legler et al., 1999; Smith et al, 1998; Varner, 1999).
A recent report on the incidence trends of adult primary intracerebral tumors in Denmark, Finland, Norway, and Sweden suggested that the average annual increase in incidence overall was 0.6% for men and 0.9% for women between 1969 and 1998 (Lönn et al., 2004a). The increase in incidence was confined to the late 1970s and early 1980s, coinciding with the introduction and widespread use of improved diagnostic methods, and was most apparent in the 60- to 79-year age group. The authors go on to report that since 1983 the incidence of adult primary intracerebral tumors has remained relatively stable for both men and women. Another recent study that investigated incidence trends of childhood and adult brain/CNS tumors in Norway from 1970 through 1999 reported that the overall rate increased during the study period. A continuing increase in the five-year period up to 1999 may be seen in the age groups 0–4 years and ⩾60 years, but the study indicated a trend of leveling off in incidence of most tumor categories (Johannesen et al., 2004).
This present report describes overall and histology-specific incidence rate trends in a population-based series of primary benign and malignant brain and other CNS tumors in the United States, and it presents trends specific to certain age groups, gender, race, and microscopic confirmation. Our previously published incidence trends using data of the Central Brain Tumor Registry of the United States (CBTRUS)3 (Jukich et al., 2001) showed the incidence rate of brain/CNS tumors to have increased 0.9% per year between 1985 and 1994. Here, we contribute five additional years of tumor registry data to determine whether incidence rates in the United States are following the pattern of stabilization of rates seen in the European registries or whether they are continuing to change over time.
Materials and Methods
Data and Variable Definitions
Details of the data compilation have been described previously (Jukich et al., 2001). Briefly, the six following population-based state cancer registries provided data on all incident primary brain tumors diagnosed in the entire 15-year period of 1985 through 1999 and were compiled as part of CBTRUS: the Connecticut Tumor Registry, Delaware State Tumor Registry, Cancer Data Registry of Idaho, Massachusetts Cancer Registry, Montana Central Tumor Registry, and Utah Cancer Registry. Primary tumors with the following topography codes from the International Classification of Diseases for Oncology (ICDO) were included in the analysis (Percy et al., 1990): C70.0 to C70.9 (meninges), C71.0 to C71.9 (brain), C72.0 to C72.9 (spinal cord, cranial nerves, and other parts of the CNS), and C75.1 to C75.3 (pituitary gland, craniopharyngeal duct, and pineal gland). The histology subgroups, with corresponding ICDO four-digit histology codes, were defined as follows (Percy et al., 1990): glioblastoma (9440–9442, 9481); astrocytoma, NOS (9400); anaplastic astrocytoma (9401, 9411); diffuse astrocytoma (9410, 9420); pilocytic astrocytoma (9421–9422); oligodendroglioma (9450); anaplastic oligodendroglioma (9451, 9460); medulloblastoma/primitive neuroectodermal tumor (8963, 9363–9364, 9470–9473, 9501–9503); ependymoma, total (9391–9394); mixed glioma (9382); glioma, NOS (9380); meningioma (9530–9534, 9537–9539); nerve sheath (9540–9541, 9550, 9560–9561, 9570); pituitary (8022, 8040, 8140, 8146, 8246, 8260, 8270–8271, 8280–8281, 8290, 8300, 8310, 8323, 8333–8334); craniopharyngioma (9350); lymphoma (9590–9595, 9650, 9652–9655, 9659, 9661–9665, 9667, 9670–9677, 9680–9687, 9690–9696, 9698, 9701–9702, 9705–9707, 9711–9714, 9720, 9723, 9731, 9740–9741, 9766, 9827, 9830, 9861, 9930, 9970); and neoplasm, unspecified (8000–8004, 8010, 8021). All primary tumors of the brain/CNS with ICDO behavior codes 0 (benign), 1 (uncertain), and 3 (malignant) were included. Available information included year of diagnosis, age at diagnosis, gender, race, ICDO site code, and diagnostic confirmation. Data quality was assessed by use of EditWriter and EDITS software developed by the Centers for Disease Control and Prevention to edit cancer registry data (CDC, 1997). Data were edited by using a brain tumor–specific EDITS metafile, a compiled database that contains all the logic and values required to check fields of data for validity (e.g., identify illogical or impossible site, morphology, and/or behavior combinations).
Population data for each region and U.S. standard population data were obtained from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute Web site (http://seer.cancer.gov/popdata) and were made available by the U.S. Census Bureau. Incidence rates were age-adjusted by using the direct method and, by five-year age groupings, were standardized to the year 2000 U.S. standard population.
Tumors were considered “confirmed” or “not confirmed’ according to the SEER Program Code Manual (Fritz and Ries, 1998): Diagnostic confirmation codes indicating positive histology (1), positive cytology, no positive histology (2), and positive microscopic confirmation, method not specified (4) were considered microscopically confirmed. Codes indicating positive laboratory test/marker study (5), direct visualization without microscopic confirmation (6), radiography and other imaging techniques without microscopic confirmation (7), clinical diagnosis only (8), and unknown whether or not microscopically confirmed (9) were considered not microscopically confirmed.
Statistical Analysis
Descriptive information was tabulated for total numbers of tumors by age group, gender, race, tumor behavior, histology, and diagnostic confirmation. The proportion of microscopically confirmed tumors was tabulated for each year by state and age group.
Multiplicative Poisson regression, in which the log incidence rate (outcome variable) was calculated as a linear function of the variables and the data was assumed to have the Poisson error distribution, was used to statistically compare trends over time (Estève et al., 1994). Results using rates as an outcome variable in the expression model are commonly expressed as the average percent change over each time period (based on the parameter estimate for time in the model). Average annual percent change (AAPC) is a measure of the estimated yearly percent change in incidence rates over a specified time interval. Trends in histology groups were individually assessed, and factors that may influence rates by affecting classification (e.g., NOS categories) or diagnostic accuracy (e.g., microscopic confirmation) were evaluated. Models were fit by using the PROC GENMOD procedure in the SAS software system, release 8.02 (SAS Institute, 1999). The independent variable of interest was time, expressed as year of tumor diagnosis and coded as a continuous variable. Predictors were age group, gender, race, and microscopic confirmation. Age group was coded as a categorical variable for the broad age groups of 0–19, 20–64, and ⩾65 years old. Indicator variables were used for gender, race, and microscopic confirmation. Trends were expressed as AAPC over the 15-year period, with corresponding two-sided 95% CIs. Models were fit for total brain tumors and for the histologic subgroups. Hierarchical models with two-way and/or three-way interaction terms for year with age group, gender, race, and microscopic confirmation were assessed. When interaction terms were statistically significant (P < 0.05), separate AAPCs were listed for the fully adjusted model (without interaction terms), as well as for the appropriate age group, gender, or race strata (with the statistically significant interaction terms). When data became too sparse for stratification and/or three-way interaction, the highest level of stratification/interaction that gave informative results was reported. All final models were adjusted for age group, gender, race, and microscopic confirmation. Selected age-specific and age-adjusted estimates were graphed to illustrate trends in incidence over time. Incidence rates for CBTRUS data have been published elsewhere (CBTRUS, 2004–2005; Jukich, et al., 2001; Surawicz et al., 1999, 2000).
Joinpoint regression software was obtained from the Web site of the Statistical Research Applications Branch of the National Cancer Institute (srab.cancer.gov/joinpoint) and was used to identify any sharp changes in incidence occurring over the period analyzed (Kim et al., 2000). Joinpoints correspond to the point in time of a change in trend where several different lines come to a juncture. The software fits the simplest joinpoint model that the data will allow using a series of permutation tests.
Results
Description of Data
The data included 25,258 primary brain and other CNS tumors (Table 1). Nine percent of the tumors occurred in children (0–19 years), while the majority (53%) occurred in the 20- to 64-year age group, and the remaining 38% occurred in the elderly (⩾65 years). The percentage of brain tumors in this data was similar by gender, but occurrence was primarily in white persons because of the population composition of the participating states.
Table 1.
Characteristics by histology of primary brain and other CNS tumors diagnosed in 1985–1999 from six U.S. state cancer registriesa
|
Age at Diagnosis (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Histology | Total (N) | Female (%) | White (%) | 0–19 | 20–64 | ⩾65 | % Micro. Confirmed |
| Glioblastoma | 6,169 | 44 | 97 | 1 | 48 | 51 | 92 |
| Astrocytoma, total | 4,108 | 45 | 95 | 20 | 55 | 25 | 97 |
| Oligodendroglioma | 770 | 46 | 95 | 9 | 79 | 12 | 98 |
| Medulloblastoma/PNET | 477 | 40 | 93 | 70 | 29 | 1 | 99 |
| Ependymoma | 475 | 39 | 93 | 29 | 62 | 9 | 98 |
| Mixed glioma | 279 | 42 | 94 | 11 | 79 | 10 | 100 |
| Glioma, NOS | 830 | 49 | 94 | 24 | 38 | 38 | 45 |
| Meningiomab | 5,283 | 72 | 91 | 1 | 49 | 50 | 83 |
| Nerve sheathb | 1,098 | 54 | 92 | 3 | 76 | 21 | 93 |
| Pituitaryb | 1,430 | 50 | 92 | 5 | 68 | 27 | 87 |
| Craniopharyngiomab | 139 | 53 | 91 | 26 | 58 | 16 | 96 |
| Lymphoma | 1,041 | 42 | 90 | 2 | 61 | 37 | 90 |
| Neoplasm, unspecified | 951 | 55 | 95 | 7 | 28 | 65 | 20 |
| Totalc | 25,258 | 52 | 94 | 9 | 53 | 38 | 86 |
Abbreviations: NOS, not otherwise specified; micro., microscopically; PNET, primitive neuroectodermal tumor.
Study population was taken from population-based state cancer registries in Connecticut, Delaware, Idaho, Massachusetts, Montana, and Utah.
Includes data from five of six registries.
Includes histologies not listed in the table.
Overall, 86% of the tumors had a microscopically confirmed diagnosis. The percentage fluctuated from a low of 83% in 1986 to a high of 88% in 1995 and showed no significant changes by registry or era. Microscopic confirmation differed by tumor histology, ranging from 20% for neoplasm, unspecified, to 100% for mixed glioma. Overall incidence rates for brain/CNS tumors by year of diagnosis, with and without brain lymphomas, and by race are presented in Fig. 1.
Fig. 1.
Incidence rates of primary brain tumors by calendar year and ethnicity from six U.S. state cancer registries. Rates are age-adjusted to the 2000 U.S. standard population.
Overall Incidence Trends
The results of Poisson regression analysis for all primary brain/CNS tumors, with and without lymphomas, are listed in Table 2. Overall, the incidence of all primary brain/CNS tumors was modestly increasing, with an AAPC of 1.1% (95% CI, 0.8–1.4). When brain lymphomas were removed from the analysis, the overall increase in incidence remained statistically significant, though the trend was specific to whites. There were no significant interactions for age, gender, and microscopic confirmation for all primary brain/CNS tumors combined.
Table 2.
Overall incidence rate trends by AAPC for primary brain tumors from six U.S. state cancer registries*
| Subgroup | Number of Cases | AAPC (%) | 95% CLs for AAPC |
|---|---|---|---|
| All brain/CNS tumors | 25,258 | 1.1 | 0.8, 1.4 |
| All brain/CNS tumors, excluding lymphoma | 24,217 | 0.9 | 0.6, 1.2 |
| White | 22,795 | 1.0 | 0.7, 1.3 |
| Nonwhite | 1,422 | −0.5 | −1.7, 0.7 |
Abbreviation: AAPC, average annual percentage change; CLs, confidence limits.
All models are Poisson regression and were adjusted for age group, gender, race, and microscopic confirmation.
Histology-Specific Incidence Trends
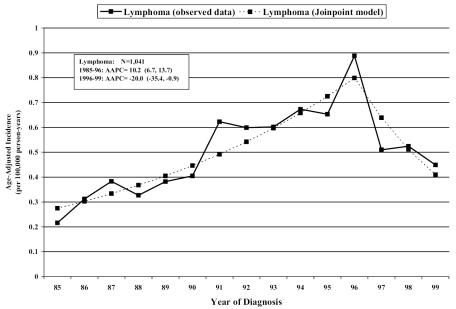
The results of Poisson regression analysis for histology-specific brain/CNS tumors are presented in Tables 3 and 4. The incidence of oligodendrogliomas, ependymomas, and brain lymphomas was increasing overall. Joinpoint regression analysis identified a sharp change in incidence over time for brain lymphomas (Fig. 2), with an increase in incidence observed from 1985 to 1996 (AAPC, 10.2; 95% confidence limits [CLs], 6.7, 13.7) and a decrease in incidence from 1996 to 1999 (AAPC, −20.0; 95% CLs, −35.4, −0.9). There were no significant trends specific to age, gender, race, or microscopic confirmation for these tumors.
Table 3.
Overall incidence rate trends by AAPC for gliomas and major subtypes from six U.S. state cancer registries
| Subgroup | Number of Cases | AAPC (%) | 95% CLs for AAPC |
|---|---|---|---|
| All gliomas | 12,701 | 0.8 | 0.4, 1.2 |
| Astrocytoma, total | 4,108 | −3.3 | −4.0, −2.6 |
| Glioblastoma | 6,169 | 2.6 | 2.0, 3.2 |
| Oligodendroglioma | 770 | 9.5 | 7.8, 11.3 |
| Ependymoma | 475 | 3.1 | 1.1, 5.3 |
| Mixed glioma | 279 | 4.4 | 1.7, 7.2 |
| Glioma, NOS | 830 | −1.3 | −2.9, 0.3 |
Abbreviations: AAPC, average annual percentage change; CLs, confidence limits; NOS, not otherwise specified.
Table 4.
Histology-specific incidence rate trends by AAPC from six U.S. state cancer registries
| Histology | Number of Cases | AAPC (%) | 95% CLs for AAPC |
|---|---|---|---|
| All gliomas, by age group | |||
| 0–19, Micro. conf. | 1162 | 0.0 | −1.2, 1.3 |
| 0–19, Not micro. conf. | 151 | −4.8 | −6.6, −3.0 |
| 20–64, Micro. conf. | 6419 | 0.8 | 0.2, 1.3 |
| 20–64, Not micro. conf. | 299 | −4.1 | −5.6, −2.6 |
| ⩾65, Micro. conf. | 4027 | 2.2 | 1.5, 2.9 |
| ⩾65, Not micro. conf. | 643 | −2.7 | −4.1, −1.3 |
| Anaplastic astrocytoma | 1059 | 2.0 | 0.6, 3.4 |
| Ages 0–19 | 78 | −4.8 | −10.0, 0.4 |
| Ages 20–64 | 688 | 0.9 | −0.9, 2.6 |
| Ages ⩾65 | 293 | 6.7 | 3.9, 9.4 |
| Pilocytic astrocytomaa | 328 | 10.3 | 7.6, 13.0 |
| Diffuse astrocytoma | 229 | −1.4 | −4.4, 1.6 |
| Ages 0–19 | 46 | −7.7 | −14.7, −9.7 |
| Ages 20–64 | 142 | −0.8 | −4.6, 3.0 |
| Ages ⩾65 | 41 | 3.7 | −3.4, 11.1 |
| Astrocytoma, NOS | 2262 | −9.0 | −10.0, −8.0 |
| Ages 0–19 | 324 | −10.2 | −12.9, −7.6 |
| Ages 20–64 | 1277 | −9.1 | −10.5, −7.8 |
| Ages ⩾65 | 661 | −8.1 | −9.9, −6.3 |
| Glioblastoma | |||
| Ages 0–19 | 65 | −1.5 | −7.1, 4.1 |
| Ages 20–64 | 2956 | 2.0 | 1.2, 2.9 |
| Ages ⩾65 | 3148 | 3.2 | 2.4, 4.0 |
| Micro. conf. | 5696 | 3.0 | 2.4, 3.6 |
| Not micro. conf. | 473 | −2.3 | −4.4, −0.3 |
| Oligodendroglioma (including anaplastic oligodendroglioma) | |||
| Ages 0–19 | 66 | 0.5 | −5.0, 6.1 |
| Ages 20–64 | 611 | 10.6 | 8.6, 12.6 |
| Ages ⩾65 | 93 | 9.6 | 4.6, 14.7 |
| Oligodendroglioma | 617 | 6.9 | 5.1, 8.8 |
| Anaplastic oligodendroglioma | 153 | 22.3 | 17.7, 27.2 |
| Mixed glioma | |||
| Ages 0–19 | 31 | −9.9 | −18.7, −1.6 |
| Ages 20–64 | 220 | 6.0 | 2.9, 9.2 |
| Ages ⩾65 | 28 | 8.4 | −0.5, 17.7 |
| Glioma, NOS | |||
| Ages 0–19, Micro. conf. | 64 | 6.4 | 2.5, 10.4 |
| Ages 0–19, Not micro. conf. | 137 | 0.2 | −3.2, 3.5 |
| Ages 20–64, Micro. conf. | 194 | −0.1 | −3.0, 2.8 |
| Ages 20–64, Not micro. conf. | 120 | −6.4 | −9.7, −3.1 |
| Ages ⩾65, Micro. conf. | 116 | 1.7 | −1.7, 5.0 |
| Ages ⩾65, Not micro. conf. | 199 | −4.6 | −7.4, −1.7 |
| Meningiomab | 5283 | 1.5 | 0.9, 2.1 |
| Micro. conf. | 4394 | 1.0 | 0.3, 1.7 |
| Not micro. conf. | 889 | 4.1 | 2.5, 5.6 |
| Nerve sheathb | 1098 | 3.7 | 2.3, 5.1 |
| Micro. conf. | 1016 | 2.5 | 1.1, 4.0 |
| Not micro. conf. | 82 | 21.0 | 14.9, 27.6 |
| Craniopharyngiomab | 139 | −0.5 | −4.4, 3.3 |
| Pituitaryb,c | 1430 | −1.8 | −3.0, −0.6 |
| Ages 0–19, white | 66 | 2.2 | −3.2, 7.6 |
| Ages 20–64, white | 894 | 0.3 | −1.2, 1.8 |
| Ages 20–64, nonwhite | 84 | −11.2 | −15.7, −6.8 |
| Ages ⩾65, white | 355 | −4.6 | −6.9, −2.2 |
Abbreviations: AAPC, average annual percentage change; CLs, confidence limits; micro. conf., microscopically confirmed.
Analysis of pilocytic astrocytomas was restricted to 0- to 19-year-olds.
Includes data from five of six registries.
Sample size of ages 0–19 and ages ⩾65 nonwhite subgroups too small to allow for reporting of statistics.
Fig. 2.
Joinpoint regression analysis from six U.S. state cancer registries identifying sharp changes in incidence over time.
The incidence of total astrocytomas (AAPC, −3.3; 95% CLs, −4.0, −2.6 [Table 3]) and neoplasm, unspecified (AAPC, −3.3; 95% CLs, −4.8, −1.9 [data not shown]) was decreasing overall. Driving the decrease in total astrocytoma incidence is the significantly decreasing incidence of astrocytoma, NOS (AAPC, −9.0; 95% CLs, −10.0, −8.0), both overall and within each of the three age groups (Table 4).
Gliomas had specific trends for age group and microscopic confirmation. Overall, gliomas in all age groups showed a statistically significant decrease in incidence for nonmicroscopically confirmed tumors (AAPC of −4.8, −4.1, and −2.7 for ages 0–19, 20–64, and ⩾65, respectively), while microscopically confirmed gliomas were significantly increasing both in the adults (AAPC, 0.8) and in the elderly (AAPC, 2.2). Mixed gliomas showed an overall increase in incidence (AAPC, 4.4), although the trend was significantly decreasing in children (AAPC, −9.9) and increasing in adults aged 20–64 (AAPC, 6.0). Incidence of microscopically confirmed glioma, NOS, was increasing in children (AAPC, 6.4), while incidence of nonmicroscopically confirmed glioma, NOS, was decreasing in the adult age groups (20–64 years; ⩾65 years), with AAPCs of −6.4 and −4.6, respectively.
Trends for selected glioma histologies and for astrocytoma subtypes are shown in Fig. 3. Overall, incidence of total astrocytomas was decreasing (AAPC, −3.3), which was largely a result of the decrease in astrocytoma, NOS (AAPC, −9.0). When excluding NOS, the incidence rate of astrocytomas was statistically significantly increasing overall and in all age groups (data not shown). The overall incidence rate of anaplastic astrocytomas was increasing, specifically in the elderly (AAPC, 6.7). Incidence of pilocytic astrocytomas was significantly increasing in children (AAPC, 10.3), while incidence of diffuse astrocytomas was significantly decreasing in children (AAPC, −7.7). Incidence of microscopically confirmed glioblastomas was increasing (AAPC, 3.0), while incidence was decreasing for those not microscopically confirmed (AAPC, −2.3).
Fig. 3.
A. Incidence rates by calendar year from six U.S. state cancer registries. A. Selected glioma subtypes. B. Astrocytomas.
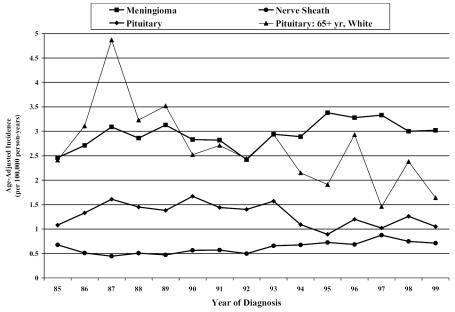
The overall incidence of meningiomas and nerve sheath tumors was increasing for both microscopically and nonmicroscopically confirmed tumors (Fig. 4). No age-, gender-, or race-specific trends were significant for these tumor histologies. Pituitary tumors, overall, expressed a significant decrease in incidence (Fig. 4). The decreasing trend was significant for nonwhites in all age groups and for whites ⩾65 years.
Fig. 4.
Incidence rates by calendar year for predominantly nonmalignant histologies from six U.S. state cancer registries.
Discussion
These analyses include all primary brain tumors regardless of behavior, allowing an analysis of trends in subgroups that are of mixed behavior or are primarily benign. A modest increase in all primary brain tumors (1.1% per year) was found and is consistent with previously published incidence trends of CBTRUS data from the years 1985–1994 (Jukich et al., 2001). This overall increase in incidence persisted after exclusion of brain lymphomas. Although the trend was specific to whites, trends in nonwhites were more unstable because of small numbers in each year. With joinpoint regression analysis, a sharp change in incidence over time was identified for lymphomas, which are likely influenced by HIV/AIDS trends. This observed decrease in incidence corresponds with a 1973–1998 SEER data report by Kadan-Lottick et al. (2002) which found that by 1998 the incidence rates of primary CNS lymphomas in the United States had decreased from a peak in 1995, especially in men under 60 years of age. This trend mirrors the decrease in AIDS rates from their peak in 1993, as well as the introduction of more effective antiretroviral therapies (Aalen et al., 1999).
Gliomas showed trend variations by age group for the histology subgroups of total gliomas; diffuse, pilocytic, and anaplastic astrocytomas; oligodendrogliomas; mixed glioma; and glioma, NOS. Overall increases in incidence for this period were observed for glioblastomas, mixed gliomas, ependymomas, and oligodendrogliomas. For five of the six registries, registry-specific AAPCs for glioblastomas were increasing, similar to the overall AAPC, varying only in magnitude of the trend, while one registry showed no significant increase, which indicated consistency of the trends across regions. Small numbers of cases prevented accurate registry-specific AAPCs for other tumor histologies. Some of the increase in incidence of oligodendrogliomas may be the result of significant attention being focused by neuropathologists on these tumors that have been shown to be chemosensitive and treatable in up to 70% of anaplastic and aggressive oligodendroglioma cases (Cairncross et al., 1992; Paleologos et al., 1999). Since oligodendrogliomas have a more favorable prognosis than other astrocytic tumors, a relaxation of the diagnostic criteria, possibly in an effort “not to impede any patient from gaining a possible benefit of chemotherapy,” has resulted in more oligodendrogliomas being diagnosed (Burger, 2002; Reifenberger and Louis, 2003).
Overall negative time trends were observed for total astrocytomas and astrocytoma, NOS, over the 15-year period, although astrocytoma subtypes of pilocytic and anaplastic showed statistically significant increases in incidence. A recent analysis of SEER data found that the incidence of adult gliomas increased between 1977 and 2000, especially among the oldest patients included in the study (Hess et al., 2004). With results similar to those from our analysis of CBTRUS data, the SEER analysis of adult gliomas demonstrated overall increases in incidence over time for glioblastomas and oligodendrogliomas, as well as anaplastic astrocytomas, and decreases in incidence for astrocytoma, NOS. However, the authors go on to report that their analysis indicated that brain cancer incidence may be stabilizing, which could suggest that glioma incidence increases may be an artifact.
Analysis of predominantly nonmalignant histologies indicated a decrease in incidence over this period for pituitary tumors. In a study of brain tumor incidence by histologic subtypes in Japan for the period 1973–1993, pituitary tumors showed rapid increases during the early 1980s and reached a plateau in trend afterward (Kaneko et al., 2002). The periods of the CBTRUS data overlap with only the latter years of the Japanese data, where the plateau was reached, and extend to more recent years. There have been no other reports of a decrease in pituitary tumor incidence in the most recent periods. Meningiomas and nerve sheath tumors demonstrated increases in incidence over the 1985–1999 period, trends that persisted upon analysis of microscopically confirmed cases. Relative increases in incidence rates for nonmicroscopically confirmed meningiomas and nerve sheath tumors might reflect the greater tendency to diagnose such tumors with MRI and follow them with periodic scans to monitor growth rate, rather than proceed directly to surgery. A few studies of cellular telephone use and nerve sheath tumors have now been completed (Christensen et al., 2004; Hardell et al., 2003; Inskip et al., 2001; Lönn et al., 2004b; Muscat et al., 2002) with no clear consensus emerging. As cellular telephone technology is relatively new, we do not yet have the long-term follow-up on their possible biological effects. The increase in incidence of nerve sheath tumors needs more investigation, as no causative factors have been identified (Propp et al., 2006 [this issue]).
Major changes in brain tumor classification have occurred that may have influenced the reporting of brain tumor data. For example, with regard to nerve sheath tumors, the second edition of the ICDO, published in 1990, differed from the first edition in that tumors occurring in the cranial nerves were split into four topography codes as opposed to being grouped into one topography code (Percy et al., 1990). In addition, the World Health Organization revised its histology classification scheme for tumors of the CNS (Kleihues et al., 1993a). This is a guideline used by neuropathologists for histologic typing of brain tumors (Kleihues et al., 1993b). Several of the new entities in the 1993 WHO recoding did not have corresponding four-digit histology codes in the ICDO at that time (Kleihues et al., 1993a). Histologic criteria have also changed over time, and depending on the experience and preference of the pathologist, differential classification of glial tumors may occur (Aldape et al., 2000; Castillo et al., 2004; Coons et al., 1997; Giannini et al., 2001). Exactly how these changes have affected the reporting of brain tumor data and histology-specific incidence rates remains unclear.
Fifteen years of recent data does not allow for the analysis of historical incidence trends that may have been impacted by diagnostic improvements that occurred over the last 25–30 years. Also, pre-1990 incidence rates may have been affected by revisions in the WHO histologic classification scheme for CNS tumors and changes in ICDO coding. Histologic criteria, which are both qualitative and subjective, have also changed over time. Variation in diagnosis, coding, and reporting practices is possible between each of the six CBTRUS collaborating state cancer registries included in this analysis. Nevertheless, AAPCs for meningiomas and nerve sheath tumors by registry either were in the same direction as the overall AAPC for that histology or showed no statistically significant trend, with the only variation being in magnitude, but not direction, of trend. In addition, the large increases in incidence rates over this 15-year period for nonmicroscopically confirmed meningiomas and nerve sheath tumors may reflect changes in case ascertainment. Awareness of the importance of benign brain tumor collection was brought to the forefront by CBTRUS with their initial survey of registries in 1992 (Carol Kruchko, CBTRUS, personal communication) and may have influenced active ascertainment of these tumors, although this issue deserves further attention. Finally, these data results largely reflect patterns for the Northeastern region of the United States, based on the populations of the contributing states, and are likely more informative for whites than for other racial groups.
Despite these possible limitations, this analysis contributes 15 years of brain/CNS tumor incidence data, the majority of which represent the period following the introduction and widespread use of diagnostic improvements such as CT and MRI scans. CBTRUS data includes information on all primary brain/CNS tumors, both malignant and benign, offering a more comprehensive analysis of temporal trends in incidence. Furthermore, this report adds to the limited amount of population-based data on brain/CNS tumors overall and, in particular, the categories of predominantly nonmalignant tumors and neoplasm, unspecified tumors. Beginning in January 2004, the Benign Brain Tumor Cancer Registries Amendment Act (Public Law 107–260) required all cancer surveillance registries to expand their primary brain tumor data collection to include tumors of benign and uncertain behavior. In the future, incidence rate trend data will be available for all primary (malignant and nonmalignant) brain tumors from the entire U.S. population.
This analysis documented a modest overall increase in brain/CNS tumor incidence rates, which remained even when excluding brain lymphomas. By using join-point regression analysis, incidence of brain lymphomas was found to be decreasing from 1996 to 1999, which may be a result of HIV/AIDS trend influence. Negative trends seen for glioma, NOS, and astrocytoma, NOS, tumor incidence, as well as the corresponding positive incidence trends in some glioma and astrocytoma subgroups, most likely reflect improvements in diagnostic technology and classification. However, increases seen in ependymomas, meningiomas, and nerve sheath tumors are less likely to be related to improvements in diagnosis and deserve further attention.
Acknowledgments
The authors gratefully acknowledge the Central Brain Tumor Registry of the United States and the collaborators at state registries who provided data for this analysis: Anthony Polednak, Connecticut Tumor Registry; Leroy Hathcock, Delaware State Tumor Registry; Stacey Carson, Cancer Data Registry of Idaho; Susan Gershman, Massachusetts Cancer Registry; Debbi Lemons, Montana Central Tumor Registry; and Rosemary Dibble, Utah Cancer Registry.
Footnotes
This work was conducted under the financial support of the Central Brain Tumor Registry of the United States.
Abbreviations used are as follows: AAPC, average annual percentage change; CBTRUS, Central Brain Tumor Registry of the United States; CLs, confidence limits; ICDO, International Classification of Diseases for Oncology; NOS, not otherwise specified; SEER, Surveillance, Epidemiology, and End Results.
References
- Aalen OO, Farewell VT, De Angelis D, Day NE, Gill ON. New therapy explains the fall in AIDS incidence with a substantial rise in number of persons on treatment expected. AIDS. 1999;13:103–108. doi: 10.1097/00002030-199901140-00014. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Neugut AI, Bruce JN. Trends in incidence of primary malignant brain tumors in USA, 1981–1990. Int J Epidemiol. 1995;24:1078–1085. doi: 10.1093/ije/24.6.1078. [DOI] [PubMed] [Google Scholar]
- Aldape K, Simmons ML, Davis RL, Miike R, Wiencke J, Barger G, Lee M, Chen P, Wrensch M. Discrepancies in diagnoses of neuroepithelial neoplasms: The San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88:2342–2349. [PubMed] [Google Scholar]
- Blair V, Birch JM. Patterns and temporal trends in the incidence of malignant disease in children: II. Solid tumours of childhood. Eur J Cancer. 1994;30A:1498–1511. doi: 10.1016/0959-8049(94)00275-a. [DOI] [PubMed] [Google Scholar]
- Bunin GR, Feuer EJ, Witman PA, Meadows AT. Increasing incidence of childhood cancer: Report of 20 years experience from the Greater Delaware Valley Pediatric Tumor Registry. Paediatr Perinat Epidemiol. 1996;10:319–338. doi: 10.1111/j.1365-3016.1996.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Burger PC. What is an oligodendroglioma? Brain Pathol. 2002;12:257–259. doi: 10.1111/j.1750-3639.2002.tb00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross JG, Macdonald DR, Ramsay DA. Aggressive oligodendroglioma: A chemosensitive tumor. Neurosurgery. 1992;31:78–82. doi: 10.1227/00006123-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Castillo MS, Davis FG, Surawicz T, Bruner JM, Bigner S, Coons S, Bigner DD. Consistency of primary brain tumor diagnoses and codes in cancer surveillance systems. Neuroepidemiology. 2004;23:85–93. doi: 10.1159/000073980. [DOI] [PubMed] [Google Scholar]
- CBTRUS, Central Brain Tumor Registry of the United States (2004–2005) Primary Brain Tumors in the United States, Statistical Report, 1997–2001, Years Data Collected Chicago: Central Brain Tumor Registry of the United States.
- Christensen HC, Schuz J, Kosteljanetz M, Poulsen HS, Thomsen J, Johansen C. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159:277–283. doi: 10.1093/aje/kwh032. [DOI] [PubMed] [Google Scholar]
- Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Davis DL, Ahlbom A, Hoel D, Percy C. Is brain cancer mortality increasing in industrialized countries? Am J Ind Med. 1991;19:421–431. doi: 10.1002/ajim.4700190402. [DOI] [PubMed] [Google Scholar]
- Desmeules M, Mikkelsen T, Mao Y. Increasing incidence of primary malignant brain tumors: Influence of diagnostic methods. J Natl Cancer Inst. 1992;84:442–445. doi: 10.1093/jnci/84.6.442. [DOI] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention (1997) EDITS [computer software]. Version 2.00. Atlanta, Ga.: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Available at http://www.cdc.gov/cancer/npcr/edits
- Estève, J., Benhamou, E., and Raymond, L. (1994) Statistical Methods in Cancer Research, Volume IV. Descriptive Epidemiology Lyon: International Agency for Research on Cancer. [PubMed]
- Forman AD. Re: Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1999;91:648–649. doi: 10.1093/jnci/91.7.648. [DOI] [PubMed] [Google Scholar]
- Fritz, A.G., and Ries, L.A. (Eds.) (1998) The SEER Program Code Manual, 3rd ed. Bethesda, MD: Cancer Statistics Branch, Surveillance Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health.
- Giannini C, Scheithauer BW, Weaver AL, Burger PC, Kros JM, Mork S, Graeber MB, Bauserman S, Buckner JC, Burton J, Riepe R, Tazelaar HD, Nascimento AG, Crotty T, Keeney GL, Pernicone P, Altermatt H. Oligodendrogliomas: Reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol. 2001;60:248–262. doi: 10.1093/jnen/60.3.248. [DOI] [PubMed] [Google Scholar]
- Greig NG, Ries LG, Yancik R, Rapoport SI. Increasing annual incidence of primary malignant brain tumors in the elderly. J Natl Cancer Inst. 1990;82:1621–1624. doi: 10.1093/jnci/82.20.1621. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Davis S, Severson RK, Fang JY, Ross JA, Robison LL. Trends in cancer incidence among children in the US. Cancer. 1996;78:532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hardell L, Hansson Mild K, Sandstrom M, Carlberg M, Hallquist A, Pahlson A. Vestibular schwannoma, tinnitus and cellular telephones. Neuroepidemiology. 2003;22:124–129. doi: 10.1159/000068745. [DOI] [PubMed] [Google Scholar]
- Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- Hjalmers U, Kulldorff M, Wahlqvist Y, Lannering B. Increased incidence rates but no space-time clustering of childhood astrocytoma in Sweden, 1973–1992. Cancer. 1999;85:2077–2090. [PubMed] [Google Scholar]
- Inskip PD, Tarone RE, Hatch EE, Wilcosky TC, Shapiro WR, Selker RG, Fine HA, Black PM, Loeffler JS, Linet MS. Cellular-telephone use and brain tumors. N Engl J Med. 2001;344:79–86. doi: 10.1056/NEJM200101113440201. [DOI] [PubMed] [Google Scholar]
- Johannesen TB, Angell-Andersen E, Tretli S, Langmark F, Lote K. Trends in incidence of brain and central nervous system tumors in Norway, 1970–1999. Neuroepidemiology. 2004;23:101–109. doi: 10.1159/000075952. [DOI] [PubMed] [Google Scholar]
- Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-Oncology. 2001;3:141–151. doi: 10.1093/neuonc/3.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95:193–202. doi: 10.1002/cncr.10643. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Nomura K, Yoshimura T, Yamaguchi N. Trend of brain tumor incidence by histological subtypes in Japan: Estimation from the Brain Tumor Registry of Japan, 1973–1993. J Neurooncol. 2002;60:61–69. doi: 10.1023/a:1020239720852. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates [erratum in Stat Med. (2001) 20, 655]. . Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kleihues, P., Burger, P.C., and Scheithauer, B.W. (1993a) Histologic Typing of Tumours of the Central Nervous System, 2nd ed. Berlin: Springer-Verlag.
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumors. Brain Pathol. 1993b;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS, Linet MS. Brain and other central nervous system cancers: Recent trends in incidence and mortality [erratum in J. Natl. Cancer Inst. (1999) 91, 1693]. . J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- Lönn S, Klaeboe L, Hall P, Mathiesen T, Auvinen A, Christensen HC, Johansen C, Salminen T, Tynes T, Feychting M. Incidence trends of adult primary intracerebral tumors in four Nordic countries. Int J Cancer. 2004a;108:450–455. doi: 10.1002/ijc.11578. [DOI] [PubMed] [Google Scholar]
- Lönn S. Ahlbom A, Hall P, Feychting M. Mobile phone use and the risk of acoustic neuroma. Epidemiology. 2004b;15:653–659. doi: 10.1097/01.ede.0000142519.00772.bf. [DOI] [PubMed] [Google Scholar]
- Mao Y, Desmeules M, Semenciw RM, Hill G, Gaudette L, Wigle DT. Increasing brain cancer rates in Canada. Can Med Assoc J. 1991;145:1583–1591. [PMC free article] [PubMed] [Google Scholar]
- McKinney PA, Ironside JW, Harkness EF, Arango JC, Doyle D, Black RJ. Registration quality and descriptive epidemiology of childhood brain tumours in Scotland 1975–90. Br J Cancer. 1994;70:973–979. doi: 10.1038/bjc.1994.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosso ML, Colombo R, Giordano L, Pastore G, Terracini B, Magnani C. Childhood Cancer Registry of the Province of Torino, Italy: Survival, incidence, and mortality over 20 years. Cancer. 1992;69:1300–1306. doi: 10.1002/cncr.2820690538. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Malkin MG, Shore RE, Thompson S, Neugut AI, Stellman SD, Bruce J. Handheld cellular telephones and risk of acoustic neuroma. Neurology. 2002;58:1304–1306. doi: 10.1212/wnl.58.8.1304. [DOI] [PubMed] [Google Scholar]
- Paleologos NA, Macdonald DR, Vick NA, Cairncross JG. Neoadjuvant procarbazine, CCNU, and vincristine for anaplastic and aggressive oligodendroglioma. Neurology. 1999;53:1141–1143. doi: 10.1212/wnl.53.5.1141. [DOI] [PubMed] [Google Scholar]
- Percy, C., Van Holten, V., and Muir, C.M. (Eds.) (1990) International Classification of Diseases for Oncology, 2nd ed. Geneva: World Health Organization.
- Polednak AP. Time trends in incidence of brain and central nervous system cancers in Connecticut. J Natl Cancer Inst. 1991;83:1679–1681. doi: 10.1093/jnci/83.22.1679. [DOI] [PubMed] [Google Scholar]
- Polednak AP, Flannery JT. Brain, other central nervous system, and eye cancer. Cancer. 1995;75 (suppl.):330–337. doi: 10.1002/1097-0142(19950101)75:1+<330::aid-cncr2820751315>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Propp JM, McCarthy BJ, Davis FG, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro-Oncology. 2006;8:1–11. doi: 10.1215/S1522851704001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37:67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Louis DN. Oligodendroglioma: Toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- SAS Institute (1999) SAS System software. Release 8.02. Cary, N.C.: SAS Institute, Inc.
- Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- Steinberg EP. The status of MRI in 1986: Rates of adoption in the United States and elsewhere. Am J Roentgenol. 1986;147:453–455. doi: 10.2214/ajr.147.3.453. [DOI] [PubMed] [Google Scholar]
- Steinberg EP, Sisk JE, Locke KE. X-ray CT and magnetic resonance imagers. Diffusion patterns and policy issues. N Engl J Med. 1985;313:859–864. doi: 10.1056/NEJM198510033131405. [DOI] [PubMed] [Google Scholar]
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG the collaborating registries of the Central Brain Tumor Registry of the United States. Descriptive epidemiology of primary brain and CNS tumors: Results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro-Oncology. 1999;1:14–25. doi: 10.1093/neuonc/1.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surawicz TS, McCarthy BJ, Jukich PJ, Davis FG. The accuracy and completeness of primary brain and central nervous system tumor data: Results from the Central Brain Tumor Registry of the United States. J Registry Manage. 2000;27:51–55. [Google Scholar]
- Varner A. Re: Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1999;91:973–974. doi: 10.1093/jnci/91.11.973a. [DOI] [PubMed] [Google Scholar]
- Werner MH, Phuphanich S, Lyman GH. The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer. 1995;76:1634–1642. doi: 10.1002/1097-0142(19951101)76:9<1634::aid-cncr2820760921>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]