Abstract
We compare survival estimates for population-based glioma cases by using two diagnostic coding schemes, (1) the International Classification of Diseases, Oncology, second edition (ICD-O-2) as reported by the Surveillance, Epidemiology, and End Results (SEER) program and (2) central neuropathology review diagnosis based on the World Health Organization II classification. In addition, among review categories, we estimate survival in relation to several patient demographic and treatment factors. Eligible cases included adults residing in the San Francisco Bay SEER Area with newly diagnosed, histologically confirmed glioma during the years 1991–1994 and 1997–1999. The study group included participating subjects for whom subsequent central neuropathology review confirmed glioma. We determined treatments, vital status, and other factors by using registry, interview, medical record, and active follow-up data. Survival differences between anaplastic astrocytoma (AA) and astrocytoma were apparent from review diagnoses (median months of survival for AA, 13.0 [95% CI, 9.9–19.5], and astrocytoma, 101.3 [95% CI lower limit, 42.1; upper limit not yet reached]), but not with ICD-O-2 diagnoses reported by SEER (median months of survival for AA, 16.6 [95% CI, 12.0–20.7], and astrocytoma, not otherwise specified, 17.2 [95% CI, 10.6–71.6]). This finding emphasizes the need for improvements in coding for nonglioblastoma astrocytomas to provide better population survival estimates. When review diagnosis was used, younger age and resection (vs. biopsy) were statistically significant for all histology groups analyzed by multivariable Cox proportional hazard models. Additional statistically significant variables were as follows: among 517 glioblastoma patients, radiation treatment and being married; among 105 AA patients, inclusion of chemotherapy in the initial treatment; and among 106 patients with nonanaplastic oligodendroglial tumors, college education. Further consideration of impact of marital status, education, and other social factors in glioma survival may be warranted.
Keywords: glioblastoma multiforme, glioma, neuropathology review, population-based study, SEER, survival
Others and we have reviewed the epidemiology of brain cancer, of which glioma is the most common form (Berleur and Cordier, 1995; Bondy et al., 1994; Bunin, 2000; Davis and McCarthy, 2000; Davis et al., 1999; Inskip et al., 1995; Preston-Martin, 1996; Wrensch et al., 2002). Glioma refers to tumors thought to be of glial cell origin and includes astrocytic tumors (WHO classification astrocytoma grades I, II [astrocytoma], III [AA, anaplastic astrocytoma], and IV [GM, glioblastoma]), oligodendrogliomas, ependymomas, and mixed gliomas (CBTRUS, 2002; Kleihues and Cavenee, 1997; Louis and Stemmer-Rachamimov, 2000). Survival from GM, the most common form of glioma in adults, is very poor, with median survival of only 3.5 months for those 65 or older at diagnosis, and only 10 months for those under age 65, according to Surveillance, Epidemiology, and End Results (SEER) data for 1981–1991 (Davis et al., 1998). Furthermore, survival for those diagnosed with GM has not demonstrably improved in the United States for more than 30 years (Davis et al., 1998). Histologic type and grade, age, extent of resection, tumor location, radiation therapy, and some chemotherapy protocols have been consistently and convincingly linked to survival (CBTRUS, 2002; Curran et al., 1993; Davis et al., 1998, 1999; Horn et al., 1999; Lamborn et al., 2004; Levin et al., 2001; Scott et al., 1998a). Analyses of GM and AA patients enrolled in Radiation Therapy Oncology Group trials and other multi-institutional clinical trials have also shown that KPS at diagnosis and other measures of mental and physical functionality (Curran et al., 1993; Scott et al., 1998a, b) may be associated with survival. Studies from Radiation Therapy Oncology Group study groups and other clinical trial groups provide useful information on prognostic factors from cases whose pathology has been centrally reviewed and for patients who qualify for and are treated in clinical trials. However, since the majority of patients do not enter clinical trials, the results cannot be readily generalized to the general population of patients with glioma.
A multinational survival study from 17 European registries (1985–1989) showed that women had somewhat better survival from primary malignant brain tumors than did men in all except two countries (Sant et al., 1998), with relative five-year survival rates of 20% for women and 17% for men. Ethnicity also seems to be linked with survival, with African Americans having higher five-year survival rates than whites (38% vs. 30%) (Davis et al., 1999). Studies based on population registry data have a disadvantage in that pathologic diagnoses are subject to considerable variability depending on numerous factors such as the pathologist’s neuropathology training (Aldape et al., 2000) and the time period and the place in which the diagnosis was made, as diagnostic criteria have varied over time and geographic region (Coons et al., 1997; Karak et al., 2000). Population registries also do not generally have as complete treatment data as are available in clinical trials.
Investigators are currently trying to identify and understand tumor markers or other patient characteristics that might influence survival or response to treatment (e.g., Bhowmick et al., 2004; Cairncross et al., 1998; Freije et al., 2004; Kyritsis et al., 1995; Lin et al., 1998; Nigro et al., 2005; Okcu et al., 2004; Puduvalli et al., 2000; Sano et al., 1999; Sigurdson et al., 1998; Simmons et al., 2001; Tang et al., 2005; Wei et al., 1997).
In this article, we make the following comparisons and estimates. (1) We compare survival of population-based cases from the San Francisco Bay Area in which patients were interviewed and in which central neuropathology review was conducted (the study group) to survival of those who were otherwise eligible but not included in the study group. (2) Within the study group, we compare survival estimates based on the International Classification of Diseases, Oncology, second edition (SEER ICD-O-2), diagnoses and those based on the central neuropathology review diagnosis. (3) For histologic groups with sufficient numbers of cases, we estimate survival in relation to several patient demographic and treatment factors.
Methods
The UCSF Committee for Human Research approved methods for both the initial subject recruitment and follow-up for survival. Individual hospital institutional review boards also approved methods for medical record abstraction.
Subjects
Eligibility criteria and ascertainment methods for the glioma cases have been previously described (Wiemels et al., 2002; Wrensch et al., 1997). Briefly, any adult (age >20) newly diagnosed with glioma (ICD-Omorphology codes 9380–9481) between August 1991 and April 1994 (series 1) and May 1997 and August 1999 (series 2) who resided in any of six San Francisco Bay Area counties at the time of diagnosis was eligible to participate. Potentially eligible cases were identified by using a rapid case ascertainment (RCA) program available through a SEER participating registry, the Northern California Cancer Center (Fremont) (NCCC). Median time from diagnosis to recruitment was 98 days. The study group included consenting patients or their proxies who were interviewed about a variety of factors, who gave written consent to obtain and review pathology specimens and records, and whose review confirmed an eligible diagnosis. The remaining cases (referred to as SEER eligible only) were not included in the study group either because consent was not obtained, central neuropathology review was not possible or revealed a diagnosis not eligible for the study, or cases were identified after the study closed.
Neuropathology Review
Pathology records and specimens were obtained from diagnosing hospitals, and a neuropathologist reviewed all tumors: Richard Davis (University of California, San Francisco [UCSF]) reviewed cases diagnosed between 1991 and 1994 (series 1), and Kenneth Aldape reviewed cases diagnosed between 1997 and 1999 (series 2). Tumors were classified according to the WHO criteria described by Kleihues et al. (1993a, b) with use of a coding form developed for this study. GM corresponds to WHO grade IV, AA to WHO grade III, and astrocytoma to WHO grade II. The corresponding ICD-O-2 codes are 9440 and 9401 for GM and AA, respectively. For astrocytoma, there is not an exact correspondence between the WHO criteria grade II and the ICD-O-2 code 9400. Details of the review of series 1 were previously published by Aldape et al. (2000), who also developed criteria for grouping ICD-O-2 histologic categories used by SEER to compare to the categories from the neuropathology review.
Determination of Vital Status
In December 2002, NCCC-SEER initially provided vital status data they routinely obtain through California State mortality tapes and the National Death Index. For those not identified as deceased, we sent a letter to the last known address and followed this with a telephone call. We searched the California Department of Motor Vehicles, UCSF medical records, and Internet search engines for those whose contact information was no longer valid. We also updated mortality from SEER in July 2004. For those contacted and determined to be alive (n = 152), the date of returned postcard or the last phone contact was their last known date alive. For six cases in which we could not locate the patient, we used the date of last contact as determined by NCCC-SEER. To summarize, patient survival time was either date of death or date of last contact. For patients not known to be dead, the patient was censored as of the date of last contact.
Determination of Treatment Information
Three sources of data, NCCC-SEER, medical record abstraction, and clinical trials database from UCSF, were used to assign treatment and other clinical characteristics. NCCC-SEER treatment information includes summary data for surgery, radiation, and chemotherapy. According to SEER guidelines, NCCC collects curative treatment information that is planned and given within the first course of therapy (within the first four months) following diagnosis. If the first plan of treatment fails, and subsequent therapy is planned and given, that treatment is not recorded by NCCC. Primary treatment data as summarized by NCCC-SEER was categorized as follows for this study: surgery—biopsy only or resection (the method of coding data by SEER precluding reliable classification into subtotal versus total resection); radiation therapy—none noted, external beam, implants, isotopes, or not otherwise specified; and chemotherapy—none noted, single or double agent, or not otherwise specified.
Medical record abstraction variables included KPS at time of diagnosis, tumor location, surgical resection (total, partial, or biopsy), radiation (types: external beam, radiosurgery, or brachytherapy, and total dose), chemotherapy (type and whether given before radiation [neoadjuvant] or during or after radiation [adjuvant]), and date of first relapse. KPS was not available for many subjects and is not included in most analyses in this article. The UCSF Neuro-Oncology Service database contained these data for patients entered in clinical trials. If any of the three data sources (SEER-NCCC, medical records, and UCSF Neuro-Oncology Service) indicated a resection, surgery was classified as resection; cases not classified as having resection were classified as having biopsy only. Radiation treatment was coded as given if that was reported in any of the three sources of information. If any of the three sources indicated chemotherapy being given, it was coded as given, with the following exceptions. Chemotherapy treatment status as recorded in SEER was recoded from “given” to “not given” for patients for whom the only chemotherapy agent listed in the hard-copy abstracts provided by NCCC was hydroxyurea or 5-bromo-2′-deoxyuridine (BUDR), which are radiation sensitizers, or the date chemotherapy was first given came on or after the date of relapse noted in the medical record or clinical trial database.
Data Management and Statistical Methods
We estimated median or lowest quartile months of survival and 95% confidence limits (CLs) and percent of subjects alive at two years by histologic diagnosis from the two coding systems (SEER ICD-O-2 and central review) with Kaplan–Meier life-table methods (Kalbfleisch and Prentice, 1980) using SAS PROC LIFETEST (SAS Institute, 1990). Lowest quartile is presented for histologies in which median months of survival have not yet been reached. We compared distributions of time to death for the study group with time-to-death distributions for SEER-eligible-only diagnostic subgroups that included at least 30 cases, using Cox proportional hazards regression models (Cox, 1972) estimated with SAS PROC PHREG (Stokes et al., 1995), adjusting for age at diagnosis, gender, and white versus nonwhite ethnicity. For the study group, we also computed Cox regressions to compare distributions of time to death as a function of age and other treatment and demographic factors of interest. Cox proportional hazards regression estimates a hazard ratio (HR) for each factor included in the model. For example, the HR associated with having radiation therapy is the probability of death at any point in time for a person alive at that point in time who has had radiation therapy divided by the probability of death at that time for a patient, also alive at that point, who did not have radiation therapy. In the multivariable model, which includes multiple independent variables, the probability is adjusted for the other factors in the model. Initially, age was evaluated as a prognostic factor. All other variables were analyzed by using age-adjusted models. Age-adjusted models were run for diagnostic categories with a minimum of 30 subjects, and multivariable models were used for homogeneous diagnostic categories with a minimum of at least 100 subjects. The associations of marital status with treatment variables were evaluated with age-, gender-, and ethnicity- adjusted odds ratios by using SAS PROC LOGISTIC and with KPS using age- gender-, and ethnicity-adjusted means by using PROC GLM. Results were considered statistically significant if P ⩽ 0.05. No adjustment was made for multiple comparisons.
Results
Comparison of SEER-Eligible Cases and the Study Group
The NCCC RCA program reported 1349 potentially eligible subjects during the study recruitment periods (1991–1994 and 1997–1999), and the SEER registry tape obtained in July 2004 reported 1316 subjects as fulfilling eligibility criteria. The two sources identified a total of 1549 individuals; since 200 of these were identified by SEER after study recruitment ended, no attempt to contact these subjects was made. Of the remaining 1349, a total of 226 were found to be ineligible because of age, a prior glioma diagnosis (i.e., recurrent disease), diagnosis date before the ascertainment period, or wrong county of residence. This left 1123 subjects, of whom we interviewed 900 patients or their proxies (80%), 211 refused, and 12 could not be contacted. Pathology review could not be completed for 14, either because consent was not obtained or because the pathology materials were insufficient or could not be obtained, and 13 cases were determined to be ineligible after review. The study group therefore included 873 (900 – 27) subjects interviewed with central neuropathology review and confirmed glioma. Six of these subjects were not included on the SEER tape, but discussions with the registry could not resolve why they had been omitted. For this article, we consider 1322 subjects as eligible (1316 on the SEER July 2004 tape plus six identified through RCA with confirmed central pathology review), of whom 873 (66%) are in the study group and 449 (34%) are not (Table 1).
Table 1.
Comparisons of subjects eligible for and included in the San Francisco Bay Area Adult Glioma Study (SFBGS), 1991–2000, with survival follow-up through July 2004
| SEER Eligible Cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Not Included in SFBGS1 |
Included in SFBGS1 |
||||||||
| SEER ICD-O-2 Histology Groups2 | N (%) | N Died | Median Age at Diagnosis | Median Months of Survival 95% CL | N (%) | N Died | Median Age at Diagnosis | Median Months of Survival 95% CL | Hazard Ratio3Included/Not Included in SFBGS 95% CL |
| Glioblastoma multiforme | 252 (56) | 241 | 66 | 5.2
4.6–6.1 |
500 (57) | 492 | 62 | 7.3
6.7–8.2 |
0.83 0.71–0.97 |
| Anaplastic astrocytoma | 48 (11) | 34 | 48 | 16.3
10.0–43.5 |
117 (13) | 94 | 49 | 16.6
12.0–20.7 |
1.2
0.83–1.8 |
| Astrocytoma | 0 | NA | 2 (0) | 25 | NA | ||||
| Astrocytoma, NOS | 47 (10) | 34 | 46 | 13.5
9.2–52.4 |
62 (7) | 43 | 55 | 17.2
10.6–71.6 |
0.53 0.32–0.87 |
| Lowest quartile months of survival 95% CL | Lowest quartile months of survival 95% CL | ||||||||
| Fibrillary astrocytoma | 5 (1) | 3 | 31 | 12.6
10.8–52.2 |
8 (1) | 3 | 33 | 38.3
7.0–*** |
NC |
| Anaplastic oligodendroglioma | 12 (3) | 6 | 46 | 13.9
6.7–*** |
19 (2) | 13 | 46 | 8.8
7.9–19.8 |
1.3
0.45–3.6 |
| Oligodendroglioma | 28 (6) | 5 | 37 | 92.5
65.9–*** |
74 (8) | 19 | 41 | 91.4
45.1–*** |
1.3
0.44–3.7 |
| Anaplastic oligoastrocytoma | NA | NA | NA | NA | NA | ||||
| Oligoastrocytoma | 14 (3) | 5 | 38 | 71.4
41.2–*** |
43 (5) | 14 | 36 | 54.7
25.0–*** |
1.0
0.34–2.9 |
| Ependymoma | 11 (2) | 6 | 55 | 22.3
2.8–68.1 |
10 (1) | 5 | 43 | 15.1
0.1–*** |
NC |
| Juvenile pilocytic astrocytoma | 7 (2) | 2 | 23 | 30.1
0.3–*** |
14 (2) | 2 | 33 |
*** 80.7–*** |
NC |
| Medulloblastoma | 8 (2) | 4 | 34 | 74.6
40.2–118.8 |
11 (1) | 2 | 29 |
*** 5.1–*** |
NC |
| Median months of survival 95% CL | Median months of survival 95% CL | ||||||||
| Other | 17 (4) | 13 | 45 | 9.1
4.0–39.6 |
13 (2) | 10 | 45 | 11.0
4.1–24.5 |
1.1
0.43–2.9 |
| Total | 449 | 353 | 58 | 873 | 697 | 55 | |||
| % Female | 43% | 44% | |||||||
| % White | 79% | 90% | |||||||
Abbreviations: CL, confidence limits; ICD-O-2, International Classification of Diseases-Oncology, 2nd ed.; NA, not available; NC, not calculated because fewer than 30 subjects total; NOS, not otherwise specified; SEER, Surveillance, Epidemiology, and End Results; SFBGS, San Francisco Bay Area Adult Glioma Study.
Eligible cases are those reported in the SEER registry tape, July 2004, with histologic newly diagnosed glioma during the study catchment time and area. Subjects included in the study are those who participated and for whom study neuropathology review confirmed glioma diagnosis.
SEER diagnoses are based on ICD-O-2 codes as follows with categories combined by Kenneth Aldape in accordance with those used by the Central Brain Tumor Registry of the United States (www.cbtrus.org): Glioblastoma multiforme includes glioblastoma multiforme 9440 (n = 731), giant cell glioma 9441 (n = 3) and gliosarcoma 9442 (n = 18); anaplastic astrocytoma includes anaplastic astrocytoma 9401 (n = 146) and gemistocytic astrocytoma 9411 (n = 19); astrocytoma includes only protoplasmic astrocytoma 9410 (n = 2); astrocytoma, NOS, 9400 (n = 109); fibrillary astrocytoma 9420 (n = 13); anaplastic oligodendroglioma 9451 (n = 31); oligodendroglioma 9450 (n = 102); mixed oligoastrocytoma 9382 (n = 57); ependymoma includes ependymoma, NOS, 9391 (n = 17) and anaplastic ependymoma 9392 (n = 4); juvenile pilocytic astrocytoma 9421 (n = 21); medulloblastoma includes 9470 (n = 13), desmoplastic medulloblastoma 9471 (n = 1), and primitive neuroectodermal tumors (PNET) 9473 not of the cerebrum (including overlapping sites) (n = 5); and other includes glioma, NOS, 9380 (n = 20), subependymoma 9383 (n = 2), choroid plexus papilloma 9390 (n = 1), pleomorphic xanthoastrocytoma 9424 (n = 1), astroblastoma 9430 (n = 1), and PNET 9473 in the cerebrum (n = 5). Survival data and demographic data are from the SEER registry tape.
Hazard ratios from Cox regressions are adjusted for age, gender, and ethnicity within histologic groups with 30 or more cases. Boldface values are statistically significant.
Lowest quartile or upper 95% confidence limit not yet reached.
Compared to subjects in SEER-eligible-only cases, the 873 subjects in the study group were, on average, younger at diagnosis (Table 1) and were somewhat more likely to be white. Among patients with SEER-eligible diagnoses of GM or astrocytoma, not otherwise specified (NOS), those in the study group had significantly better survival than did those not in the study group (age-, gender-, ethnicity-adjusted HR = 0.83 [0.71–0.97] for GM; HR = 0.53 [0.32–0.87] for astrocytoma, NOS [Table 1]). There were no significant survival differences between the two groups for AA, oligodendroglial tumors, or other glioma. However, HR estimates for some of these tumors were of the same order of magnitude as observed for GM, but with the study group having poorer survival as compared to the SEER-eligible group (HRs between 1.2 and 1.3 [Table 1]).
Study Group Survival Estimates Based on Central Neuropathology Review Versus SEER ICD-O-2 Diagnosis Coding Systems
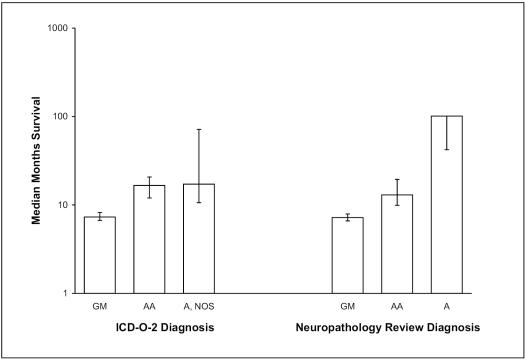
With central review diagnoses, a progressive increase can be observed in the median months of survival, from GM to AA to astrocytoma, with nonoverlapping 95% CLs (Table 2). On the other hand, while there is a comparable difference in median months of survival between GM and AA based on SEER diagnoses, the median months of survival for AA and astrocytoma, NOS, are nearly the same, with widely overlapping CLs (Table 1; see the column “Included in SFBGS”). These comparisons by diagnostic coding system also are shown in Fig. 1. Also note that the largest differences in numbers of cases categorized by the two diagnostic coding systems were for the review diagnosis category astrocytoma and the SEER ICD-O-2 category astrocytoma, NOS; specifically, for cases in the study group, review diagnosis coded 43 for astrocytoma and 1 for astrocytoma, NOS (Table 2), whereas SEER ICD-O-2 coded 2 for astrocytoma and 62 for astrocytoma, NOS (Table 1).
Table 2.
Subject characteristics within study neuropathology review diagnosis1: The San Francisco Bay Area Adult Glioma Study (SFBGS) 1991–2000, with survival follow-up through July 2004
|
SFBGS Neuropathology Review and Survival Data1 |
||||
|---|---|---|---|---|
| Review Histologic Diagnosis1 | N (%) | N Died | Median Age at Diagnosis | Median Months of Survival 95% CL |
| Glioblastoma multiforme | 519 (59) | 516 | 63 | 7.2
6.6–7.9 |
| Anaplastic astrocytoma | 105 (12) | 85 | 52 | 13.0
9.9–19.5 |
| Astrocytoma | 43 (5) | 24 | 42 | 101.3
42.1–*** |
| Astrocytoma, NOS | 1 | 1 | 59 | 12.5
*** |
| Lowest Quartile Median Months of Survival 95% CL | ||||
| Fibrillary astrocytoma | 0 | |||
| Anaplastic oligodendroglioma | 26 (3) | 14 | 50 | 18.1
14.4–47.8 |
| Oligodendroglioma | 67 (8) | 25 | 42 | 70.1
22.7–101.3 |
| Anaplastic oligoastrocytoma | 16 (2) | 10 | 42 | 28.5
10.1–74.1 |
| Oligoastrocytoma | 40 (5) | 18 | 40 | 60.6
16.4–128.1 |
| Ependymoma | 8 (1) | 2 | 40 |
*** 0.0–*** |
| Juvenile pilocytic astrocytoma | 15 (2) | 1 | 32 | *** |
| Medulloblastoma | 10 (1) | 1 | 30 |
*** 21.3–*** |
| Median Months of Survival 95% CL | ||||
| Other2 | 23 (3) | 14 | 53 | 20.0
8.6–*** |
| Total | 873 | 711 | 55 | |
| % Female | 44% | |||
| % White | 83% | |||
Abbreviation: CL, confidence limits.
Review diagnoses were based on neuropathology review by Richard Davis for cases diagnosed in 1991–1994 and by Kenneth Aldape for cases diagnosed in 1997–1999 by using criteria defined in the text. Among these, note that SEER (Surveillance, Epidemiology, and End Results) diagnosis fibrillary astrocytoma did not have a corresponding review diagnosis. Survival data are from SEER registry data plus active study follow-up, and demographic data are from questionnaires.
The 23 cases classified as “other” include dysembryoplastic neuroepithelial tumor (n = 2), glial neoplasm (n = 1), glioma, not otherwise specified (NOS) (n = 1), gliosarcoma (n = 4), low-grade glioma (n = 2), malignant glioma (n = 2), malignant neoplasm (n = 1), primitive neuroectodermal tumor (n = 1), pleomorphic xanthoastrocytoma (n = 2), subependymal giant cell astrocytoma (n = 1), anaplastic glioma (n = 1), subependymoma (n = 1), other (n = 1), and ganglioglioma (n = 3).
Lowest quartile or upper 95% confidence limit not yet reached. Survival data are from SEER registry data plus active study follow-up, and demographic data are from
Fig 1.
Median months of survival and 95% confidence limits (CLs) for San Francisco Bay Area Adult Glioma Study subjects diagnosed from 1991 to 2000 and followed through June 2004, according to SEER ICD-O-2 and the study neuropathology review diagnosis of astrocytic gliomas. Note that the upper 95% CL for astrocytoma in the Neuropathology Review Diagnosis data has not yet been reached. Abbreviations: A, astrocytoma; A, NOS, astrocytoma not otherwise specified; AA, anaplastic astrocytoma; GM, glioblastoma.
Study Group Age-Adjusted and Multivariable Survival Analyses
Study Group GM
Two GM patients could not be included in multivariable analyses because they lacked data on education. In univariate (age) or age-adjusted analyses of 517 patients, younger age, resection, radiation therapy, chemotherapy, being married at the time of diagnosis, and participation in a UCSF treatment protocol were significantly associated with improved survival (Table 3). In multivariable analyses including gender and ethnicity and all these variables (except participation in a UCSF treatment protocol), younger age, resection, and radiation therapy were highly statistically significant (P < 0.001). Being married at the time of diagnosis was significant (P = 0.05), and diagnosis at an academic hospital was nearly significantly associated with improved survival (P = 0.07) (Table 3). For chemotherapy, the HR approached the null in multivariable analysis. In a separate multivariable analysis including the same variables except diagnosis at an academic hospital, participation in a UCSF treatment protocol was not statistically significantly associated with survival (HR = 0.85; P = 0.21) (Table 3, note 4). Marital status was not related to either chemotherapy or extent of surgery, but married subjects were about twice as likely to have received radiation treatment (age-, gender-, ethnicity- adjusted odds ratio for receiving radiation therapy for married versus unmarried cases was 2.01 [95% CI, 1.23–3.29]). The multivariable analysis in Table 3 shows, however, that the association of marital status with survival exists (with a similar HR) even when adjusted for radiation treatment. In a subset of 428 GM cases with KPS scores, married patients had a mean KPS of 71 as compared to 64 for unmarried patients (P < 0.003). In a model including variables shown in Table 3 plus KPS, the HR (95% CI) for marital status moved toward the null (0.94 [0.76–1.16]), and higher KPS was significantly associated with better survival HR (0.73 [0.69–0.78]).
Table 3.
Cox regression of survival among glioblastoma patients, San Francisco Bay Area Adult Glioma Study, 1991–20001
| Demographic or Treatment Factor | N2(517) | N Died3(514) | Median Months of Survival4 | Percent Surviving Two Years4 | Univariate HR | Multivariable HR5 | Multi-P6 |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (10 years)7 | 1.40 (1.30–1.50) | 1.27 (1.18–1.37) | <0.001 | ||||
| 20–29 | 6 | 6 | 16 | 33 | |||
| 30–39 | 27 | 24 | 11 | 15 | |||
| 40–49 | 75 | 75 | 13 | 11 | |||
| 50–59 | 120 | 120 | 10 | 10 | |||
| 60–69 | 113 | 113 | 7 | 6 | |||
| 70–79 | 140 | 140 | 4 | 1 | |||
| 80–89 | 33 | 33 | 3 | 0 | |||
| 90–99 | 3 | 3 | 3 | 0 | |||
| Age-Adjusted HR | |||||||
| Ethnicity | |||||||
| White | 434 | 433 | 7 | 7 | |||
| Nonwhite | 83 | 81 | 10 | 7 | 0.93 (0.73–1.18) | 0.88 (0.69–1.13) | 0.32 |
| Gender | |||||||
| Female | 226 | 225 | 6 | 6 | |||
| Male | 291 | 289 | 8 | 8 | 0.94 (0.79–1.12) | 0.96 (0.80–1.15) | 0.67 |
| Surgery | |||||||
| Biopsy | 141 | 141 | 4 | 1 | |||
| Resection | 376 | 373 | 9 | 9 | 0.56 (0.46–0.68) | 0.56 (0.46–0.69) | <0.001 |
| Chemotherapy | |||||||
| No | 406 | 405 | 6 | 5 | |||
| Yes | 111 | 109 | 10 | 13 | 0.80 (0.64–1.00) | 0.96 (0.77–1.21) | 0.75 |
| Radiation | |||||||
| No | 94 | 94 | 2 | 0 | |||
| Yes | 423 | 420 | 9 | 8 | 0.20 (0.15–0.25) | 0.21 (0.16–0.27) | <0.001 |
| Diagnosis at an academic hospital | |||||||
| No | 398 | 397 | 7 | 6 | |||
| Yes | 119 | 117 | 10 | 11 | 0.87 (0.71–1.08) | 0.82 (0.66–1.02) | 0.07 |
| College graduate | |||||||
| No | 326 | 324 | 6 | 6 | |||
| Yes | 191 | 190 | 9 | 8 | 0.84 (0.70–1.01) | 0.89 (0.74–1.07) | 0.20 |
| Married | |||||||
| No | 168 | 167 | 5 | 5 | |||
| Yes | 349 | 347 | 8 | 8 | 0.78 (0.64–0.94) | 0.83 (0.68–1.00) | 0.05 |
| UCSF protocol | |||||||
| No | 437 | 435 | 7 | 6 | |||
| Yes | 80 | 79 | 10 | 11 | 0.78 (0.61–1.00) | See note 5 | |
Abbreviations: HR, hazard ratio; UCSF, University of California, San Francisco.
Boldface values are statistically significant. Median (range) months of follow-up for those alive is 138 (82–145).
Number of cases.
Number deceased.
Kaplan-Meier estimates of median months of survival and percent surviving two years or more.
Multivariable model includes age, ethnicity, gender, surgery, chemotherapy, radiation, being diagnosed at an academic institution (i.e., Stanford or UCSF), college graduation, and marital status. In an alternative multivariable model that included all these variables except diagnosis at an academic hospital and included being on a UCSF protocol, the HR for being on a UCSF protocol was 0.85 (0.65–1.10; P = 0.21).
Pvalue for each variable in the multivariable model.
HR for 10-year age variable calculated by using continuous age data.
Study Group AA
Among 105 patients in the study group with AA, younger age, resection versus biopsy, chemotherapy, and radiation therapy were significantly associated with improved survival in univariate or age-adjusted analyses (Table 4). In multivariable analyses, younger age (P < 0.001), chemotherapy (P = 0.01), and resection (P = 0.002) were the only variables significantly associated with improved survival (Table 4), although having radiation therapy was nearly significant with an estimated twofold improved survival (P = 0.06).
Table 4.
Cox regression of survival among anaplastic astrocytoma patients, San Francisco Bay Area Adult Glioma Study, 1991–20001
| Demographic or Treatment Factor | N2(105) | N Died3(85) | Median Months of Survival4 | Percent Surviving Two Years4 | Univariate HR | Multivariable HR5 | Multi-P6 |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (10 years)7 | 1.68 (1.44–1.95) | 1.52 (1.26–1.83) | <0.001 | ||||
| 20–29 | 6 | 3 | ** | 83 | |||
| 30–39 | 25 | 16 | 37 | 64 | |||
| 40–49 | 18 | 13 | 36 | 56 | |||
| 50–59 | 23 | 21 | 8 | 4 | |||
| 60–69 | 21 | 20 | 5 | 10 | |||
| 70–79 | 8 | 8 | 3 | 13 | |||
| 80–89 | 4 | 4 | 3 | 0 | |||
| 90–99 | |||||||
| Age-Adjusted HR | |||||||
| Ethnicity | |||||||
| White | 80 | 64 | 14 | 34 | |||
| Nonwhite | 25 | 21 | 10 | 32 | 1.19 (0.72–1.96) | 1.01 (0.59–1.74) | 0.97 |
| Gender | |||||||
| Female | 50 | 40 | 15 | 36 | |||
| Male | 55 | 45 | 12 | 31 | 0.92 (0.60–1.42) | 0.93 (0.59–1.46) | 0.75 |
| Surgery | |||||||
| Biopsy | 33 | 33 | 5 | 12 | |||
| Resection | 72 | 52 | 20 | 43 | 0.45 (0.28–0.73) | 0.43 (0.25–0.74) | 0.002 |
| Chemotherapy8 | |||||||
| No | 69 | 63 | 8 | 25 | |||
| Yes | 36 | 22 | 26 | 50 | 0.50 (0.30–0.84) | 0.49 (0.28–0.84) | 0.01 |
| Radiation8 | |||||||
| No | 12 | 10 | 2 | 25 | |||
| Yes | 93 | 75 | 16 | 34 | 0.43 (0.22–0.86) | 0.50 (0.25–1.03) | 0.06 |
| Diagnosis at an academic hospital | |||||||
| No | 76 | 63 | 11 | 26 | |||
| Yes | 29 | 22 | 30 | 52 | 1.10 (0.64–1.88) | 1.18 (0.66–2.11) | 0.58 |
| College graduate8 | |||||||
| No | 62 | 51 | 11 | 27 | |||
| Yes | 43 | 34 | 17 | 42 | 0.97 (0.62–1.51) | 0.86 (0.53–1.39) | 0.53 |
| Married | |||||||
| No | 38 | 33 | 13 | 32 | |||
| Yes | 67 | 52 | 14 | 34 | 0.76 (0.49–1.18) | 1.05 (0.65–1.69) | 0.85 |
| UCSF protocol | |||||||
| No | 90 | 76 | 12 | 32 | |||
| Yes | 15 | 9 | 21 | 47 | 0.64 (0.31–1.34) | See note 4 | |
Abbreviations: HR, hazard ratio; UCSF, University of California, San Francisco.
Boldface values are statistically significant.
Number of cases.
Number deceased.
Kaplan-Meier estimates of median months of survival and percent surviving 2 years or more. Median (range) months of survival for those alive is 81 (21–151).
Median months of survival not yet reached.
Multivariable model includes age, ethnicity, gender, surgery, chemotherapy, radiation, being diagnosed at an academic institution (i.e., Stanford or UCSF), college graduation, and marital status. In an alternative multivariable model that included all these variables except diagnosis at an academic hospital and included being on a UCSF protocol, the HR for being on a UCSF protocol was 1.11 (0.50–2.45; P = 0.80).
P value for each variable in the multivariable model.
HR for 10-year age variable calculated by using continuous age data.
Unadjusted HRs were 0.35, 0.55, and 0.83 for chemotherapy, radiation, and graduating college; generally matching the age and multivariable adjusted results and consistent with the relative differences in median survival.
Study Group Oligodendroglial Tumors
The proportion of subjects diagnosed with oligodendroglioma versus oligoastrocytoma changed between series, likely reflecting somewhat different diagnostic criteria of the two review pathologists. Specifically, in series 1, 34 patients (7%) were centrally reviewed as oligodendroglioma (including anaplastic oligodendroglioma) and 47 (10%) as oligoastrocytoma (including anaplastic). In series 2, 58 patients (14%) were centrally reviewed as oligodendroglioma, and only 10 (2%) had review diagnosis of oligoastrocytoma. Because the total percentage of gliomas with oligodendroglial component was similar for the two series (17% of tumors in series 1 and 16% of tumors in series 2) and because there were too few tumors in the oligoastrocytoma category for separate analysis, we estimated univariate and age-adjusted HRs for tumors diagnosed as oligodendroglioma and for tumors with any oligodendroglial component (Table 5 for those not considered to be anaplastic and Table 6 for those considered to be anaplastic oligodendroglial tumors).
Table 5.
Cox regression of survival among nonanaplastic oligodendroglioma and nonanaplastic oligoastrocytoma patients, San Francisco Bay Area Adult Glioma Study, 1991–20001
| Oligodendroglioma | Oligodendroglioma and Oligoastrocytoma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic or Treatment Factor | N2(66) | N Died3(25) | Median Months of Survival4 | Percent Surviving Two Years4 | Univariate HRPValue | N2(106) | N Died3(43) | Median Months of Survival4 | Percent Surviving Two Years4 | Univariate HRPValue |
| Age at diagnosis (10 years)5 |
1.69 (1.26–2.28) 0.0005 |
1.67 (1.33–2.11) <0.0001 |
||||||||
| 20–29 | 8 | 2 | 80 | 88 | 14 | 2 | ** | 93 | ||
| 30–39 | 21 | 5 | ** | 90 | 34 | 10 | ** | 91 | ||
| 40–49 | 18 | 5 | ** | 100 | 29 | 11 | 128 | 97 | ||
| 50–59 | 10 | 6 | 131 | 80 | 17 | 11 | 131 | 71 | ||
| 60–69 | 5 | 4 | 21 | 40 | 7 | 5 | 61 | 57 | ||
| 70–79 | 3 | 2 | 10 | 33 | 4 | 3 | 12 | 25 | ||
| 80–89 | 1 | 1 | 6 | 0 | 1 | 1 | 6 | 0 | ||
| Age-Adjusted HRPValue | Age-Adjusted HRPValue | |||||||||
| Ethnicity | ||||||||||
| White | 55 | 22 | 127 | 84 | 90 | 37 | 131 | 83 | ||
| Nonwhite | 11 | 3 | ** | 82 | 1.00 (0.29–3.43)
0.99 |
16 | 6 | 147 | 88 | 0.63 (0.26–1.54)
0.32 |
| Gender | ||||||||||
| Female | 25 | 9 | 127 | 80 | 41 | 17 | 127 | 80 | ||
| Male | 41 | 16 | 131 | 85 | 0.68 (0.29–1.59)
0.37 |
65 | 26 | 136 | 86 | 0.55 (0.29–1.04)
0.07 |
| Surgery | ||||||||||
| Biopsy | 7 | 5 | 21 | 43 | 16 | 13 | 29 | 56 | ||
| Resection | 59 | 20 | 131 | 88 | 0.29 (0.10–0.82)
0.02 |
90 | 30 | 151 | 89 |
0.29 (0.15–0.56) 0.0002 |
| Chemotherapy | ||||||||||
| No | 56 | 24 | 127 | 82 | 88 | 39 | 131 | 83 | ||
| Yes | 10 | 1 | ** | 90 | 0.29 (0.04–2.16)
0.23 |
18 | 4 | ** | 89 | 0.73 (0.26–2.07)
0.55 |
| Radiation | ||||||||||
| No | 20 | 3 | ** | 95 | 25 | 4 | ** | 92 | ||
| Yes | 46 | 22 | 101 | 78 | 2.31 (0.68–7.82)
0.18 |
81 | 39 | 128 | 81 | 2.19 (0.77–6.18)
0.14 |
| Diagnosis at an academic hospital | ||||||||||
| No | 48 | 22 | 101 | 81 | 74 | 31 | 131 | 82 | ||
| Yes | 18 | 3 | 151 | 89 | 0.32 (0.09–1.15)
0.08 |
32 | 12 | 136 | 88 | 0.83 (0.42–1.65)
0.59 |
| College graduate | ||||||||||
| No | 29 | 14 | 127 | 72 | 48 | 25 | 127 | 75 | ||
| Yes | 37 | 11 | 131 | 92 | 0.50 (0.22–1.13)
0.10 |
58 | 18 | 151 | 91 |
0.47 (0.25–0.87) 0.02 |
| Married | ||||||||||
| No | 25 | 7 | ** | 84 | 43 | 14 | 136 | 84 | ||
| Yes | 41 | 18 | 101 | 83 | 1.52 (0.63–3.66)
0.35 |
63 | 29 | 127 | 84 | 1.37 (0.72–2.60)
0.34 |
| UCSF protocol | ||||||||||
| No | 61 | 24 | 127 | 84 | 99 | 40 | 136 | 84 | ||
| Yes | 5 | 1 | ** | 80 | 0.63 (0.08–4.90)
0.65 |
7 | 3 | ** | 86 | 1.63 (0.48–5.46)
0.62 |
Abbreviations: HR, hazard ratio; UCSF, University of California, San Francisco.
Boldface values are statistically significant.
Number of cases.
Number deceased.
Kaplan-Meier estimates of median months of survival and percent surviving two years or more.
Median months of survival not yet reached.
HR given for 10-year age groups calculated by using continuous age data. Median (range) months of follow-up for those alive is 75 (29–154) for oligodendroglioma and 83 (29–154) for oligodendroglioma and oligoastrocytoma.
Table 6.
Cox regression of survival among anaplastic oligodendroglioma and anaplastic oligoastrocytoma patients, San Francisco Bay Area Adult Glioma Study, 1991–20001
| Demographic or Treatment Factor | N2(42) | N Died3(24) | Median Months of Survival4 | Percent Surviving Two Years4 | Univariate HR | PValue |
|---|---|---|---|---|---|---|
| Age at diagnosis (10 years)5 | 1.92 (1.41–2.62) | <0.0001 | ||||
| 20–29 | 4 | 1 | ** | 100 | ||
| 30–39 | 9 | 2 | ** | 100 | ||
| 40–49 | 10 | 6 | 54 | 70 | ||
| 50–59 | 9 | 6 | 40 | 67 | ||
| 60–69 | 5 | 4 | 12 | 40 | ||
| 70–79 | 4 | 4 | 15 | 25 | ||
| 80–89 | 1 | 1 | 0 | 0 | ||
| Age-Adjusted HR | PValue | |||||
| Ethnicity | ||||||
| White | 37 | 22 | 67 | 68 | ||
| Nonwhite | 5 | 2 | ** | 80 | 0.74 (0.17–3.19) | 0.68 |
| Gender | ||||||
| Female | 19 | 11 | 48 | 68 | ||
| Male | 23 | 13 | 77 | 70 | 0.79 (0.35–1.8) | 0.58 |
| Surgery | ||||||
| Biopsy | 5 | 5 | 17 | 20 | ||
| Resection | 37 | 19 | 77 | 76 | 0.26 (0.08–0.79) | 0.02 |
| Chemotherapy6 | ||||||
| No | 24 | 13 | 77 | 67 | ||
| Yes | 18 | 11 | 58 | 72 | 2.79 (1.09–7.13) | 0.03 |
| Radiation6 | ||||||
| No | 5 | 2 | ** | 60 | ||
| Yes | 37 | 22 | 67 | 70 | 1.06 (0.23–4.78) | 0.94 |
| Diagnosis at an academic hospital | ||||||
| No | 22 | 15 | 53 | 64 | ||
| Yes | 20 | 9 | 77 | 75 | 0.81 (0.34–1.92) | 0.63 |
| College graduate | ||||||
| No | 24 | 15 | 44 | 58 | ||
| Yes | 18 | 9 | 91 | 83 | 0.74 (0.31–1.74) | 0.49 |
| Married | ||||||
| No | 16 | 9 | 67 | 63 | ||
| Yes | 26 | 15 | 66 | 73 | 0.56 (0.23–1.36) | 0.20 |
| UCSF protocol | ||||||
| No | 35 | 19 | 74 | 66 | ||
| Yes | 7 | 5 | 45 | 86 | 2.29 (0.78–6.75) | 0.13 |
Abbreviations: HR, hazard ratio; UCSF, University of California, San Francisco.
Boldface values are statistically significant. Median (range) months of follow-up for those alive is 74 (63–150).
Number of cases.
Number deceased.
Kaplan-Meier estimates of median months of survival and percent surviving two years or more.
** Median months of survival not yet reached.
HR given for 10-year age groups calculated by using continuous age data.
Note that unadjusted HR for chemotherapy = 1.17 and for radiation = 1.47; thus, age adjustment had a marked effect on HR estimates.
In analyses of 66 patients with nonanaplastic oligodendroglioma, younger age and resection were significantly associated with improved survival (Table 5). In analyses of 106 patients with tumors with any nonanaplastic oligodendroglial tumors (40 with oligoastrocytoma plus the 66 with oligodendroglioma described above), younger age, resection, and having graduated from college were significantly associated with improved survival in univariate or age-adjusted analyses (Table 5). For patients with anaplastic oligodendroglial tumors, younger age, resection, and not having chemotherapy were significantly associated with improved survival in univariate or age-adjusted analyses (Table 6). We did not conduct separate survival analyses of other histologic categories because the numbers of subjects were too small for meaningful analysis.
Discussion
Representativeness of Study Group Participants
Differences in survival within SEER diagnostic categories among the study group and SEER-only cases could indicate systematic bias in ascertainment or recruitment of subjects. We found that the survival time of the study group was somewhat better for the most common glioma diagnosis, GM, and for astrocytoma, NOS; thus, there appears to be a modest bias in our study group for recruitment of those with better survival. Because of the often rapid mortality associated with GM, it is not unexpected that, even with RCA, a proportion of subjects would die prior to contact or that their families might be less willing to participate as proxies. For example, in this study 252 (34%) of 752 SEER ICD-O-2 eligible GM cases were not included in the study (Table 1). Of these, 92 were reported to us after the study closed. Of those included, proxies participated on behalf of 197 (40%) of 519 review diagnosis GM cases. It is a significant and ongoing challenge to enroll population-based subjects (or their proxies) with such rapidly fatal cancers as GM. For the lower grade tumors, such as AA, anaplastic oligodendroglioma, and oligodendroglioma, the somewhat poorer survival of the study group compared to that of the SEER-only cases could be due to differences among hospitals in rapid reporting of cases. Thus, there appears to be a modest, though not statistically significant, bias in our study for recruitment of those with somewhat poorer survival in these diagnostic groups.
Comparison of the SEER and Centrally Reviewed Diagnostic Categories Among the Study Group
Comparison of the study group survival estimates by using the central review diagnostic categories versus the SEER ICD-O-2 diagnoses could indicate the extent to which differential classification of tumors might influence survival estimates. In this study, for astrocytic tumors, the review diagnosis codes performed better than the SEER ICD-O-2 codes at discriminating survival. In particular, median survival for the SEER category astrocytoma, NOS, was virtually indistinguishable from that for AA, whereas with the central review diagnoses, astrocytoma had substantially better survival than AA. These findings highlight the often expressed need (Aldape et al., 2000; Bruner et al., 1997; Coons et al., 1997; Karak et al., 2000; McCarthy et al., 2002) for specialist neuropathology review to accurately classify astrocytic tumors (especially those not classified as GM) in order to provide meaningful survival information through population resources. Mechanisms for instituting population-wide neuropathology review and/or revising ICD-O coding criteria for certain subgroups of glioma should be explored and evaluated.
Treatment and Demographic Factors Associated with Survival in the Study Group
As expected, survival of GM patients was significantly improved by younger age at diagnosis, surgical resection versus biopsy only, and radiation therapy in multivariable analysis (CBTRUS, 2002–2003; Curran et al., 1993; Davis et al., 1998, 1999; Horn et al., 1999; Lamborn et al., 2004; Levin et al., 2001; Scott et al., 1998a). In the study group, although chemotherapy and treatment on a UCSF protocol were favorable prognostic indicators in age-adjusted analyses, they moved toward the null in multivariable analysis, suggesting that patients with a more favorable prognosis might have been preferentially provided chemotherapy or placed on a protocol. Interestingly, being married at diagnosis was a favorable prognostic indicator in multivariable analysis, although in the subset of patients with KPS scores, the association moved toward the null when KPS was included in the model. Also, since the association of marital status with GM was of moderate statistical significance (P = 0.05) and was not apparent for AA or nonanaplastic oligodendroglial tumors, the possibility remains that this association could be due to chance, given the number of comparisons made in this report. If not due to chance, the fact that the association of marital status and GM survival appeared to be confounded with KPS at time of diagnosis suggests that the improved survival for married GM patients might result from earlier diagnosis while their KPS was higher. However, other aspects of marriage such as social support might influence KPS and subsequent survival; in this circumstance, KPS could be an intermediate causal factor (not a confounder) by which marital status might influence survival. Although the impact of social factors has been extensively studied in relation to survival for other cancer sites (Banerjee et al., 2004; Charalampopoulou et al., 2004; De Boer et al., 1999; Rosengren and Wilhelmsen, 2004; Smedslund and Ringdal, 2004; Soler-Vila et al., 2003; Spiegel et al., 1989; Ward et al., 2004), little information is available about the association of social factors and glioma survival. Thus, our results also point to the need for further exploration and evaluation of these factors in clinical trials and epidemiologic investigations of brain tumors.
For the approximately 100 participants in the study group with AA, only surgical resection versus biopsy, chemotherapy, and younger age remained as significantly favorable prognostic indicators after multivariable analysis. However, statistical power for some of the other variables may have been weak, and it is worth noting that there was an estimated twofold benefit of radiation therapy after adjustment for other factors. No notable benefits for education, marital status, or diagnosis at an academic hospital or treatment on a UCSF protocol were noted in the multivariable analysis.
For the 106 patients with nonanaplastic oligodendroglial tumors, younger age and surgical resection versus biopsy were significantly associated with better survival. Interestingly, those patients who were college graduates also showed significantly better survival in age-adjusted comparisons. For the 42 patients with anaplastic oligodendroglial tumors, only younger age and surgical resection were statistically significant, but the statistical power for other variables was low, given the small numbers of cases. Clearly, multi-institutional studies will be needed to clarify other factors associated with this relatively rare form of glioma. We cannot assess the impact of tumor loss of 1p or 19q on survival (such tumors are known to be associated with more favorable survival [van den Bent, 2004]), as these data are not routinely available, and we also cannot assess the extent to which loss of 1p or 19q might be confounded with other variables considered. Given that loss of 1p or 19q influences response to chemotherapy, lack of information on these tumor markers precludes further discussion of our finding that those not given chemotherapy had better survival.
As noted in the introduction and above, survival estimates from population registry and clinical trials have different strengths and limitations: Registry data lack consistent and predictive pathology diagnoses for some histologies and have limited treatment data, whereas clinical trials, having stringent pathology criteria and detailed treatment data, are not readily generalizable because many glioma patients are not treated in these trials. Given that this article reports results from a cohort and not a clinical trial, a general limitation of the cohort approach is noteworthy: Patients with particularly good or poor prognoses may be given different treatments, and those with especially poor survival may not live long enough to institute the recommended treatments. This would tend to inflate the observed benefit of certain treatments in this cohort design in comparsion to the corresponding benefits observed in clinical trials.
Summary and Conclusions
To summarize, most of the prognostic variables identified were as expected because of other population or clinical studies of glioma. Comparisons of the study group with SEER-eligible-only cases indicated that recruitment somewhat favored GM patients and astrocytoma, NOS, patients with better survival. Within the study group, comparison of survival estimates using the SEER ICD-O-2 codes versus central review diagnosis for non-GM astrocytomas supports efforts both to standardize and improve neuropathologic coding criteria used by registries and perhaps to have brain tumors reviewed by pathologists with specialty training in neuropathology and specific interest/experience in CNS tumor diagnosis. Finally, the findings that having graduated from college in cases of patients with oligodendroglial tumors and being married at time of diagnosis in cases of GM patients are associated with improved survival, if not due to chance from multiple comparisons or simple confounding by KPS, suggest that these factors and perhaps other social factors deserve further consideration in epidemiological and clinical studies of brain tumors.
Acknowledgments
Thanks to Richard Davis for pathology review of series 1 cases and the pathology departments of the following medical centers for providing tumor specimens for review: Alexian Brothers Medical Center, Alta Bates Summit Medical Center, Brookside, California Pacific Medical Center, Doctors Medical Center Pinole/San Pablo, Eden Medical Center, El Camino Hospital, Good Samaritan Hospital, Alameda County Medical Center Highland Hospital, John Muir Medical Center, Kaiser Redwood City, Kaiser San Francisco, Kaiser Santa Teresa, Community Hospital of Los Gatos, Los Medanos Hospital, Marin General Hospital, Merrithew Memorial Hospital, Mills Peninsula Hospital, Mt. Diablo Medical Center, Mt. Zion Medical Center, Naval Hospital, O’Connor Hospital, Ralph K. Davies Medical Center, Saint Louise Regional Hospital, San Francisco General, San Jose Medical Center, San Leandro Hospital, San Mateo County Health Center, San Ramon Regional Medical Center, Santa Clara Valley Medical Center, Sequoia Hospital, Seton Medical Center, St. Francis Memorial Hospital, St. Luke’s Hospital, St. Rose Hospital, Stanford, University of California, San Francisco, Valley Livermore Memorial, Veterans Palo Alto, San Francisco VA Medical Center, and Washington Hospital. We also thank Jennette Sison and Michelle Moghadassi for assistance in manuscript preparation.
Footnotes
This work was supported by National Institutes of Health grants CA52689, CA097257, CA89032, ES06717, and ES04705. Additional funding was provided by a gift from the Robert J. and Helen H. Glaser Family Foundation.
Abbreviations used are as follows: AA, anaplastic astrocytoma; BUDR, 5-bromo-2′-deoxyuridine; GM, glioblastoma; HR, hazard ratio; ICD-O-2, International Classification of Diseases, Oncology, second edition; NCCC, Northern California Cancer Center; NOS, not otherwise specified; RCA, rapid case ascertainment; RPA, recursive partitioning analysis; SEER, Surveillance, Epidemiology, and End Results; SFBGS, San Francisco Bay Area Adult Glioma Study; UCSF, University of California, San Francisco.
References
- Aldape K, Simmons ML, Davis RL, Miike R, Wiencke J, Barger G, Lee M, Chen P, Wrensch M. Discrepancies in diagnoses of neuroepithelial neoplasms: The San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88:2342–2349. [PubMed] [Google Scholar]
- Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004;22:2567–2575. doi: 10.1200/JCO.2004.11.141. [DOI] [PubMed] [Google Scholar]
- Berleur MP, Cordier S. The role of chemical, physical, or viral exposures and health factors in neurocarcinogenesis: Implications for epidemiologic studies of brain tumors. Cancer Causes Control. 1995;6:240–256. doi: 10.1007/BF00051796. [DOI] [PubMed] [Google Scholar]
- Bhowmick DA, Zhuang Z, Wait SD, Weil RJ. A functional polymorphism in the EGF gene is found with increased frequency in glioblastoma multiforme patients and is associated with more aggressive disease. Cancer Res. 2004;64:1220–1223. doi: 10.1158/0008-5472.can-03-3137. [DOI] [PubMed] [Google Scholar]
- Bondy M, Wiencke J, Wrensch M, Kyritsis AP. Genetics of primary brain tumors: A review. J Neurooncol. 1994;18:69–81. doi: 10.1007/BF01324606. [DOI] [PubMed] [Google Scholar]
- Bruner JM, Inouye L, Fuller GN, Langford LA. Diagnostic discrepancies and their clinical impact in a neuropathology referral practice [see Comments] Cancer. 1997;79:796–803. doi: 10.1002/(sici)1097-0142(19970215)79:4<796::aid-cncr17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Bunin G. What causes childhood brain tumors? Limited knowledge, many clues. Pediatr Neurosurg. 2000;32:321–326. doi: 10.1159/000028961. [DOI] [PubMed] [Google Scholar]
- Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473–1439. doi: 10.1093/jnci/90.19.1473. [DOI] [PubMed] [Google Scholar]
- CBTRUS, Central Brain Tumor Registry of the United States (2002–2003) Primary Brain Tumors in the United States: Statistical Report, 1995–1999. Chicago: CBTRUS.
- Charalampopoulou A, Petridou E, Spyridopoulos T, Dessypris N, Oikonomou A, Athanasiadou-Piperopoulou F, Baka M, Kalmanti M, Polychronopoulou S, Trichopoulos D. An integrated evaluation of socioeconomic and clinical factors in the survival from childhood acute lymphoblastic leukaemia: A study in Greece. Eur J Cancer Prev. 2004;13:397–401. doi: 10.1097/00008469-200410000-00007. [DOI] [PubMed] [Google Scholar]
- Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Davis FG, McCarthy BJ. Epidemiology of brain tumors. Curr Opin Neurol. 2000;13:635–640. doi: 10.1097/00019052-200012000-00004. [DOI] [PubMed] [Google Scholar]
- Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- Davis FG, McCarthy B, Jukich P. The descriptive epidemiology of brain tumors. Neuroimaging Clin N Am. 1999;9:581–594. [PubMed] [Google Scholar]
- De Boer MF, Ryckman RM, Pruyn JF, Van den Borne HW. Psychosocial correlates of cancer relapse and survival: A literature review. Patient Educ Couns. 1999;37:215–230. doi: 10.1016/s0738-3991(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- Horn B, Heideman R, Geyer R, Pollack I, Packer R, Goldwein J, Tomita T, Schomberg P, Ater J, Luchtman-Jones L, Rivlin K, Lamborn K, Prados M, Bollen A, Berger M, Dahl G, McNeil E, Patterson K, Shaw D, Kubalik M, Russo C. A multi-institutional retrospective study of intracranial ependymoma in children: Identification of risk factors. J Pediatr Hematol Oncol. 1999;21:203–211. doi: 10.1097/00043426-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Inskip PD, Linet MS, Heineman EF. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17:382–414. doi: 10.1093/oxfordjournals.epirev.a036200. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch, J., and Prentice, R. (1980) The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons.
- Karak AK, Singh R, Tandon PN, Sarkar C. A comparative survival evaluation and assessment of interclassification concordance in adult supratentorial astrocytic tumors. Pathol Oncol Res. 2000;6:46–52. doi: 10.1007/BF03032658. [DOI] [PubMed] [Google Scholar]
- Kleihues, P., and Cavenee, W.K. (Eds.) (1997) Pathology and Genetics of Tumours of the Nervous System. Lyon, France: International Agency for Research on Cancer.
- Kleihues, P., Burger, P.C., and Scheithauer, B.W. (1993a) Histological Typing of Tumours of the Central Nervous System, 2nd ed. (WHO International Histological Classification of Tumours series). Berlin: Springer-Verlag.
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993b;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Kyritsis AP, Bondy ML, Hess KR, Cunningham JE, Zhu D, Amos CJ, Yung WK, Levin VA, Bruner JM. Prognostic significance of p53 immunoreactivity in patients with glioma. Clin Cancer Res. 1995;1:1617–1622. [PubMed] [Google Scholar]
- Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro-Oncology. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, V.A., Leibel, S.A., and Gutin, P.H. (2001) Neoplasms of the central nervous system. In: DeVita, V.T., Jr., Hellman, S., and Rosenberg, S.A. (Eds.), Cancer: Principles and Practice of Oncology, 6th ed., Vol. 2. Philadelphia: Lippincott, Williams and Wilkins, pp. 2100–2160.
- Lin H, Bondy ML, Langford LA, Hess KR, Delclos GL, Wu X, Chan W, Pershouse MA, Yung WK, Steck PA. Allelic deletion analyses of MMAC/PTEN and DMBT1 loci in gliomas: Relationship to prognostic significance. Clin Cancer Res. 1998;4:2447–2454. [PubMed] [Google Scholar]
- Louis, D.N., and Stemmer-Rachamimov, A.O. (2000) Pathology and classification. In: Bernstein, M., and Berger, M. (Eds.), Neuro-Oncology: The Essentials New York: Thieme Medical Publishers, pp. 18–29.
- McCarthy BJ, Surawicz T, Bruner JM, Kruchko C, Davis F. Consensus Conference on Brain Tumor Definition for registration. Neuro-Oncology. 2002;4:134–145. doi: 10.1215/15228517-4-2-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro JM, Misra A, Zhang L, Smirnov I, Colman H, Griffin C, Ozburn N, Chen M, Pan E, Koul D, Yung WK, Feuerstein BG, Aldape KD. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65:1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- Okcu MF, Selvan M, Wang LE, Stout L, Erana R, Airewele G, Adatto P, Hess K, Ali-Osman F, Groves M, Yung AW, Levin VA, Wei Q, Bondy M. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S. Epidemiology of primary CNS neoplasms. Neurol Clin. 1996;14:273–290. doi: 10.1016/s0733-8619(05)70256-5. [DOI] [PubMed] [Google Scholar]
- Puduvalli VK, Kyritsis AP, Hess KR, Bondy ML, Fuller GN, Kouraklis GP, Levin VA, Bruner JM. Patterns of expression of Rb and p16 in astrocytic gliomas, and correlation with survival. Int J Oncol. 2000;17:963–969. doi: 10.3892/ijo.17.5.963. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Wilhelmsen L. Cancer incidence, mortality from cancer and survival in men of different occupational classes. Eur J Epidemiol. 2004;19:533–540. doi: 10.1023/b:ejep.0000032370.56821.71. [DOI] [PubMed] [Google Scholar]
- Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK, Steck PA. Differential expression of MMAC/PTEN in glioblastoma multiforme: Relationship to localization and prognosis. Cancer Res. 1999;59:1820–1824. [PubMed] [Google Scholar]
- Sant M, van der Sanden G, Capocaccia R. Survival rates for primary malignant brain tumours in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:2241–2247. doi: 10.1016/s0959-8049(98)00336-0. [DOI] [PubMed] [Google Scholar]
- SAS Institute (1990) SAS Procedures Guide: Version 6. Cary, NC: SAS Institute, Inc.
- Scott CB, Scarantino C, Urtasun R, Movsas B, Jones CU, Simpson JR, Fischbach AJ, Curran WJ., Jr Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: A report using RTOG 90-06. Int J Radiat Oncol Biol Phys. 1998a;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, Mac-Kinnon JA, Sutherland G, Cairncross JG, Forsyth P. Long-term glioblastoma multiforme survivors: A population-based study. Can J Neurol Sci. 1998b;25:197–201. doi: 10.1017/s0317167100034016. [DOI] [PubMed] [Google Scholar]
- Sigurdson AJ, Bondy ML, Hess KR, Toms SA, Kyritsis AP, Gu J, Wang LE, Wang X, Adatto P, Bruner JL, Yung WK, Levin VA, Wei Q. Gamma-ray mutagen sensitivity and survival in patients with glioma. Clin Cancer Res. 1998;4:3031–3035. [PubMed] [Google Scholar]
- Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG, 2nd and, Aldape K. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–1128. [PubMed] [Google Scholar]
- Smedslund G, Ringdal GI. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. J Psychosom Res. 2004;57:123–131. doi: 10.1016/S0022-3999(03)00575-0. [DOI] [PubMed] [Google Scholar]
- Soler-Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and white breast cancer patients: A population-based study. Cancer. 2003;98:1299–1308. doi: 10.1002/cncr.11670. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- Stokes, M.E., Davis, C.S., and Koch, G.G. (1995) Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute, Inc.
- Tang J, Shao W, Tevfik Dorak M, Li Y, Miike R, Lobashevsky E, Wiencke JK, Wrensch M, Kaslow RA, Cobbs CS. Positive and negative associations of human leukocyte antigen variants with the onset and prognosis of adult glioblastoma multiforme. Cancer Epidemiol Biomarkers Prev. 2005;14:2040–2044. doi: 10.1158/1055-9965.EPI-05-0136. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ. Advances in the biology and treatment of oligodendrogliomas. Curr Opin Neurol. 2004;17:675–680. doi: 10.1097/00019052-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- Wei Q, Bondy ML, Mao L, Gaun Y, Cheng L, Cunningham J, Fan Y, Bruner JM, Yung WK, Levin VA, Kyritsis AP. Reduced expression of mismatch repair genes measured by multiplex reverse transcription-polymerase chain reaction in human gliomas. Cancer Res. 1997;57:1673–1677. [PubMed] [Google Scholar]
- Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–615. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R, Wiencke J, Neuhaus J. Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. 1997;145:581–593. doi: 10.1093/oxfordjournals.aje.a009154. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro-Oncology. 2002;4:278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]