Abstract
Vestibular schwannomas, commonly termed acoustic neuromas, arise from the vestibular branch of the eighth cranial nerve (acoustic nerve) and are benign, slow-growing brain tumors that negatively impact patient quality of life. They are thought to account for the majority of intracranial nerve sheath tumors. To describe incidence rate patterns and trends of primary nerve sheath tumors of the brain/CNS and the subset of vestibular schwannomas in two population-based incidence registries, data were obtained from 11 Central Brain Tumor Registry of the United States (CBTRUS) collaborating state registries and the Los Angeles County Cancer Surveillance Program (LACCSP) (1975–1998). Average annual incidence rates were tabulated by age, gender, race, year, and region and were age-adjusted to the year 2000 U.S. standard population. Multiplicative Poisson regression models were used to compare trends in primary nerve sheath tumors of the brain/CNS overall and in subgroups, including vestibular schwannomas, controlling for age, gender, race, microscopic confirmation, and region. Join-point regression analysis was used to identify any sharp changes in incidence over time. The overall incidence of primary nerve sheath tumors of the brain/CNS was 1.1 per 100,000 person-years (CBTRUS, 1995–1999 and LACCSP, 1995–1998). The incidence of vestibular schwannomas was similar for both data sets: 0.6 per 100,000 person-years (CBTRUS, 1995–1999) and 0.8 per 100,000 person-years (LACCSP, 1995–1998). Moreover, the incidence of primary nerve sheath tumors of the brain/CNS overall (CBTRUS, 1985–1999 and LACCSP, 1975–1998) and of vestibular schwannomas (CBTRUS, 1992–1999 and LACCSP, 1992–1998) increased over time. However, the incidence of benign schwannomas in sites other than the acoustic nerve either decreased (CBTRUS, 1992–1999) or experienced no significant change (LACCSP, 1992–1998). While improvements in diagnosis and reporting may explain some of these trends, further consideration of potential etiologic factors may be warranted.
Keywords: acoustic neuroma, average annual percentage change, incidence trends, nerve sheath tumors, vestibular schwannoma
Vestibular schwannomas, commonly termed acoustic neuromas, arise from the vestibular branch of the eighth cranial nerve and are benign, slow-growing brain tumors that negatively impact patient quality of life. Clinical and hospital-based studies have been completed, and vestibular schwannomas are thought to account for the majority of intracranial nerve sheath tumors (Pitts et al., 1996).
In the United States, the Central Brain Tumor Registry of the United States (CBTRUS)3 has reported an incidence rate for all primary nerve sheath tumors of the brain/CNS of 0.8 per 100,000 person-years for the years 1990–1994 and 1.1 per 100,000 person-years for the years 1995–1999 (CBTRUS, 1998, 2002–2003). In Los Angeles County, Preston-Martin et al. (1989a) reported incidence rates of intracranial nerve sheath tumors of 0.6 and 0.7 per 100,000 person-years in males and females, respectively (1972–1985 data). In a trend analysis using CBTRUS data, Jukich et al. (2001) found that the incidence rate of primary nerve sheath tumors of the brain/CNS increased almost 6% per year in males between 1985 and 1994. This was a statistically significant increase, in contrast to the 0.9% increase observed for all primary brain/CNS tumors as a group. Increases in the incidence of vestibular schwannomas over the last few decades have also been observed in Denmark and other countries (Howitz et al., 2000; Tos et al., 1999).
Various factors may have influenced the observed increases over time in the incidence of nerve sheath tumors and vestibular schwannomas in particular. These factors include the introduction and subsequent use of CT and MRI to diagnose tumors, an increased awareness among clinicians of the diagnosis of vestibular schwannoma, increased access to CT and MRI, increased diagnostic testing of symptomatic elderly by physicians, and/or a true increase in the incidence of these tumors. The introduction and subsequent availability of CT in the early 1970s and MRI in the early 1980s enabled clinicians to make noninvasive diagnoses, which led to identification of brain tumors previously undiagnosed. Older age groups are more likely to have been affected by the increased use of noninvasive techniques as well as changes in Medicare coverage for CT and MRI (Gibson et al., 1984; Modan et al., 1992). These widespread changes may have led to increases in incidental and noninvasive diagnoses of brain tumors, especially in the over-65-year age group, which is covered by Medicare. Improvements in diagnostic technology and diagnostic alertness among clinicians have also impacted the observed incidence of brain tumors (Desmeules et al., 1992; Mosso et al., 1992; Radhakrishnan et al., 1995).
Major changes in brain tumor classification have also occurred. The first edition of the International Classification of Diseases for Oncology (ICDO), a coding system used by cancer registries in the classification of tumors, was published in 1976 (WHO, 1976). This edition of the ICDO included topography and morphology sections, but had only one site code for tumors occurring in the cranial nerves (192.0). The second edition of the ICDO was published in 1990 and included a new scheme to code tumors by site (topography) (Percy et al., 1990). Tumors occurring in the cranial nerves were now split into four topography codes: C72.2 (olfactory nerve), C72.3 (optic nerve), C72.4 (acoustic nerve), and C72.5 (cranial nerve, not otherwise specified [NOS]) (Percy et al., 1990). Registry data prior to 1992 were recoded to reflect the new scheme (e.g., 192.0 converted to C72.5). Another change was the revision of the WHO’s histology classification scheme for tumors of the central nervous system (Kleihues et al., 1993a). This scheme serves as a guideline in surgical pathology and is used by neuropathologists for histologic typing of brain tumors (Kleihues et al., 1993b). Several of the new entities in the 1993 WHO recoding did not have corresponding four-digit histology codes in the ICDO (Kleihues et al., 1993a). The effect of these coding changes on the reporting of brain tumor data is unknown.
This report describes the incidence rate patterns by age, gender, race, and time in two population-based incidence registries: the CBTRUS and the Los Angeles County Cancer Surveillance Program (LACCSP). We assess data on all nerve sheath tumors, contrasting vestibular schwannomas with those benign schwannomas not coded to the eighth cranial nerve, to understand overall and subgroup patterns and to determine if the previously reported increase in intracranial primary nerve sheath tumors primarily reflects a trend in the subgroup of vestibular schwannomas. If so, more extensive etiologic studies evaluating potential environmental causes of these tumors will be better justified.
Materials and Methods
Data Collection
Data on all nerve sheath tumors were obtained from three sources: (1) data on all incident primary nerve sheath tumors of the brain/CNS diagnosed from 1995 to 1999 compiled from seven CBTRUS collaborating regions (Arizona, Colorado, Maine, Massachusetts, Minnesota, New York, and North Carolina), (2) data on all incident primary nerve sheath tumors of the brain/CNS diagnosed from 1985 to 1999 compiled from four CBTRUS collaborating regions (Connecticut, Delaware, Idaho, and Montana), and (3) data on all incident primary nerve sheath tumors of the brain/CNS diagnosed from 1975 to 1998 compiled from LACCSP.
These data included tumors with the following ICDO site codes (Percy et al., 1990): C70.0–C70.9 (meninges), C71.0–C71.9 (brain), and C72.0–C72.9 (spinal cord, cranial nerves, and other parts of CNS). All primary nerve sheath tumors of the brain/CNS with ICDO behavior codes 0 (benign), 1 (uncertain), and 3 (malignant) were included. Available information included year of diagnosis, age at diagnosis, gender, race, five-digit ICDO-2 morphology code (histology plus behavior), ICDO site code, and diagnostic confirmation. Data quality was assessed by use of EDITS computer software developed by the Centers for Disease Control and Prevention (CDC) to edit cancer registry data (CDC, 1997).
Population data for each region were obtained from the NCI Surveillance, Epidemiology, and End Results (SEER) program (http://seer.cancer.gov) and were made available by the U.S. Census Bureau.
Data were analyzed by major age groups (0–19, 20–44, 45–64, and 65+ years) and by gender (male and female). Cases were also grouped by race, but given the small numbers of nonwhites, stratification was limited to whites and nonwhites. A variable for microscopic confirmation was included. Tumors were considered “confirmed” or “not confirmed” according to the SEER Program Code Manual (Fritz et al., 1998): Cases with diagnostic confirmation codes 1, 2, or 4—respectively indicating positive histology, positive cytology but no positive histology, and positive microscopic confirmation with method not specified—were considered microscopically confirmed; cases with codes indicating positive laboratory test/marker study (5), direct visualization without microscopic confirmation (6), radiography and other imaging techniques without microscopic confirmation (7), clinical diagnosis only (8), or unknown whether or not microscopically confirmed (9) were considered not microscopically confirmed.
Statistical Analysis
To clarify classification of vestibular schwannomas in the population-based registry data, we assessed data on all primary nerve sheath tumors of the brain/CNS, contrasting those benign schwannomas coded to the eighth cranial nerve with those coded to all other brain/CNS sites to understand overall and subgroup patterns. Data were categorized as follows: all primary nerve sheath tumors of the brain/CNS (sites C70.0–C72.9 and histology 9540–9570) and the nerve sheath tumor subgroups of benign schwannomas (morphology 9560/0), vestibular schwannomas (site C72.4 and morphology 9560/0), and benign schwannomas in sites other than the acoustic nerve (sites other than C72.4 and morphology 9560/0). A detailed description of the nerve sheath tumor categories can be found in Table 1.
Table 1.
Brain and CNS nerve sheath tumors and subgroups by site and morphology
| Type of Tumor | ICDO Topography Code | Site | ICDO-2 Histology Code | Histology |
|---|---|---|---|---|
| Nerve sheath tumors | C70.0–C72.9 | Brain, meninges, spinal cord, cranial nerves, and other parts of the CNS | 9540–9570 | Neurofibroma, NOS; neurofibromatosis, NOS; neurofibrosarcoma; melanotic neurofibroma; plexiform neurofibroma; schwannoma, NOS (neurilemmoma, NOS); neurinomatosis; neurilemmoma, malignant; triton tumor, malignant; neuroma, NOS |
| Subgroups of Nerve Sheath Tumors | ||||
| Schwannoma, benign | C70.0–C72.9 | Brain, meninges, spinal cord, cranial nerves, and other parts of the CNS | 9560/0 | Schwannoma, NOS |
| Mutually Exclusive Subgroups of Benign Schwannomas | ||||
| Vestibular schwannoma | C72.4 | Acoustic nerve | 9560/0 | Schwannoma, NOS |
| Nonvestibular schwannoma | C70.0–C72.9, excluding C72.4 | Brain, meninges, spinal cord, cranial nerves (excluding acoustic nerve), and other parts of the CNS | 9560/0 | Schwannoma, NOS |
Abbreviations: ICDO, International Classification of Diseases for Oncology; NOS, not otherwise specified.
Descriptive statistics were tabulated for nerve sheath tumors overall and tumor subgroups by age group, gender, race, and microscopic confirmation status for CBTRUS 1995–1999 data and LACCSP 1995–1998 data. Overall average annual incidence rates and average annual incidence rates by age group, gender, and race were determined for all nerve sheath tumors and the subgroup of vestibular schwannomas. Incidence rates were age adjusted by using the year 2000 U.S. standard population.
Trends in incidence rates over time were analyzed for CBTRUS 1985–1999 data and LACCSP 1975–1998 data. Because of changes in coding of site of tumor (ICDO topography code), the time frame of analysis for vestibular schwannomas was limited to the years 1992 and forward. Incidence rates by calendar year were computed. Trends were analyzed for primary nerve sheath tumors of the brain/CNS overall and for the subgroups of benign schwannomas, vestibular schwannoma, and nonvestibular schwannoma, controlling for age, gender, race, and microscopic confirmation. Trends are expressed as average annual percentage change with corresponding two-sided 95% confidence intervals. Multiplicative Poisson regression models were used to statistically compare trends in incidence rates over time (Estève et al., 1994).
Multiplicative Poisson regression models were fit by using the “proc genmod” procedure in SAS software (release 8.02) (SAS Institute, 1999). Models were fit for all nerve sheath tumors and for the tumor subgroups. The variable of interest was time, expressed as year of tumor diagnosis and coded as a continuous variable. All models were adjusted for age, gender, race, and microscopic confirmation. Age group (0–19, 20–44, 45–64, or 65+) was coded as a categorical variable. Indicator variables were used for gender (male, female), race (white, non-white), and microscopic confirmation (microscopically confirmed, not microscopically confirmed). Hierarchical models with two- or four-way interaction terms for year with age group, gender, race, and microscopic confirmation were assessed. Statistically significant (P < 0.05) interaction terms indicated a different trend over time for the corresponding variable value. Statistically significant (P < 0.05) interaction terms were included in the final model. When more than one interaction term was significant (P < 0.05) for a particular subgroup, appropriate interaction terms were entered into a hierarchical model. When interaction terms were included in final models, separate average annual percentage changes were listed for the fully adjusted model (without interaction terms), as well as for the appropriate microscopic confirmation, race, gender, and age group strata (with the statistically significant [P < 0.05] interaction terms). Estimates are given for multiple strata within a specific tumor type when statistically significant (P < 0.05) interaction was detected. P values are indicated when appropriate.
Joinpoint regression software was obtained from the Web site of the NCI Statistical Research Applications Branch (http://srab.cancer.gov/joinpoint) and was used to identify any sharp changes in incidence occurring over the time periods analyzed (Kim et al., 2000).
Data were summarized in tabular and graphic forms to best reflect any statistically significant (P < 0.05) variations in age, gender, race, microscopic confirmation status, time, and region that emerged. The patterns observed in CBTRUS regions and Los Angeles County were compared and contrasted in order to enhance the reliability of the patterns that emerged.
Results
Average Annual Incidence Rates
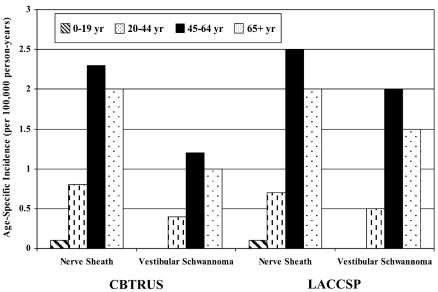
The CBTRUS data set included 2811 cases of nerve sheath tumors diagnosed from 1995 through 1999. Eighty-nine percent of the nerve sheath tumors were benign schwannomas, 57% of the benign schwannomas were vestibular schwannomas (i.e., located in the acoustic nerve), and 84% of nerve sheath tumors and 77% of vestibular schwannomas were microscopically confirmed. The incidence of nerve sheath tumors was 1.1 per 100,000 person-years, and the incidence of vestibular schwannomas was 0.6 per 100,000 person-years (Table 2). The age-, gender-, and race-specific incidence of all primary nerve sheath tumors and vestibular schwannoma is shown in Table 2. Age-specific rates are illustrated in Fig. 1. Incidence of vestibular schwannoma was similar among males and females, higher in whites than in nonwhites, lowest among the 0- to 19-year age group, and highest among the 45- to 64-year age group. Patterns of incidence among nerve sheath tumors by gender, race, and age were similar to those of vestibular schwannomas.
Table 2.
Average annual incidence rates, overall and by gender and race, CBTRUS (1995–1999) and LACCSP (1995–1998)a
|
Gender |
Race |
|||||
|---|---|---|---|---|---|---|
| Type of Tumor | Total Number of Tumors (N) | Overall Rate (CI) | Male | Female | White | Nonwhite |
| CBTRUS, 1995–1999 | ||||||
| Nerve sheath | 2,811 | 1.08 (1.04–1.12) | 1.10 (1.04–1.16) | 1.07 (1.02–1.13) | 1.13 (1.08–1.17) | 0.56 (0.48–0.63) |
| Vestibular schwannoma | 1,424 | 0.55 (0.52–0.58) | 0.56 (0.52–0.60) | 0.55 (0.51–0.58) | 0.58 (0.55–0.61) | 0.23 (0.18–0.28) |
| LACCSP, 1995–1998 | ||||||
| Nerve sheath | 352 | 1.11 (0.99–1.22) | 1.15 (0.97–1.32) | 1.07 (0.91–1.23) | 1.21 (1.08–1.36) | 0.68 (0.50–0.87) |
| Vestibular schwannoma | 256 | 0.82 (0.71–0.92) | 0.83 (0.68–0.99) | (0.66–0.94)0.80 | 0.89 (0.77–1.01) | 0.51 (0.36–0.67) |
Rates are per 100,000 person-years and are age adjusted to the year 2000 U.S. standard population.
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; LACCSP, Los Angeles County Cancer Surveillance Program.
Fig. 1.
Average annual incidence rates by age at diagnosis, CBTRUS, years 1995–1999, and LACCSP, years 1995–1998.
The LACCSP data set included 352 cases of nerve sheath tumors diagnosed from 1995 through 1998. Vestibular schwannomas accounted for 256 of these cases. Ninety-eight percent of nerve sheath tumors of the brain/CNS were benign schwannomas. Seventy-four percent of the benign schwannomas were vestibular schwannomas. Ninety-seven percent of nerve sheath tumors and 96% of vestibular schwannomas were microscopically confirmed. The incidence of nerve sheath tumors was 1.1 per 100,000 person-years, and the incidence of vestibular schwannomas was 0.8 per 100,000 person-years (Table 2). Incidence of vestibular schwannomas was similar among males and females, higher in whites than in nonwhites, lowest among the 0- to 19-year age group, and highest among the 45- to 64-year age group (Fig. 1). Patterns of incidence among nerve sheath tumors by gender, race, and age were similar to those of vestibular schwannomas for both regions.
Time Trends
In the period 1985–1999, 733 cases of nerve sheath tumors, of which 682 cases were benign schwannomas, were diagnosed in the geographic areas of CBTRUS. Within the time frame 1992–1999, 200 cases of vestibular schwannomas and 250 cases of nonvestibular schwannomas were diagnosed. In the period 1975–1998, 1745 cases of nerve sheath tumors, of which 1693 cases were benign schwannomas, were diagnosed in Los Angeles County. Within the time frame 1992–1998, 417 cases of vestibular schwannomas and 149 cases of non-vestibular schwannomas were diagnosed. The distribution of select variables for cases of primary nerve sheath tumor, benign schwannoma, vestibular schwannoma, and nonvestibular schwannoma in the CBTRUS and LACCSP data is shown in Table 3. A greater proportion of benign schwannoma data were vestibular schwannomas (i.e., located in the acoustic nerve) for LACCSP data as compared to CBTRUS data (74% vs. 44%, 1992+ data). More than 97% of nerve sheath tumors and 97% of vestibular schwannomas were microscopically confirmed for LACCSP data, whereas more than 92% of nerve sheath tumors but about 80% of vestibular schwannomas were microscopically confirmed for CBTRUS data.
Table 3.
Characteristics of subjects included in analysis of time trends, CBTRUS (1985–1999) and LACCSP (1975–1998)
| Time Period | Type of Tumor | Total No. of Tumors | Percent Female | Percent White | Median Age at Diagnosis (years) | Percent Microscopically Confirmed |
|---|---|---|---|---|---|---|
| CBTRUS | ||||||
| 1985–1999 | Nerve sheath | 733 | 52.5 | 93.2 | 52 | 92.6 |
| Schwannoma, benign | 682 | 52.9 | 93.1 | 53 | 92.2 | |
| 1992–1999 | Vestibular schwannoma | 200 | 50.5 | 93.5 | 55 | 80.5 |
| Nonvestibular schwannoma | 250 | 50.0 | 92.0 | 52 | 96.4 | |
| LACCSP | ||||||
| 1975–1998 | Nerve sheath | 1745 | 52.3 | 82.5 | 52 | 97.4 |
| Schwannoma, benign | 1693 | 52.3 | 82.6 | 52 | 97.5 | |
| 1992–1998 | ||||||
| Vestibular schwannoma | 417 | 52.8 | 78.7 | 52 | 97.4 | |
| Nonvestibular schwannoma | 149 | 48.3 | 81.9 | 47 | 99.3 | |
Abbreviations: CBTRUS, Central Brain Tumor Registry of the United States; LACCSP, Los Angeles County Cancer Surveillance Program.
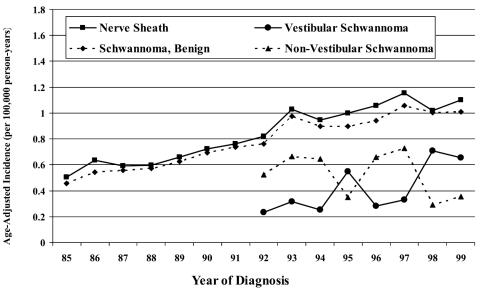
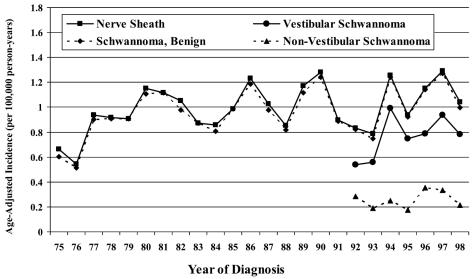
The annual incidence rates ranged from 0.5 to 1.2 per 100,000 person-years for nerve sheath tumors (1985–1999) and 0.2 to 0.7 per 100,000 person-years for vestibular schwannomas (1992–1999) for CBTRUS data. For LACCSP data, the annual incidence rates ranged from 0.5 to 1.3 per 100,000 person-years for nerve sheath tumors (1975–1998) and 0.5 to 1.0 per 100,000 person-years for vestibular schwannomas (1992–1998). Overall incidence by calendar year is presented in Figs. 2 and 3.
Fig. 2.
Incidence rates by calendar year, CBTRUS, years 1985–1999.
Fig. 3.
Incidence rates by calendar year, LACCSP, years 1975–1998.
The results of Poisson regression analysis for CBTRUS data (nerve sheath tumor and benign schwannoma, 1985–1999; vestibular and nonvestibular schwannomas, 1992–1999) are summarized in Table 4. Incidence of nerve sheath tumors increased about 5% per year (95% confidence interval [CI], 3.7%–7.1%) and incidence of vestibular schwannomas increased about 14% per year (95% CI, 8.2%–20.8%). Statistically significant increasing trends were observed for nerve sheath tumors overall (P < 0.0001) and the subgroups of benign schwannomas (P < 0.0001) and vestibular schwannomas (P < 0.0001). Nonvestibular schwannomas exhibited a statistically significant decreasing trend (P = 0.0411). Variations in time trends specific to microscopic confirmation status for nerve sheath tumors, benign schwannomas, and vestibular schwannomas were identified (P values for interaction terms [year × microscopic confirmation] were P < 0.0001, P < 0.0001, and P = 0.0037, respectively). Statistically significant positive trends for nerve sheath tumors overall (P < 0.0001), benign schwannomas (P < 0.0001), and vestibular schwannomas (P = 0.0038) persisted upon analysis of the subset of cases with microscopic confirmation. Acceleration in incidence was observed for tumors without microscopic confirmation. The data exhibited statistically significant increasing time trends specific to microscopic confirmation (P value for interaction term [year × microscopic confirmation] was P = 0.0037) and race (P value for interaction term [year × race] was P = 0.0078) for vestibular schwannomas. No significant time trends specific to age groups, gender, and race were observed for nerve sheath tumors, benign schwannomas, and nonvestibular schwannomas (P values for interaction terms were P > 0.05).
Table 4.
Incidence rate trendsa,b by AAPC, CBTRUS (1985–1999)
| Type of Tumor | No. of Cases | AAPC (%) | 95% CI |
|---|---|---|---|
| 1985–1999 | |||
| Nerve sheathc | 733 | 5.4 | 3.7, 7.1 |
| Microscopically confirmedc | 679 | 4.1 | 2.3, 5.9 |
| Not microscopically confirmedc | 54 | 27.8 | 19.3, 37.3 |
| Schwannoma, benignc | 682 | 5.4 | 3.7, 7.2 |
| Microscopically confirmedc | 629 | 4.0 | 2.2, 5.8 |
| Not microscopically confirmedc | 53 | 28.7 | 20.0, 38.5 |
| 1992–1999 | |||
| Vestibular schwannomac | 200 | 14.4 | 8.2, 20.8 |
| Microscopically confirmedc | 161 | 10.2 | 3.3, 17.2 |
| Not microscopically confirmedc | 39 | 34.8 | 19.0, 52.4 |
| Whitec | 187 | 12.2 | 5.8, 18.7 |
| Microscopically confirmedc | 149 | 7.3 | 0.3, 14.5 |
| Not microscopically confirmedc | 38 | 34.1 | 18.2, 51.7 |
| Nonwhitec,d | 13 | 64.3 | 29.3, 112.0 |
| Nonvestibular schwannomac | 250 | −5.7 | −11.1, −0.2 |
Abbreviations: AAPC, average annual percentage change; CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval.
Poisson regression models are adjusted for gender, race, age group, and microscopic confirmation.
The tumor types of nerve sheath and benign schwannoma have variation in trend by microscopic confirmation (nerve sheath, year × microscopic confirmation, P < 0.0001, and benign schwannoma, year × micropscopic confirmation, P < 0.0001), and the tumor type of vestibular schwannoma has variation in trend by race (year × race, P = 0.0078) and microscopic confirmation (year × microscopic confirmation, P = 0.0037).
The specific trend estimate is statistically significant (P < 0.05).
Sample size was insufficient to allow separate estimates by microscopic confirmation.
The results of Poisson regression analysis for LACCSP data (nerve sheath tumor and benign schwannoma, 1975–1998; vestibular and nonvestibular schwannomas, 1992–1998) are summarized in Table 5. Incidence of nerve sheath tumors increased about 1% per year (95% CI, 0.5%–1.9%), and incidence of vestibular schwannomas increased almost 6% per year (95% CI, 1.0%–10.6%). Statistically significant increasing trends for nerve sheath tumors overall (P = 0.0241) and the subgroups of benign schwannomas (P = 0.0324) and vestibular schwannomas (P = 0.0184) were observed. The data exhibited a slight, increasing trend for non-vestibular schwannomas, but it was not statistically significant (P = 0.8233). Variation in time trends specific to microscopic confirmation status for vestibular schwannomas was identified (P value for interaction term [year × microscopic confirmation] was 0.0222). The positive trend for vestibular schwannomas persisted upon analysis of the subset of cases with microscopic confirmation, but was not statistically significant (P = 0.0535). Acceleration in incidence was observed for vestibular schwannomas without microscopic confirmation. No significant time trends specific to age groups, gender, or race were observed for any tumor type (P values for all interaction terms were P > 0.05).
Table 5.
Incidence rate trendsa,b by AAPC, LACCSP (1975–1998)
| Type of Tumor | No. of Cases | AAPC (%) | 95% CI |
|---|---|---|---|
| 1975–1998 | |||
| Nerve sheathc | 1745 | 1.2 | 0.5, 1.9 |
| Schwannoma, benignc | 1693 | 1.3 | 0.6, 2.0 |
| 1992–1998 | |||
| Vestibular schwannomac | 417 | 5.8 | 1.0, 10.6 |
| Microscopically confirmed | 406 | 4.8 | –0.1, 9.7 |
| Not microscopically confirmedd | 11 | 50.9 | 15.6, 96.0 |
| Nonvestibular schwannoma | 149 | 0.9 | −7.1, 8.9 |
Abbreviations: AAPC, average annual percentage change; CI, confidence interval; LACCSP, Los Angeles County Cancer Surveillance Program.
Poisson regression models are adjusted for gender, race, age group, and microscopic confirmation.
The tumor type of vestibular schwannoma has variation in trend by microscopic confirmation (year × microscopic confirmation, P = 0.0222).
The specific trend estimate is statistically significant (P < 0.05).
No statistically significant changes in trend (P > 0.05) (whether in rate or direction) for any of the diagnostic and demographic subgroups for either region were observed with joinpoint regression analysis.
Discussion
This report presents a description of more than 20 years of primary malignant and nonmalignant nerve sheath tumor data, including eight years of vestibular schwannoma data. Changes in diagnostic practices and brain tumor classification, including the introduction of CT and MRI, occurred during this time period and have been taken into consideration. We analyzed data from two registries, providing different populations for which to compare and contrast incidence rates and time trends. Overall incidence rates and patterns by age, gender, and race were estimated from data from the LACCSP and 11 collaborating regions of CBTRUS, representing approximately 20% of the U.S. population. The time trend analysis was based on data from (1) the large, diverse population within the catchment area of the LACCSP and (2) four collaborating regions of CBTRUS (Connecticut, Delaware, Idaho, and Montana) that reflect approximately 4% of the U.S. population, but disproportionately reflect individuals from the Northeast and Caucasians.
Incidence of nerve sheath tumors of the brain/CNS was similar in the CBTRUS and the LACCSP data. Incidence of the subgroup vestibular schwannomas was also similar in both sets of data (0.6 per 100,000 person-years vs. 0.8 per 100,000 person-years, respectively). In addition, these rates approximate estimates of vestibular schwannoma in Denmark (range, 0.2–0.8 per 100,000 person-years during the years 1960 to 1998 [Howitz et al., 2000]; 0.78 per 100,000 [Tos et al., 1999]) and Sweden (0.5 per 100,000 during 1977–1981 and 1.0 per 100,000 during 1992–1995 [Hardell et al., 2003]). For both registries, patterns of incidence rates of nerve sheath tumors, and specifically vestibular schwannomas, were similar among males and females, higher in whites than in nonwhites, lowest among the 0- to 19-year age group, and highest among the 45- to 64-year age group.
Time trends differed in the two race categories for vestibular schwannomas, although this result was not robust because of the small number of nonwhites in the four geographic regions of the CBTRUS data. Overall positive trends were observed in cases with microscopic confirmation and those without microscopic confirmation. The steep increase in incidence rates over time for nonmicroscopically confirmed tumors as compared to those with microscopic confirmation may have reflected changes in case ascertainment; however, the number of nonmicroscopically confirmed tumors was too small per year to evaluate this further.
During an examination of pathology reports in Los Angeles County, it was observed that approximately 90% of intracranial nerve sheath tumors arose in the eighth cranial nerve (S.P.-M., personal communication, 2002). A preliminary analysis of CBTRUS data looking at neurilemmomas and neuromas suggested that only 45% were coded as acoustic nerve tumors. As this differs from clinical texts (Pitts et al., 1996), it was important to clarify classification of vestibular schwannoma in population-based registry data. For the CBTRUS 1995–1999 data set, 53% of nerve sheath tumors of the brain/CNS (intra- and extracranial) were coded to the acoustic nerve, whereas for the LACCSP 1995–1998 data set, 73% of nerve sheath tumors of the brain/CNS (intra- and extracranial) were coded to the acoustic nerve. Excluding nerve sheath tumors coded to the spinal cord and cauda equina (i.e., extracranial tumors) resulted in 64% of nerve sheath tumors being coded to the acoustic nerve for CBTRUS 1995–1999 and 91% of nerve sheath tumors being coded to the acoustic nerve for LACCSP 1995–1998.
The vestibular schwannoma data for both CBTRUS and LACCSP should have been unaffected by the diagnostic changes brought about by the introduction of CT and MRI, as the time frame of the analyses was 1992 and forward. The CBTRUS nerve sheath tumor data covered a period of 15 years (1985–1999), which may have been impacted by the effects of the introduction of MRI, which occurred in the early 1980s. Increases observed over the 24 years (1975–1998) of nerve sheath tumor data from LACCSP could have been impacted by changes in diagnostic practice with the introduction of the CT scan in the 1970s and MRI in the early 1980s. However, no sharp changes in incidence over time (whether in rate of incidence or direction of trend) for any of the tumor types for any of the data were detected by joinpoint regression analysis, which lends weight to the assertion that diagnostic improvements throughout the time frames of analysis seem unlikely to have altered or strongly impacted the entire pattern of trends. For example, no plateauing of rates at a level detectable by joinpoint regression analysis occurred in the 1990s (after CT/MRI introduction). Also, analysis of 1992+ nerve sheath tumor data and data for the subgroup of benign schwannomas revealed positive time trends for that post-CT/MRI time frame for both CBTRUS and LACCSP data, suggesting continued and steady diagnostic improvements over time, improved case ascertainment, or the presence of an unexplained increase.
There were many similarities in time trends between the CBTRUS and LACCSP data. For instance, both sets of data exhibited (1) statistically significant (P < 0.05) trends of increased incidence of nerve sheath tumors of the brain/CNS overall and for the subgroups of benign schwannomas and vestibular schwannomas; (2) positive trends in incidence of vestibular schwannomas, which persisted upon analysis of the subset of cases with microscopic confirmation; (3) no variation in trends of incidence by age, gender, or race for nerve sheath tumors, benign schwannomas, and nonvestibular schwannomas; (4) no sharp changes in incidence over time; and (5) positive trends for nerve sheath tumors, primarily reflecting the trends of the subgroup of vestibular schwannomas. These similarities support the likelihood that the observations are real.
Variations in diagnosis, coding, and reporting practices among regions may explain some of the differences in incidence and patterns between regions. This report documented that a greater proportion of nerve sheath tumors were benign schwannomas, a greater proportion of benign schwannomas were vestibular schwannomas, and a greater proportion of nerve sheath tumors and the subgroup of vestibular schwannomas were microscopically confirmed for the LACCSP data than for the CBTRUS data. Reporting of benign brain tumors has not been required or standardized on a national level until recently (NAACCR, 2003; Public Law 107–260, 2002), and for the data reported here, reporting and coding practices of the registries likely differed. With the standardization of reporting for all primary brain tumors, differences in rates and proportions between registries should diminish. In addition, nerve sheath tumors overall and the subgroups of vestibular schwannomas and benign schwannomas showed patterns of greater increased incidence of nonmicroscopically confirmed tumors than of microscopically confirmed tumors. This suggests an acceleration in the diagnosis of these tumors by radiologic or clinical means as compared to surgical confirmation. Other tumor subtypes with statistically significant (P < 0.05) increasing trends exhibited no difference by diagnostic confirmation for the LACCSP data. Clinicians in the Los Angeles County area may rely upon microscopic confirmation for diagnosis more frequently than do clinicians in other regions.
Incidence rates may have been affected by changes in classification/coding (e.g., revisions made to the WHO histologic classification scheme for CNS tumors and topography coding in the ICDO). The unstable trends seen for vestibular schwannomas and nonvestibular schwannomas are most likely due to misclassification by the registries of vestibular schwannomas as nonvestibular schwannomas and vice versa. The small number of nerve sheath tumors in comparison to all cancers collected by registries may result from unfamiliarity with these tumors. The possibility exists that the tumor location of some benign schwannomas of the acoustic nerve was miscoded to the cerebellum, NOS; cranial nerve, NOS; and other nonspecific sites. Comparison of data from 11 regions of CBTRUS and LACCSP (1995–1998/1999) revealed a high proportion of benign schwannomas with tumor site coded to cerebellum, NOS, and/or cranial nerve, NOS, for some CBTRUS regions (data not shown). The ICDO topography code for cerebellum, NOS, includes the cerebellopontine angle, while the ICDO topography code for cranial nerve, NOS, includes cranial nerves other than the olfactory, optic, and acoustic nerves. The cerebellopontine angle is bordered by the cerebellum, pons, and temporal bone, and cranial nerves 6–11 pass through it, allowing vestibular schwannomas to grow and compress the brainstem. Use of this terminology in the pathology report for these tumors may result in misclassification to this site. Similarly, those unfamiliar with the terminology associated with the acoustic nerve may inadvertently miscode this tumor to cranial nerve, NOS. Because of the close proximity of vestibular schwannomas to the cerebellopontine angle and other cranial nerves, and the variations in terminology of these anatomical structures, it is plausible that some benign schwannomas of the acoustic nerve may have been miscoded to other sites.
Despite methodological differences among studies that have examined time trends in primary nerve sheath tumors of the brain and vestibular schwannomas, this analysis supports previous reports that suggest an overall increase in incidence rates of nerve sheath tumors, primarily reflecting the subgroup of vestibular schwannomas (Hardell et al., 2003; Howitz et al., 2000; Tos et al., 1999). In addition, this report details decreasing trends in nonvestibular schwannomas, possibly due to an increase in correct classification of vestibular schwannomas.
Implications of Research
Etiologic studies of vestibular schwannomas are few. One case-control study of these tumors in men (Preston- Martin et al., 1989b) found an association between occupations involving exposure to extremely loud noise and acoustic neuromas. A dose-response relationship supported this observation. Men working in loud noise situations for 20 or more years were two times more likely to develop these tumors than individuals who did not have jobs involving loud noise. No study has attempted to replicate these results.
A recent focus on these and other brain tumors has developed because of their proximity to the site of exposure to radiofrequency radiation emitted by cellular phones and concerns about the relationship between cellular phone use and brain tumor development. The International Agency for Research on Cancer is conducting a large study of acoustic neuromas (and other brain tumors) to test the hypothesis that these tumors may be associated with the use of cellular telephones. Two lines of evidence support this work. First, vestibular schwannomas are located within the direct line of the radiofrequency exposures that occur near the ear (Davis and Preston-Martin, 1998). Second, in a cohort study of individuals exposed to high-dose radiation, the relative risks associated with developing nerve sheath tumors were very high (odds ratio [OR] = 18.8), higher than meningiomas (OR = 9.5) or gliomas (OR = 2.6) (Ron et al., 1988). This suggests that the acoustic nerve may be particularly radiosensitive, legitimizing investigations in the lower radiofrequency range of exposures.
Several studies of cellular telephone use and vestibular schwannomas have been completed (Christensen et al., 2004; Hardell et al., 2003; Inskip et al., 2001; Johansen et al., 2001; Lonn et al., 2004; Muscat et al., 2002). Most of these studies have produced no evidence that cellular telephone use increases the overall risk for vestibular schwannoma (Christensen et al., 2004; Inskip et al., 2001; Johansen et al., 2001; Lonn et al., 2004; Muscat et al., 2002). Hardell et al. (2003) found an increased risk for vestibular schwannoma in individuals who used analog phones for more than one year (OR = 3.45; 95% CI, 1.77–6.76) and for more than five years (OR = 3.71; 95% CI, 1.61–8.56). Lonn et al. (2004) found that risk may be increased in those who have used cell phones for at least 10 years (OR = 1.9; 95% CI, 0.9–4.1), but this was not confirmed in the study by Christensen et al. (2004). It has been pointed out that numerous study design issues have been associated with these case-control studies (Kundi et al., 2004) and that these studies may not be sufficient to evaluate the risks of vestibular schwannoma among long-term heavy users of cell phones (Inskip et al., 2001). The increase in incidence of vestibular schwannomas needs further investigation, as no definitive causative factors have been identified.
This analysis documented an increase in incidence of vestibular schwannomas, as well as primary nerve sheath tumors of the brain and CNS overall, lending (modest) support to the emerging hypothesis regarding an environmental cause of these rare tumors, which requires further elucidation. However, steady improvements in diagnosis and reporting could also explain some or all of the patterns observed. Recommendations regarding the collection of data on these patients include emphasizing the importance of (1) ensuring accurate coding of location (primary site) and morphology of nerve sheath tumors by registries; (2) the avoidance of possible mis-classification of the primary site of benign schwannomas of the acoustic nerve (i.e., vestibular schwannomas) to the cerebellum, NOS; cranial nerve, NOS; and nonspecific or more general site categories; and (3) ensuring complete case ascertainment (e.g., active vs. passive case reports that may reflect only microscopically confirmed cases). These recommendations may improve the accuracy of incidence rates reported by registries and used by patients and their families, clinicians, and researchers. This research provides support for the further studies of vestibular schwannomas that may aid in the understanding of the causes of these tumors and may provide direction for preventive strategies in subsequent generations.
Acknowledgments
This work was conducted under the financial support of a grant from the National Brain Tumor Foundation. The authors wish to acknowledge the Los Angeles County Cancer Surveillance Program and the collaborating registries of the Central Brain Tumor Registry of the United States that provided data for this analysis: Dennis Deapen, Los Angeles County Cancer Surveillance Program; Georgia Yee, Arizona Cancer Registry; Randi Rycroft, Colorado Central Cancer Registry; Anthony Polednak, Connecticut Tumor Registry; Leroy Hathcock, Delaware Cancer Registry; Stacey Carson, Cancer Data Registry of Idaho; Castine Verrill, Maine Cancer Registry; Susan Gershman, Massachusetts Cancer Registry; Sally Bushhouse, Minnesota Cancer Surveillance System; Debbi Lemons, Montana Central Tumor Registry; Maria Schymura, New York State Cancer Registry; and Dale Herman, North Carolina Central Cancer Registry.
Footnotes
This work was conducted under the financial support of an epidemiology grant from the National Brain Tumor Foundation.
Abbreviations used are as follows: CBTRUS, Central Brain Tumor Registry of the United States; CI, confidence interval; ICDO, International Classification of Diseases for Oncology; LACCSP, Los Angeles County Cancer Surveillance Program; NOS, not otherwise specified; OR, odds ratio; SEER, Surveillance, Epidemiology, and End Results program.
References
- CBTRUS, Central Brain Tumor Registry of the United States (1998) 1997 Annual Report. Chicago, Ill.: Central Brain Tumor Registry of the United States.
- CBTRUS, Central Brain Tumor Registry of the United States (2002–2003) Primary Brain Tumors in the United States: Statistical Report, 1995–1999. Chicago, Ill.: Central Brain Tumor Registry of the United States.
- CDC, Centers for Disease Control and Prevention (1997) EDITS (computer software). Version 2.00. Atlanta, Ga.: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Available at http://www.cdc.gov/cancer/npcr/edits
- Christensen HC, Schuz J, Kosteljanetz M, Poulsen HS, Thomsen J, Johansen C. Cellular telephone use and risk of acoustic neuroma. Am J Epidemiol. 2004;159:277–283. doi: 10.1093/aje/kwh032. [DOI] [PubMed] [Google Scholar]
- Davis, F.G., and Preston-Martin, S. (1998) Epidemiology: Incidence and survival. In: Bigner, D.D., McClendon, R.E., and Bruner, J.M. (Eds.), Russell and Rubinstein’s Pathology of Tumors of the Nervous System, 6th ed. London: Oxford University Press, pp. 5–45.
- Desmeules M, Mikkelsen T, Mao Y. Increasing incidence of primary malignant brain tumors: Influence of diagnostic methods. J Natl Cancer Inst. 1992;84:442–445. doi: 10.1093/jnci/84.6.442. [DOI] [PubMed] [Google Scholar]
- Estève, J., Benhamou, E., and Raymond, L. (1994) Statistical Methods in Cancer Research. Vol. 4: Descriptive Epidemiology. Lyon: International Agency for Research on Cancer. [PubMed]
- Fritz, A., and Ries, L. (Eds.) (1998) The SEER Program Code Manual, 3rd ed. Bethesda, Md.: Cancer Statistics Branch, Surveillance Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health.
- Gibson RM, Levit KR, Lazenby H, Waldo DR. National health expenditures, 1983. Health Care Financ Rev. 1984;6:1–29. [PMC free article] [PubMed] [Google Scholar]
- Hardell L, Hansson Mild K, Sandstrom M, Carlberg M, Hallquist A, Pahlson A. Vestibular schwannoma, tinnitus and cellular telephones. Neuroepidemiology. 2003;22:124–129. doi: 10.1159/000068745. [DOI] [PubMed] [Google Scholar]
- Howitz MF, Johansen C, Tos M, Charabi S, Olsen JH. Incidence of vestibular schwannoma in Denmark, 1977–1995. Am J Otol. 2000;21:690–694. [PubMed] [Google Scholar]
- Inskip PD, Tarone RE, Hatch EE, Wilcosky TC, Shapiro WR, Selker RG, Fine HA, Black PM, Loeffler JS, Linet MS. Cellular- telephone use and brain tumors. N Engl J Med. 2001;344:79–86. doi: 10.1056/NEJM200101113440201. [DOI] [PubMed] [Google Scholar]
- Johansen C, Boice J, Jr, McLaughlin J, Olsen J. Cellular telephones and cancer—a nationwide cohort study in Denmark. J Natl Cancer Inst. 2001;93:203–207. doi: 10.1093/jnci/93.3.203. [DOI] [PubMed] [Google Scholar]
- Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-Oncology. 2001;3:141–151. doi: 10.1093/neuonc/3.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993a;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Kleihues, P., Burger, P.C., and Scheithauer, B.W. (1993b) Histologic Typing of Tumours of the Central Nervous System. Berlin: Springer-Verlag.
- Kundi M, Mild K, Hardell L, Mattsson MO. Mobile telephones and cancer—a review of epidemiological evidence. J Toxicol Environ Health B Crit Rev. 2004;7:351–384. doi: 10.1080/10937400490486258. [DOI] [PubMed] [Google Scholar]
- Lonn S, Ahlbom A, Hall P, Feychting M. Mobile phone use and the risk of acoustic neuroma. Epidemiology. 2004;15:653–659. doi: 10.1097/01.ede.0000142519.00772.bf. [DOI] [PubMed] [Google Scholar]
- Modan B, Wagener DK, Feldman JJ, Rosenberg HM, Feinleib M. Increased mortality from brain tumors: A combined outcome of diagnostic technology and change of attitude toward the elderly. Am J Epidemiol. 1992;135:1349–1357. doi: 10.1093/oxfordjournals.aje.a116246. [DOI] [PubMed] [Google Scholar]
- Mosso ML, Colombo R, Giordano L, Pastore G, Terracini B, Magnani C. Childhood cancer registry of the province of Torino, Italy: Survival, incidence and mortality over 20 years. Cancer. 1992;69 :1300–1306. doi: 10.1002/cncr.2820690538. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Malkin MG, Shore RE, Thompson S, Neugut AI, Stellman SD, Bruce J. Handheld cellular telephones and risk of acoustic neuroma. Neurology. 2002;58:1304–1306. doi: 10.1212/wnl.58.8.1304. [DOI] [PubMed] [Google Scholar]
- NAACCR, North American Association of Central Cancer Registries (2003) NAACCR 2004 Implementation Guidelines. Available at http://wwwnaaccrorg Accessed April 14, 2004.
- Percy, C., Van Holten, V., and Muir, C.M. (Eds.) (1990) International Classification of Diseases for Oncology, 2nd ed. Geneva: World Health Organization.
- Pitts, L.H., Jackler, R.K., and Fuller, G.N. (1996) Intracranial nerve sheath tumors. In: Levin, V.A. (Ed.), Cancer in the Nervous System. New York: Churchill Livingstone, pp. 199–210.
- Preston-Martin S. Descriptive epidemiology of primary tumors of the brain, cranial nerves and cranial meninges in Los Angeles County. Neuroepidemiology. 1989a;8:283–295. doi: 10.1159/000110196. [DOI] [PubMed] [Google Scholar]
- Preston-Martin S, Thomas DC, Wright WE, Henderson BE. Noise trauma in the aetiology of acoustic neuromas in men in Los Angeles County, 1978–1985. Br J Cancer. 1989b;59:783–786. doi: 10.1038/bjc.1989.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Law 107–260 (2002) Benign Brain Tumor Cancer Registries Amendment Act. Available at http://frwebgateaccessgpogov/cgi-bin/getdoccgi?dbname=107_cong_public_laws&docid=f:publ260107 Accessed April 14, 2004.
- Radhakrishnan K, Mokri B, Parisi JE, O’Fallon WM, Sunku J, Kurland LT. The trends in incidence of primary brain tumors in the population of Rochester, Minnesota. Ann Neurol. 1995;37:67–73. doi: 10.1002/ana.410370113. [DOI] [PubMed] [Google Scholar]
- Ron E, Modan B, Boice JD, Jr, Alfandary E, Stovall M, Chetrit A, Katz L. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- SAS Institute (1999) SAS System software: Release 8.02. Cary, N.C.: SAS Institute, Inc.
- Tos M, Charabi S, Thomsen J. Incidence of vestibular schwannomas. Laryngoscope. 1999;109:736–740. doi: 10.1097/00005537-199905000-00011. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1976) International Classification of Diseases for Oncology, 1st ed. Geneva: World Health Organization.