Abstract
Preclinical studies support the concept that inhibition of protein kinase C (PKC) by tamoxifen (TAM) should provide both antineoplastic effects and radiosensitization. High-dose TAM (80 mg/m2 p.o. daily in divided doses) was given with and after conventional radiotherapy (XRT) to inhibit PKC-mediated signaling, which is known to be enhanced in glioblastoma (GBM). Seventy-seven patients were accrued between December 2000 and December 2001; two were ineligible and not included in the efficacy results. Pretreatment characteristics of the patients included the following: 52% were less than 60 years of age, 39% had a Zubrod score of 0, 70% had minor or no neurological symptoms, and 65% were Radiation Therapy Oncology Group–recursive partition analysis (RPA) class III and IV. Eighty-six percent of patients achieved acceptable dosing of TAM. Notable toxicity included late radiation grade 3 in two patients and thromboembolic events in 16 patients (two grade 2, 10 grade 3, three grade 4, and one grade 5), for an incidence of 20.8% (which is lower than expected, based on the literature for deep vein thrombophlebitis in GBM patients not receiving TAM). Median survival time (MST) was 9.7 months as compared (by three different statistical methodologies) to the historical GBM control database of 1457 RPA class III, IV, and V drug/ XRT-treated patients. After controlling for RPA class IV, the MST was 11.3 months, which compares to the historical RPA control of 11.3 months (P = 0.37). The results obtained do not exhibit a substantial advance over those of previous studies with various XRT/drug doublets, including BCNU. However, as TAM does not have significant overlapping toxicities with most other drugs, its testing in a combined modality approach with other medications may be justified in future clinical trials. Historically, the incidence of thromboembolic events in GBM patients is approximately 30%. The lower-than-expected incidence seen here has also been observed in other high-dose TAM GBM studies. We speculate that TAM inhibited the PKC-mediated phosphorylation of coagulation factors.
Keywords: glioblastoma multiforme, radiation, tamoxifen
Current results with all methodologies for the treatment of glioblastoma multiforme (GBM)3 continue to be disappointing. The use of surgical resection, radiation therapy, and chemotherapy produces a median survival of less than one year (Robins et al., 2003). Surgery and radiation may have reached maximal effectiveness. Chemotherapy has the potential to improve survival, but increases in survival have been marginal to date (Robins et al., 2003).
A series of studies have suggested that the proliferation of high-grade gliomas is in part dependent on the activation of protein kinase C (PKC)–mediated pathways (Baltuch et al. 1995; Gelmann, 1997; Leslie et al., 1994; Mastronardi et al., 1998a, b; Weller et al., 1997). Thus, blocking this enzyme, which is known to be involved in signal transduction, provides a novel approach to inhibiting glioma cell growth. Beyond this, recent investigations have suggested that inhibition of PKC can enhance the ionizing effects of irradiation (Chmura et al., 1997; Tsuchida and Urano, 1997). Relevant to this, the antiestrogen tamoxifen (TAM) is a significant PKC inhibitor (Baltuch et al., 1995; Mastronardi et al., 1998a, c; Weller et al., 1997). At concentrations severalfold higher than that used in its traditional role as an estrogen receptor– binding agent, TAM can block glioma cell lines in vitro. By extrapolation, oral dosing in excess of 80 mg/m2 can achieve a serum concentration in a putative therapeutic range. In this regard, Couldwell et al. (1996) reported both safety and efficacy (i.e., responses in 4 of 20 GBM patients) in a small series at a TAM dose of 160 to 200 mg per day. The results of this study, as well as those of smaller series and anecdotal reports, are consistent with continued investigation of TAM in this patient population (Chang et al., 1998; Cloughesy et al., 1997; Gelmann, 1997; Mastronardi et al., 1998b; Pollack et al., 1997).
Based on the aforementioned considerations, the Radiation Therapy Oncology Group (RTOG) initiated a phase 2 trial of high-dose TAM for patients newly diagnosed with GBM in December 2000. TAM was given during and after radiation. The primary end point of this study was overall survival. A secondary end point of the study was toxicity. Relative to this, it was recognized from the onset of the trial that patients with GBM were highly predisposed to thromboembolic phenomena (Brisman and Mendell, 1973; Hamilton et al., 1994; Iberti et al., 1994; Kayser-Gatchalian and Kayser, 1975; Millac, 1967; Nathanson and Savitsky, 1952; Sawaya and Highsmith 1988, 1992; Sawaya et al., 1992; Shlebak and Smith, 1997) and that there is a defined risk of thromboembolic disease in patients receiving TAM. Thus, the incidence of thromboembolic disease was carefully monitored throughout the study. This report summarizes the results of the phase 2 trial.
Patients and Methods
To be eligible for the protocol, patients were required to have histologically proven, supratentorial GBM, with an estimated survival of at least eight weeks and a KPS ⩾70 (Zubrod 0 and 1). Patients were required to have had preoperative and postoperative, contrast-enhanced MRI or CT scan prior to the initiation of radiotherapy. Patients must have recovered from surgery. Laboratory requirements included the following: absolute neutrophil count ⩾1500/mm3, platelets ⩾100,000 mm3, blood urea nitrogen ⩽25, creatinine ⩽1.5 mg/dl, bilirubin ⩽2.0 mg/ dl, hemoglobin ⩾10 g/dl, and serum glutamic-pyruvic transaminase or serum glutamic-oxaloacetic transaminase ⩽2 × normal range. Protocol exclusion included major medical or psychiatric illness, prior malignancy or endometrial hyperplasia, acquired immune deficiency, and pregnant or lactating women. All patients signed a study-specific consent form prior to registration.
Treatment
The radiation treatment was as follows: 60.0 Gy in 30 fractions × 2.0 Gy. For the first 46 Gy/23 fractions, the treatment volume included the volume of contrast-enhancing lesion and surrounding edema on preoperative CT/MRI scan plus a 2-cm margin. If no edema was present, the margin was 2.5 cm. After 46.0 Gy, the treatment volume included the contrast-enhancing lesion (without edema) on the presurgery MRI/CT scan plus a 2.5-cm margin.
Administration of TAM began on day 1 of radiotherapy. The dose was escalated 20 mg per day until the target dose was established, which was 80 mg/m2 orally (in four individual doses, 20 mg/m2 every 6 h). TAM administration was continued until disease progression.
Statistical Considerations
The sample size of this study was calculated by using the Dixon–Simon (1988) method for the comparison of survival against a historical control. With at least 68 recursive partition analysis (RPA) class III, IV, and V patients whose cases were followed over 18 months, there is at least an 80% probability of detecting a minimum of 50% improvement in median survival time (MST) (at the 0.05 significance level) as compared to the RTOG glioma data. Survival and progression-free survival were estimated by using the Kaplan–Meier (1958) method, and testing with historical control was performed by using one-sided log-rank statistic (Mantel, 1966) looking for the superiority of TAM. Overall survival was also fitted by using the Cox (1972) proportional hazard model.
Results
Seventy-seven patients were enrolled between December 6, 2000, and December 28, 2001. Of these, two were ineligible (i.e., histology showed renal cell carcinoma in one patient, and one patient had a Zubrod score of 3) and are not included in the efficacy analysis. All 77 patients were assessed for treatment toxicity, but the ineligible cases were excluded from the efficacy analyses. At the time of analysis, six patients were alive, and two of those six had less than 18 months of follow-up. Pretreatment characteristics are summarized in Table 1.
Table 1.
Pretreatment characteristics*
| Characteristic | Patients (N = 75) |
|---|---|
| Age | |
| <60 | 39 (52%) |
| ⩾60 | 36 (48%) |
| Zubrod score | |
| 0 | 29 (39%) |
| 1 | 46 (61%) |
| Gender | |
| Male | 47 (63%) |
| Female | 28 (37%) |
| Race | |
| White | 69 (92%) |
| Hispanic | 3 (4%) |
| Black | 3 (4%) |
| Neurological function | |
| No symptoms | 13 (17%) |
| Minor symptoms | 40 (53%) |
| Moderate (fully active) | 13 (17%) |
| Moderate (not fully active) | 8 (11%) |
| Missing | 1 (1%) |
| Prior surgery | |
| Biopsy | 22 (29%) |
| Partial resection | 28 (37%) |
| Total resection | 25 (33%) |
| MMSE | |
| 10–23 | 10 (13%) |
| 24–30 | 61 (81%) |
| Missing | 4 (5%) |
| RPA class | |
| III | 4 (5%) |
| IV | 45 (60%) |
| V | 26 (35%) |
Abbreviations: MMSE, mini–mental state exam; RPA, recursive partition analysis.
Percentages may not sum to 100% because of rounding.
TAM and acute radiotherapy toxicities are summarized in Table 2. Of particular interest in this study was the incidence of thromboembolic events (TEEs), which was 20.8%. Late radiation toxicities are summarized in Table 3.
Table 2.
Tamoxifen and acute radiotherapy toxicities (tamoxifen treatment continues until progression)
|
Grade (N = 77) |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 0 | 2 | 0 | 0 | 0 |
| Auditory/hearing | 3 | 2 | 0 | 0 | 0 |
| Blood/bone marrow | 35 | 6 | 2 | 0 | 0 |
| Cardiovascular | |||||
| Thrombosis/embolism | 0 | 2 | 10 | 3 | 1 |
| General | 5 | 5 | 0 | 0 | 0 |
| Constitutional symptoms | |||||
| Fatigue | 18 | 30 | 6 | 1 | 0 |
| Other | 13 | 6 | 1 | 0 | 0 |
| Dermatology/skin | 20 | 27 | 1 | 0 | 0 |
| Endocrine | 6 | 4 | 0 | 0 | 0 |
| Gastrointestinal | |||||
| Constipation | 2 | 3 | 1 | 0 | 0 |
| Nausea/vomiting | 16 | 13 | 1 | 0 | 0 |
| Anorexia | 10 | 8 | 3 | 0 | 0 |
| Other | 10 | 9 | 0 | 0 | 0 |
| Hemorrhage | 0 | 0 | 1 | 0 | 0 |
| Hepatic | 3 | 1 | 4 | 0 | 0 |
| Infection/febrile neutropenia | 1 | 3 | 3 | 0 | 0 |
| Metabolic/laboratory | 10 | 1 | 4 | 0 | 0 |
| Musculoskeletal | 1 | 2 | 1 | 0 | 0 |
| Neurology | 6 | 12 | 6 | 0 | 0 |
| Ocular/visual | 3 | 2 | 0 | 0 | 0 |
| Pain | |||||
| Headache | 10 | 8 | 2 | 0 | 0 |
| Other | 8 | 5 | 1 | 0 | 0 |
| Pulmonary | 2 | 7 | 1 | 0 | 0 |
| Renal/genitourinary | 0 | 2 | 0 | 0 | 0 |
| Sexual reproductive function | 1 | 0 | 3 | 0 | 0 |
| Worst toxicity | 5 | 33 | 27 | 4 | 1 |
Table 3.
Late radiation toxicities
|
Grade (N = 62) |
|||
|---|---|---|---|
| Toxicity | 1 | 2 | 3 |
| Brain | 3 | 1 | 2* |
| Eye | 3 | 0 | 0 |
| Skin (within field) | 9 | 4 | 0 |
| Subcutaneous tissue | 3 | 0 | 0 |
| Other | 5 | 3 | 0 |
| Maximum per patient | 8 | 6 | 2 |
Description of brain toxicity Days from Radiotherapy Start
Seizures secondary to tumor and necrosis 283
Complete aphasia; decreased vision, hearing, and balance 532
Seventy patients had sufficiently documented TAM treatment delivery records and were reviewed by the study chair for quality assurance. Forty-nine patients (70%) received drug according to protocol, and 11 (16%) had acceptable variations. Ten (14%) had an unacceptable deviation (i.e., failure to achieve a dose within 31% of the target dose after 21 days of protocol treatment).
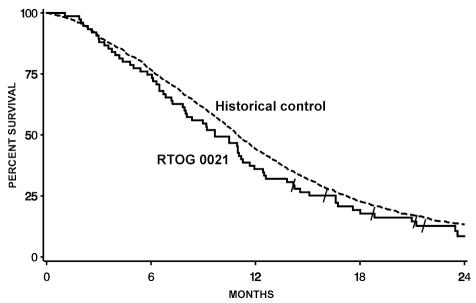
Table 4 summarizes overall survival. Figure 1 shows the comparison of overall survival of patients on this study to 1457 patients from the RTOG database of past GBM studies. The P value of the comparison is 0.94. Additionally, a random subgroup of patients from the RTOG historical database was selected such that the overall RPA class distribution of survival for the subgroup matched that of the patients on this study (P = 0.70). In a Cox regression model stratified by RPA class, the hazard ratio of this study compared to the historical database was found to be 1.16, with a 95% CI of 0.91–1.48. Overall survival was 11.3 months for this study and for the RTOG RPA class IV historical control (P value one-sided log-rank = 0.37). Similarly, for RPA class V, the study value was 6.2 months versus 8.6 months for the historical control (P value one-sided log-rank = 0.92). Progression-free survival was 2.9 months for the entire patient cohort; for RPA classes IV and V, it was 3.0 and 2.5 months, respectively.
Table 4.
Overall survival*
| Months | Survival | No. at Risk |
|---|---|---|
| 0 | 100% | 75 |
| 6 | 75% | 56 |
| 12 | 36% | 27 |
| 18 | 19% | 13 |
| 24 | 9% | 4 |
Median survival time was 9.7 months. Of 75 patients, 65 were dead.
Fig. 1.
Overall survival comparison of Radiation Therapy Oncology Group (RTOG) protocol BR-0021 patients and the RTOG historical control database of patients with glioblastoma multiforme.
Discussion
As reviewed above, a series of studies have suggested the potential of TAM as an antineoplastic agent for high-grade glioma. The virtue of this trial resides in its evaluation of this agent in a large cooperative group setting with a defined histologic patient population. Further, the application of the RTOG RPA analysis provides a systematic comparison of the data obtained to a matched and reproducible historical control (Curran et al., 1993; Seiferheld et al., 2002). The results derived from this study, and their analysis taken collectively, do not exhibit a substantial difference in comparison to the results of previous RTOG studies of patients with newly diagnosed GBM who received radiation therapy and various adjuvant drugs, including BCNU (N,N′-bis [2-chloroethyl]-N-nitrosourea, carmustine). Indeed, the first results showing a drug (i.e., temozolomide) significantly impacting this clinical setting in the past three decades were reported by Stupp et al. (2004) at the 2004 American Society of Clinical Oncology meeting.
The toxicity observed in this trial (summarized in Tables 2 and 3) is consistent with that in earlier GBM experiences with TAM alone and in combination with other drugs. The study most comparable to this trial was reported by Muanza et al. (2000). This was a pilot toxicity study of 12 GBM patients in which high-dose TAM was given with and after radiotherapy. There was one episode of deep vein thrombophlebitis (DVT) reported in that trial. In considering their experience, as well as ours, a general discussion of TEEs in patients with high-grade glioma is relevant. Brain tumor patients are highly predisposed to thromboembolic phenomena (Brisman and Mendell, 1973) and have demonstrated an 8.4% incidence of pulmonary emboli (which is almost three times the incidence seen in nonmalignant neurosurgical patients). Similarly, the incidence of DVT in such patients is 27.5%, compared to 17% in a controlled neurosurgical group (Kayser-Gatchalian and Kayser, 1975). Sawaya et al. (1992), using fibrinogen I 125 scanning, demonstrated DVTs in 60% of patients with GBM. Interestingly, the presence of DVTs did not correlate with time of surgery, length of operation, ambulatory status, or occurrence in a paretic limb. It has been suggested that malignant brain tumors release a factor responsible for this predisposition to coagulopathy (Sawaya and Highsmith, 1992). Earlier work suggested that increased platelet adhesiveness in malignant brain tumors is consistent with this supposition (Millac, 1967; Nathanson and Savitsky, 1952). More recent work further supports the concept of an increased coagulable state of brain tumor patients (Hamilton et al., 1994; Iberti et al., 1994). To address this concern, Canadian and American cooperative group trials are now in progress testing the use of prophylactic low-molecular-weight heparin.
Relative to the aforementioned discussion, there is a defined increased risk of thromboembolic disease in patients receiving low-dose TAM (Shlebak and Smith, 1997). In an attempt to explicate this complication, Love et al. (1992) studied antithrombin III levels, fibrinogen levels, and platelet count changes with adjuvant TAM therapy in breast cancer patients; they did not, however, find an obvious correlation to the observation of TAM-induced thromboembolic disease.
Thus, at the onset of this clinical trial, it was logical to assume there might be an increased risk if this drug were introduced in high doses to a patient population predisposed to thromboembolic disease. (In this regard, a report by Broniscer et al. [2000] regarding brainstem gliomas in children treated with high-dose TAM was reassuring; these investigators did not find an increase in the expected incidence of thromboembolic disease in a series of 29 patients.) Ultimately, as our trial concluded, the incidence of thromboembolic problems (i.e., 20.8%) was less than the approximately 30% expected in a prospectively followed population of GBM patients. As this RTOG study was reaching its accrual goal, Tremont-Lukats et al. (2002) reported an exhaustive review of the literature regarding TEE in glioma patients receiving TAM. The review encompassed 15 studies and 381 adult and pediatric patients. These authors found an incidence of TEE lower than that observed by Pritchard et al. (1996) for breast cancer patients receiving chemotherapy in addition to TAM, that is, 13.5%. Thus, there is an anecdotal suggestion (not supported by phase 3 data) that the addition of high-dose TAM may prevent TEE. Although our experience is clearly consistent with the literature, these results are counterintuitive. A review of the coagulation literature provides a mechanistic explanation for the conjecture that high-dose TAM may be protective against TEE: Protein kinase C (for which TAM acts as an inhibitor) is involved in the phosphorylation of coagulation factors (I, II, VIII), and as a consequence this phosphorylation can be limited by PKC inhibitors (Abe, 1993). Further, there is evidence that cytokine activation of the coagulation cascade relative endothelial cell tissue factor expression also involves protein PKC mediation (Terry and Callahan, 1996; Xuereb et al., 2000).
It is obvious from the foregoing discussion that TAM does not have significant overlapping toxicities with most other drugs. Thus, its testing in a combined modality approach with other medications, for example, temozolomide, may be justified in future clinical trials. In this context, in order to gain further insight into the potential value of TAM, a corollary study will be done. Over the next year, we will assay tissue samples derived from patients treated in this study. The aim of this testing will be to determine PKC amplification and evaluate whether it correlates with survival and/or time to progression.
Footnotes
Supported by RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 grants from NCI. The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Abbreviations used are as follows: BCNU, N,N′-bis(2-chloroethyl)-N-nitrosourea (carmustine); DVT, deep vein thrombophlebitis; GBM, glioblastoma multiforme; MST, median survival time; PKC, protein kinase C; RPA, recursive partition analysis; RTOG, Radiation Therapy Oncology Group; TAM, tamoxifen; TEE, thromboembolic event.
References
- Abe K. A study on the participation of protein kinase C in the blood coagulation. Hokkaido Igaku Zasshi [Hokkaido J Med Sci] 1993;68:368–376. [PubMed] [Google Scholar]
- Baltuch GH, Dooley NP, Villemure JG, Yong VW. Protein kinase C and growth regulation of malignant gliomas. Can J Neurol Sci. 1995;22:264–271. doi: 10.1017/s0317167100039457. [DOI] [PubMed] [Google Scholar]
- Brisman R, Mendell J. Thromboembolism and brain tumor. J Neurosurg. 1973;38:337–338. doi: 10.3171/jns.1973.38.3.0337. [DOI] [PubMed] [Google Scholar]
- Broniscer A, Leite CC, Lanchote VL, Machado T.M, Cristofani LM. Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: Results of a Brazilian cooperative study. J Clin Oncol. 2000;18:1246–1253. doi: 10.1200/JCO.2000.18.6.1246. [DOI] [PubMed] [Google Scholar]
- Chang SM, Barker FG, II, Huhn SL, Nicholas MK, Page M, Rabbitt J, Prados MD. High dose oral tamoxifen and subcutaneous interferon alpha-2a for recurrent glioma. J Neurooncol. 1998;37:169–176. doi: 10.1023/a:1005826323652. [DOI] [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzenski E, Quintans J, Kufe DW, Weichselbaum RR. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor killing by ionizing radiation. Cancer Res. 1997;57:4340–4347. [PubMed] [Google Scholar]
- Cloughesy TF, Woods RP, Black KL, Couldwell WT, Law RE, Hinton DR. Prolonged treatment with biologic agents for malignant glioma: A case study with high dose tamoxifen. J Neurooncol. 1997;35:39–45. doi: 10.1023/a:1005895616377. [DOI] [PubMed] [Google Scholar]
- Couldwell WT, Hinton DR, Surnock AA, DeGiorgio CM, Weiner LP, Apuzzo ML, Masri L, Law RE, Weiss MH. Treatment of recurrent malignant gliomas with chronic oral high-dose tamoxifen. Clin Cancer Res. 1996;2:619–622. [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Dixon DO, Simon R. Sample size considerations for studies comparing survival curves using historical controls. J Clin Epidemiol. 1988;41:1209–1213. doi: 10.1016/0895-4356(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Gelmann EP. Tamoxifen for the treatment of malignancies other than breast and endometrial carcinoma. Semin Oncol. 1997;24(suppl.):S1-65–S1-70. [PubMed] [Google Scholar]
- Hamilton MG, Hull RD, Pineo GF. Venous thromboembolism in neurosurgery and neurology patients: A review. Neurosurgery. 1994;34:280–296. doi: 10.1227/00006123-199402000-00012. [DOI] [PubMed] [Google Scholar]
- Iberti TJ, Miller M, Abalos A, Fischer EP, Post KD, Benjamin E, Oropello JM, Wiltshire-Clement M, Rand JH. Abnormal coagulation profile in brain tumor patients during surgery. Neurosurgery. 1994;34:389–395. doi: 10.1227/00006123-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kayser-Gatchalian MC, Kayser K. Thrombosis and intracranial tumors. J Neurol. 1975;209:217–224. doi: 10.1007/BF00312543. [DOI] [PubMed] [Google Scholar]
- Leslie KK, Keefe D, Powell S, Naftolin F. Estrogen receptors are identified in the glioblastoma cell line U138MG. J Soc Gynecol Invest. 1994;1:238–244. doi: 10.1177/107155769400100311. [DOI] [PubMed] [Google Scholar]
- Love RR, Surawicz TS, Williams EC. Antithrombin III level, fibrinogen level, and platelet count changes with adjuvant tamoxifen therapy. Arch Intern Med. 1992;152:317–320. [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- Mastronardi L, Farah JO, Puzzilli F, Ruggeri A. Tamoxifen modulation of carboplatin cytotoxicity in a human U-138 glioma cell line. Clin Neurol Neurosurg. 1998a;100:89–93. doi: 10.1016/s0303-8467(98)00004-3. [DOI] [PubMed] [Google Scholar]
- Mastronardi L, Puzzilli F, Couldwell WT, Farah JO, Lunardi P. Tamoxifen and carboplatin combinational treatment of high-grade gliomas: Results of a clinical trial on newly diagnosed patients. J Neurooncol. 1998b;38:59–68. doi: 10.1023/a:1005968724240. [DOI] [PubMed] [Google Scholar]
- Mastronardi L, Puzzilli F, Ruggeri A. Tamoxifen as a potential treatment of glioma. Anti-Cancer Drugs. 1998c;9:581–586. doi: 10.1097/00001813-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Millac P. Platelet stickiness in patients with intracranial tumors. Br Med J. 1967;4:25–26. doi: 10.1136/bmj.4.5570.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanza T, Shenouda G, Souhami L, Leblanc R, Mohr G, Corns R, Langleben A. High dose tamoxifen and radiotherapy in patients with glioblastoma multiforme: A phase IB study. Can J Neurol Sci. 2000;27:302–306. doi: 10.1017/s0317167100001049. [DOI] [PubMed] [Google Scholar]
- Nathanson M, Savitsky JP. Platelet adhesive index studies in multiple sclerosis and other neurological disorders. Bull NY Acad Med. 1952;28:462–468. [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, DaRosso RC, Robertson PL, Jakacki RL, Mirro JR, Jr, Blatt J, Nicholson S, Packer RJ, Allen JC, Cisneros A, Jordan VC. A phase I study of high-dose tamoxifen for the treatment of refractory malignant gliomas of childhood. Clin Cancer Res. 1997;3:1109–1115. [PubMed] [Google Scholar]
- Pritchard KI, Paterson AH, Paul NA, Zee B, Fine S, Pater J. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. J Clin Oncol. 1996;14:2731–2737. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]
- Robins HI, Peterson CG, Mehta MP. Combined modality treatment for central nervous system malignancies. Semin Oncol. 2003;30 (suppl. 9):11–22. doi: 10.1016/s0093-7754(03)00271-9. [DOI] [PubMed] [Google Scholar]
- Sawaya, R., and Highsmith, R.F. (1988) Brain tumors and the fibrinolytic enzyme system. In: Kornblith, P.L., and Walker, M.D. (Eds.) Advances in Neuro-Oncology Mount Kisco, N.Y.: Futura Publishing Co., pp. 103–157.
- Sawaya R, Highsmith RF. Postoperative venous thromboembolism and brain tumors: Part III. Biochemical profile J Neurooncol. 1992;14:113–118. doi: 10.1007/BF00177614. [DOI] [PubMed] [Google Scholar]
- Sawaya R, Zuccarrello M, Elkalliny M, Nishiyama H. Postoperative venous thromboembolism and brain tumors: Part I. Clinical profile. J Neurooncol. 1992;14:119–125. doi: 10.1007/BF00177615. [DOI] [PubMed] [Google Scholar]
- Seiferheld WF, Mehta MP, Del Rowe J, Macdonald D, Langer C, Scott C, Curran WJ, Yung WKA. Five years of glioblastoma multiforme (GBM) phase II trials at the Radiation Therapy Oncology Group (RTOG) Proc Am Soc Clin Oncol. 2002;21:71a. (abstract 281) [Google Scholar]
- Shlebak AA, Smith DB. Incidence of objectively diagnosed thromboembolic disease in cancer patients undergoing cytotoxic chemotherapy and/or hormonal therapy. Cancer Chemother Pharmacol. 1997;39:462–466. doi: 10.1007/s002800050599. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn M, Brandes AA, Cairncross G, Lacombe D, Mirimanoff RO. Concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM). Conclusive results of a randomized phase III trial by the EORTC Brain & RT Groups and NCIC Clinical Trials Group. J Clin Oncol. 2004;22 (July 15 suppl.) (abstract 2). [Google Scholar]
- Terry CM, Callahan KS. Protein kinase C regulates cytokine-induced tissue factor transcription and procoagulant activity in human endothelial cells. J Lab Clin Med. 1996;127:81–93. doi: 10.1016/s0022-2143(96)90169-9. [DOI] [PubMed] [Google Scholar]
- Tremont-Lukats IW, Teixeira GM, Conrad C. Thromboembolic events with tamoxifen for malignant gliomas. A systematic review of clinical trials. Neuro-Oncology. 2002;4:347–348. (abstract 148) [Google Scholar]
- Tsuchida E, Urano M. The effect of UCN-01 (7-hydroxy-staurosporine), a potent inhibitor of protein kinase C, on fractionated radiotherapy or daily chemotherapy of a murine fibrosarcoma. Int J Radiat Oncol Biol Phys. 1997;39:1153–1161. doi: 10.1016/s0360-3016(97)00549-x. [DOI] [PubMed] [Google Scholar]
- Weller M, Trepel M, Grimmel C, Schabet M, Bremen D, Krajewski S, Reed JC. Hypericin-induced apoptosis of human malignant glioma cells is light-dependent, independent of bcl-2 expression, and does not require wild-type p53. Neurol Res. 1997;19:459–470. [PubMed] [Google Scholar]
- Xuereb JM, Sie P, Boneu B, Constans J. Inhibition of tissue factor synthesis by disruption of ERK kinases and PKC signaling pathways in human vascular SMCs. Thromb Haemost. 2000;84:129–136. [PubMed] [Google Scholar]