Abstract
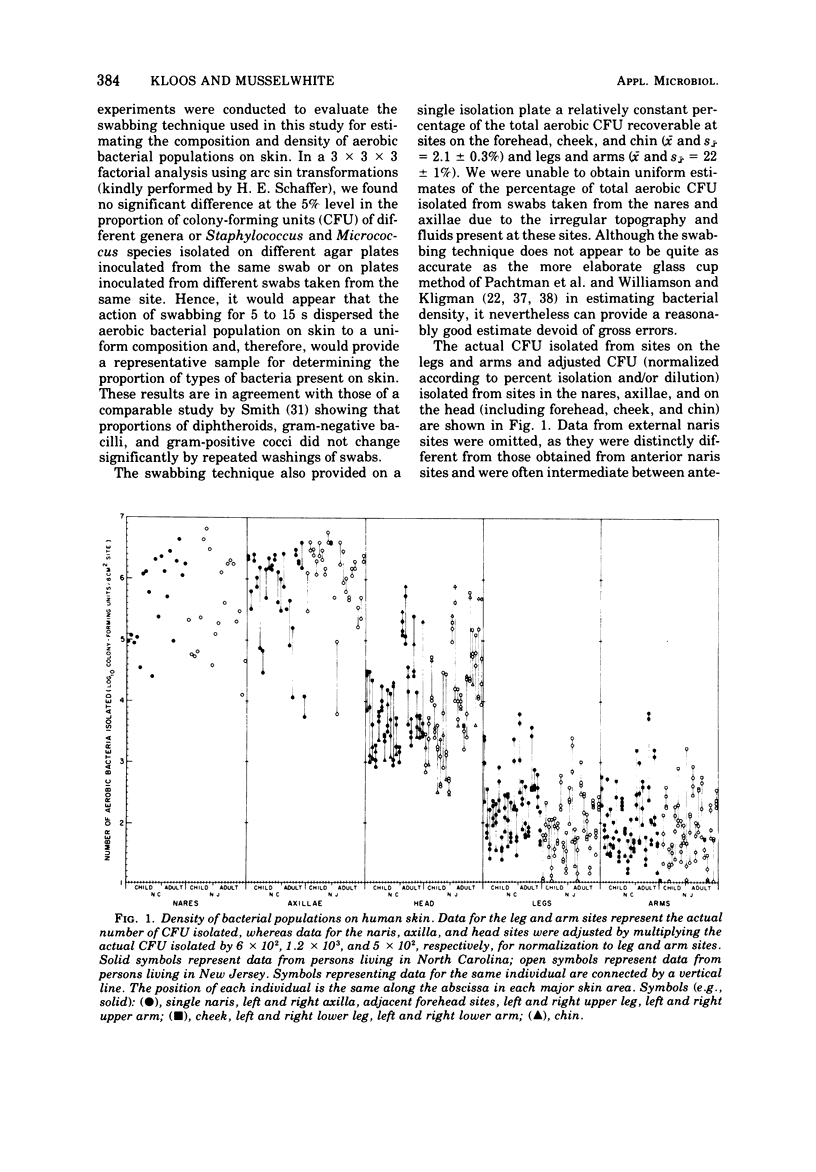
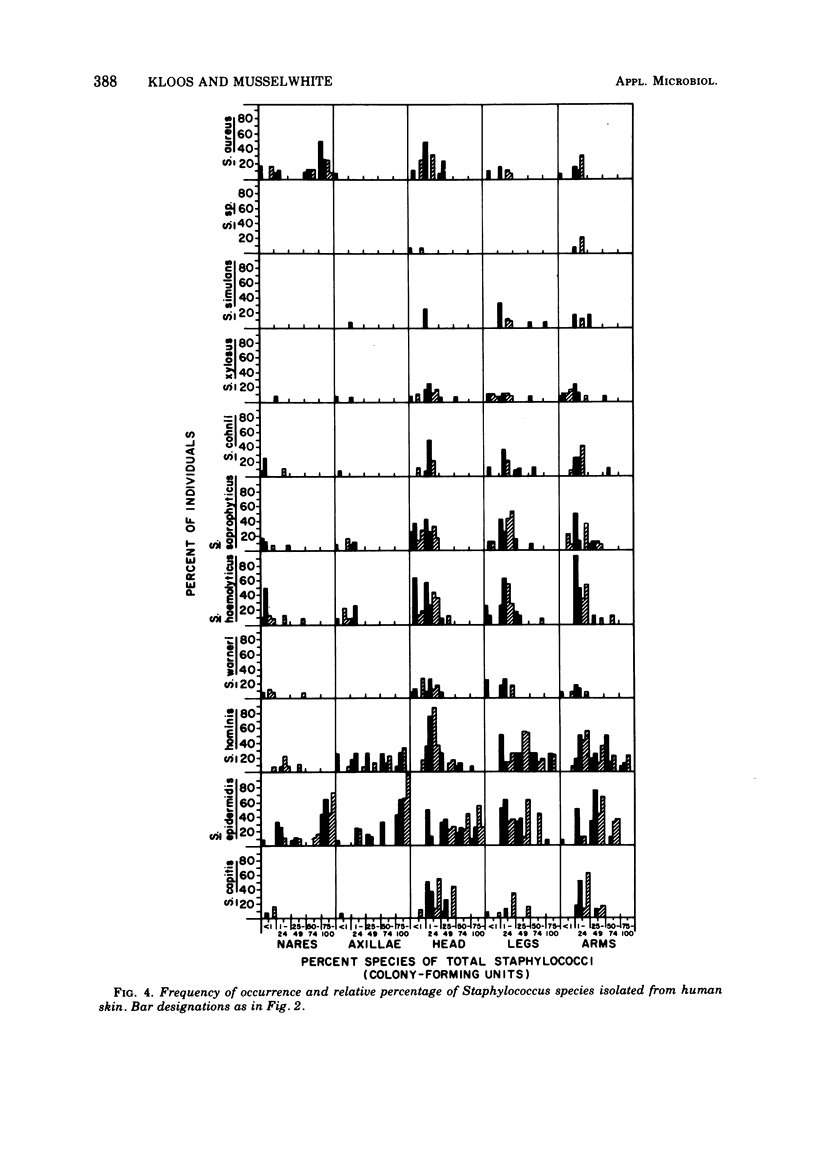
The distribution of Staphylococcus and Micrococcus species and associated coryneform bacteria, Acinetobacter, Klebsiella, Enterobacter, Bacillus, and Streptomyces on skin was determined during October 1971 from samples collected on persons living in North Carolina and New Jersey. Persistence of these organisms on skin was estimated in temporal studies conducted during the period from June 1971 to June 1972 on persons living in North Carolina. Staphylococci and coryneforms were the most predominant and persistent bacteria isolated from the nares and axillae. Staphylococci, coryneforms, micrococci, and Bacillus were the most predominant and persistent bacteria isolated from the head, legs, and arms. Acinetobacters were most frequently isolated during the warmer months of the years. Staphylococcus aureus and S. epidermidis were the most predominant and persistent staphylococci isolated from the nares, whereas S. epidermidis and S. hominis were the most predominant and persistent staphylococci isolated from the axillae, head, legs, and arms. S. capitis was often isolated from the head and arms and S. haemolyticus was often isolated from the head, legs, and arms. S. simulans, S. xylosus, S. cohnii, S. saprophyticus, S. warneri, and an unclassified coagulase-positive species were only occasionally isolated from skin. Micrococcus luteus was the most predominant and persistent Micrococcus isolated from skin and preferred regions of the head, legs, and arms. M. varians was the second most frequent Micrococcus isolated. M. lylae, M. sedentarius, M. roseus, M. kristinae, and M. nishinomiyaensis were only occasionally isolated from skin. M. lylae was most frequently isolated during the colder months of the years.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- BURGI E., NAYLOR H. B. Observations on abortive infection of Micrococcus lysodeikticus with bacteriophage. Virology. 1956 Oct;2(5):577–593. doi: 10.1016/0042-6822(56)90039-3. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A. C. Staphylococci and their classification. Ann N Y Acad Sci. 1965 Jul 23;128(1):4–25. doi: 10.1111/j.1749-6632.1965.tb11626.x. [DOI] [PubMed] [Google Scholar]
- Baird-Parker A. C. The basis for the present classification of staphylococci and micrococci. Ann N Y Acad Sci. 1974 Jul 31;236(0):7–14. doi: 10.1111/j.1749-6632.1974.tb41478.x. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. B., Kloos W. E. Use of shake cultures in a semisolid thioglycolate medium for differentiating staphylococci from micrococci. Appl Microbiol. 1972 Feb;23(2):326–331. doi: 10.1128/am.23.2.326-331.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M. Sarcina species on the skin of the human forearm. Trans St Johns Hosp Dermatol Soc. 1973;59(1):56–60. [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples R. R. Diphtheroids of normal human skin. Br J Dermatol. 1969;81(Suppl):47+–47+. doi: 10.1111/j.1365-2133.1969.tb12833.x. [DOI] [PubMed] [Google Scholar]
- Noble W. C. Distribution of the Micrococcaceae. Br J Dermatol. 1969;81(Suppl):27+–27+. [PubMed] [Google Scholar]
- Noble W. C. Skin carriage of the Micrococcaceae. J Clin Pathol. 1969 May;22(3):249–253. doi: 10.1136/jcp.22.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACHTMAN E. A., VICHER E. E., BRUNNER M. J. The bacteriologic flora in seborrheic dermatitis. J Invest Dermatol. 1954 May;22(5):389–396. doi: 10.1038/jid.1954.55. [DOI] [PubMed] [Google Scholar]
- Reeder W. J., Ekstedt R. D. Unique teichoic acid isolated from the cell walls of a strain of Staphylococcus aureus. Infect Immun. 1973 Apr;7(4):586–588. doi: 10.1128/iai.7.4.586-588.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkany I., Gaylarde C. C. Bacterial colonisation of the skin of the newborn. J Pathol Bacteriol. 1968 Jan;95(1):115–122. doi: 10.1002/path.1700950113. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kloos W. E. A simple test system for the separation of staphylococci from micrococci. J Clin Microbiol. 1975 Mar;1(3):337–338. doi: 10.1128/jcm.1.3.337-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kocur M. Classification of staphylococci based on chemical and biochemical properties. Arch Mikrobiol. 1973 Oct 4;93(1):65–85. doi: 10.1007/BF00666081. [DOI] [PubMed] [Google Scholar]
- Smith R. F. Characterization of human cutaneous lipophilic diphtheroids. J Gen Microbiol. 1969 Mar;55(3):433–443. doi: 10.1099/00221287-55-3-433. [DOI] [PubMed] [Google Scholar]
- Smith R. F. Comparative enumeration of lipophilic and nonlipophilic cutaneous diphtheroids and cocci. Appl Microbiol. 1970 Feb;19(2):254–258. doi: 10.1128/am.19.2.254-258.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville D. A., Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973 Oct;89(4):395–400. doi: 10.1111/j.1365-2133.1973.tb02994.x. [DOI] [PubMed] [Google Scholar]
- Somerville D. A., Murphy C. T. Quantitation of Corynebacterium acnes on healthy human skin. J Invest Dermatol. 1973 Apr;60(4):231–233. doi: 10.1111/1523-1747.ep12724525. [DOI] [PubMed] [Google Scholar]
- Somerville D. A. The normal flora of the skin in different age groups. Br J Dermatol. 1969 Apr;81(4):248–258. doi: 10.1111/j.1365-2133.1969.tb13976.x. [DOI] [PubMed] [Google Scholar]
- TAPLIN D., ZAIAS N. THE HUMAN SKIN AS A SOURCE OF MIMA-HERELLEA INFECTIONS. JAMA. 1963 Dec 7;186:952–955. doi: 10.1001/jama.1963.63710100030023a. [DOI] [PubMed] [Google Scholar]
- Williamson P., Kligman A. M. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965 Dec;45(6):498–503. doi: 10.1038/jid.1965.164. [DOI] [PubMed] [Google Scholar]


