Abstract
The purpose of this study was to assess the impact of early radiation therapy and extent of surgical resection on progression-free survival (PFS) and overall survival (OS) in children with WHO grade II low-grade gliomas (LGGs). We conducted a historical cohort study of 90 patients, ages 21 or younger, diagnosed with WHO grade II LGGs between 1970 and 1995. Median follow-up for surviving patients was 9.4 years (range, 0.5–22.6 years). Tests for variables correlating with OS and PFS were conducted by using log-rank tests and Cox proportional hazards models. Eleven patients underwent gross total resections (GTRs), 43 had subtotal resections, and 34 underwent biopsy only at diagnosis. Two patients underwent biopsy at time of recurrence. Of the 90 patients, 52 received radiation as part of their initial therapy following diagnosis (early-RT group). The overall five-year PFS and OS rates ± SE were 56% ± 5% and 90% ± 3%, respectively. Ten-year PFS and OS rates were 42% ± 6% and 81% ± 5%, respectively. For patients older than three years and without GTRs, administration of early radiation did not appear to influence PFS or OS (P = 0.98 and P = 0.40, respectively; log-rank test). This was confirmed by multivariate analyses (P = 0.95 and P = 0.33 for PFS and OS, respectively). Of the 11 patients with GTRs, disease progressed in only two, and all were alive with no evidence of disease at last follow-up. Patients who underwent GTRs had significantly longer PFS (P = 0.02), but did not have significantly improved OS. Excellent long-term survival rates were achieved for children with WHO grade II LGGs. We were unable to demonstrate a benefit for administering radiation as part of initial treatment. An outcome benefit was seen with greater extent of resection.
Keywords: low-grade glioma, pediatric, radiation, resection
Low-grade gliomas (LGGs) 4are the most common pediatric brain tumors, comprising 35% of childhood primary central nervous system malignancies (Halperin et al., 2005). However, many aspects of treatment remain poorly defined, particularly with respect to nonpilocytic, WHO grade II lesions (Halperin et al., 2005; Rilliet and Vernet, 2000; Schmandt and Packer, 2000; Stother et al., 2002). One such controversy is whether to administer postoperative radiation to children with WHO grade II tumors, balancing the potential effectiveness of radiation with long-term toxicities. Historically, treatment of incompletely resected tumors frequently involved postoperative radiation and/or chemotherapy. However, the morbidity of radiation therapy (RT) in children has prompted many physicians to replace early radiation with chemotherapy or observation (Avizonis et al., 1992; Chadderton et al., 1995; Cohen and Duffner, 1991; Duffner and Cohen, 1991; Ellenberg et al., 1987; Reddy and Packer, 1998; Schmandt and Packer, 2000; Watson et al., 2001).
Lack of consensus regarding the optimal treatment of pediatric grade II gliomas is in part due to the absence of randomized prospective trials. Although pediatric consortia and cooperative groups had designed a prospective, randomized, phase 3 trial (POG 9130/CCG 9891/INT 0128 trial) to evaluate neurosurgical and radiotherapeutic treatments of children with LGGs (Wisoff et al., 1996), practitioner bias forced early closure of this trial (Dhodapkar et al., 1999; Watson et al., 2001). Furthermore, most previous retrospective studies of LGGs have included limited numbers of children and grade II lesions (Bernstein et al., 1984; Bloom et al., 1990; Bowers et al., 2002; Butler et al., 1994; Desai et al., 2001; Deutsch, 1982; Dewit et al., 1984; Dohrmann et al., 1985; Erkal et al., 1997; Gajjar et al., 1997; Garcia et al., 1985; Hirsch et al., 1989; Hoffman et al., 1993; Kandil et al., 1999; Karim et al., 1998; Laws et al., 1984; Leibel et al., 1975; Lote et al., 1997; Marsa et al., 1973; Medbery et al., 1988; Mercuri et al., 1981; Pencalet et al., 1999; Pollack et al., 1995; Scanlon and Taylor, 1979; Sgouros et al., 1995; Shaw et al., 1989; Shibamoto et al., 1993; Wara, 1985; Weir and Grace, 1976; Yeh et al., 2002).
Given the relative paucity of data relating to the role of radiation therapy in pediatric grade II gliomas, we hoped to shed additional light on this and other key treatment issues by evaluating the experience of the University of California, San Francisco (UCSF). The primary focus of this study was the role of radiation therapy in the up-front treatment of pediatric grade II gliomas. In addressing this issue, we concentrated on a specific subgroup of patients for whom the use of radiation at the time of initial diagnosis is controversial, namely, children who are older than three years of age and patients who have undergone incomplete surgical resections. We reviewed our cohort of pediatric patients treated for WHO grade II LGGs and estimated differences in survival outcomes based on prognostic and therapeutic factors. In particular, we sought to assess, first, the impact of radiation therapy at initial presentation and, second, the effect of extent of resection on progression-free survival (PFS) and overall survival (OS).
Patients and Methods
We conducted a historical cohort study of 90 patients, ages 21 years and younger, with WHO grade II gliomas, diagnosed at UCSF between 1970 and 1995. All patient characteristics, treatment parameters, and survival data were extracted from clinical records, radiographic information, and active follow-up obtained from the Departments of Radiation Oncology, Neurooncology, and/or Neurosurgery at UCSF. Table 1 summarizes the clinical characteristics for the entire cohort. Patients with grade II brainstem and spinal cord lesions were excluded, as were those with prior radiation or chemotherapy, previous cancers, second concurrent malignancies, or extracranial metastases at diagnosis.
Table 1.
Patient characteristics and treatment details
|
Radiation at Diagnosis |
|||
|---|---|---|---|
| Variable | No | Yes | Total |
| Number of patients | 38 | 52 | 90 |
| Median age (range), years | 3.9 (0.5–18.0) | 12.5 (1.2–20.7) | 9.0 (0.5–20.7) |
| Median years of follow-up (range)a | 8.9 (0.5–16.4) | 10.6 (0.5–22.6) | 9.4 (0.5–22.6) |
| Patients with five years of follow-up:a No. of patients/total surviving (%) | 29/35 (83%) | 32/36 (89%) | 61/71 (86%) |
| Female:male | 17:21 | 22:30 | 39:51 |
| Tumor location | |||
| Cerebrum | 20 | 19 | 39 |
| Cerebellum | 4 | 5 | 9 |
| Midline structures | 14 | 28 | 42 |
| Histology | |||
| Astrocytoma | 25 | 43 | 68 |
| Oligodendroglioma | 6 | 1 | 7 |
| Mixed | 7 | 8 | 15 |
| Seizure at presentation: No:Yes | 16:22 | 30:22 | 46:44 |
| Extent of resection at diagnosis | |||
| Gross total resection | 11 | 0 | 11 |
| Subtotal resection | 16 | 27 | 43 |
| Biopsy only | 11 | 23 | 34 |
| No surgeryb | 0 | 2 | 2 |
| Chemotherapy at diagnosis: No:Yes | 25:13 | 42:10 | 67:23 |
| Radiation at any time: No:Yes | 25:13 | 0:52 | 25:65 |
Follow-up for surviving patients.
Two patients had no surgery at initial diagnosis. For both, biopsy was conducted at time of recurrence and confirmed grade II low-grade glioma.
All patients in the study population had pathologically confirmed WHO grade II gliomas. Subjects’ original pathological diagnoses from UCSF neuropathology were used for classification. Histology was defined by WHO criteria as grade II astrocytoma, oligodendroglioma, or mixed oligoastrocytoma. Extent of resection was based on preoperative and postoperative radiographic imaging studies as well as operative reports. Extent of resection was defined as gross total resection (GTR: all visible tumor removed and postoperative film negative for residual tumor), subtotal resection (STR: bulk tumor resection 50%–95% and residual tumor on film), and biopsy (less than 50% of the tumor volume removed). Chemotherapy details, including agents, dates, and protocols, were collected. The most common chemotherapy regimens were nitrosourea-based multiagent chemotherapy and single-agent carboplatin, as previously published (Levin et al., 2000; Prados et al., 1997). All 90 patients underwent postoperative imaging. Sixty-eight cases were followed by MRI alone, 14 by CT initially and then MRI until last follow-up, and eight by CT alone.
Patients were analyzed according to whether they received radiation at diagnosis (early RT) or did not receive radiation at diagnosis (no early RT). The decision of whether to administer early radiation was made at the discretion of the treating physician. Some patients who did not receive radiation at diagnosis subsequently were treated with radiation at the time of progression. Radiation was directed to the primary tumor volume plus margin, with a median dose of 54 Gy (range, 45–60 Gy).
We calculated PFS and OS rates from date of diagnosis. All of the patients had been diagnosed prior to 1995. Life-table methods were used to estimate PFS and OS (Kaplan and Meier, 1958). Five-year and ten-year PFS and OS estimates were calculated by the Kaplan-Meier method, with standard errors calculated by Greenwood’s formula. Elapsed time from diagnosis to an event or last follow-up was used to compute PFS and OS probabilities. In analyses of PFS, last follow-up dates for patients not experiencing an event were based on imaging studies that delineated disease status. For OS, deaths, regardless of cause, were coded as events. For PFS, an event was defined as relapse, progression, or death during the period of active follow-up. Kaplan-Meier curves for PFS and OS were calculated for the subgroups that did and did not have radiation administered at diagnosis. Additional sets of Kaplan-Meier curves were derived to determine the PFS and OS of patients by extent of resection at original diagnosis.
To assess variables influencing PFS and OS, univariate analyses using log-rank tests and proportional hazards models were performed to evaluate treatment with early radiation and extent of original tumor resection. To further verify results of univariate analyses, Cox proportional hazards multivariate analyses were conducted, including treatment with early radiation and extent of resection as well as additional variables considered possibly prognostic. The additional variables were age at diagnosis, tumor location, and tumor histology. Age was analyzed as a continuous variable. Tumor location was defined as midline versus nonmidline on the basis of radiographic and surgical findings. Histology was defined as a categorical variable, including astrocytoma, oligodendroglioma, and mixed pathology. For evaluation of extent of resection as a predictor of outcome, extent of resection was considered as a categorical variable, and for multivariate analyses, two variables were included indicating whether a patient had an STR and whether the patient had a GTR. This study was performed after approval by local human investigation committees.
Results
Demographics
Ninety patients with WHO grade II gliomas were included in the study. Table 1 delineates patient characteristics and treatment factors. The median age for all patients was 9.0 years (range, 0.5–20.7 years). Thirty-nine (43%) patients were female and 51 (57%) were male. Thirty-nine tumors (43%) were located primarily in the cerebrum, 42 (47%) in midline structures (20, thalamus; 5, hypothalamus; 2, optic pathway; 7, optic-hypothalamic; 6, pineal; 1, third ventricle; and 1, tectal plate) and nine (10%) in the cerebellum.
Histologic confirmation was obtained in all cases. Sixty-eight patients (76%) had astrocytomas, seven (8%) had oligodendrogliomas, and 15 (17%) had mixed oligoastrocytomas. Two of the 90 patients did not have biopsy confirmation at initial diagnosis, but biopsies at the time of recurrence confirmed grade II lesions. For analyses involving extent of resection, these two patients were included in the biopsy group.
Median follow-up for surviving patients was 9.4 years (range, 0.5–22.6 years). Among the 90 patients, 19 deaths were documented during the follow-up period. Of the remaining 71 surviving children, 61 had more than five years of follow-up from time of diagnosis, and 32 had more than 10 years of follow-up.
Progression-Free Survival and Overall Survival
For the entire cohort of 90 patients, the five-year PFS rate was 56% ± 5%, and the ten-year PFS rate was 42% ± 6%. Five-year and ten-year OS rates were 90% ± 3% and 81% ± 5%, respectively. The median PFS for all patients was 80.4 months (95% confidence interval [CI], 40.3–120.5 months).
Of the cohort of 90 patients, 49 experienced disease progression. The median time to progression for these 49 patients was 25.0 months (range, 2.4–259.2 months). Twelve (24.5%) of these patients progressed at five or more years after diagnosis. Of the 49 patients who progressed, at last follow-up 19 had died, five remained alive with disease, and 25 had no evidence of disease (NED). Nineteen patients experienced multiple recurrences (two or more), with seven having disease progression that led to death, three remaining alive with disease, and nine having NED at last follow-up.
The median survival time for the 19 patients who died was 69.6 months (range, 4.8–276 months). Nine (47%) of these 19 children died within five years of diagnosis. Five of the 19 died more than 10 years after their original diagnoses. Of the 49 patients whose disease progressed, a limited number had repeat tissue diagnoses at the time of progression, revealing 11 grade II, 13 grade III, and three grade IV tumors.
Impact of Early Radiation
Thirty-eight patients received no radiation at diagnosis (no-early-RT group), and 52 children did receive radiation as part of their initial treatment regimen (early-RT group). The median age of children receiving no early RT was 3.9 years (range, 0.5–18.0 years), and median age for those receiving early RT was 12.5 years (range, 1.2–20.7 years). Long-term follow-up was achieved for most surviving patients. The median follow-up for surviving patients in the no-early-RT and early-RT groups was 8.9 years (range, 0.5–16.4 years) and 10.6 years (range, 0.5–22.6 years), respectively. Follow-up of five or more years was achieved for most surviving children, 83% and 89%, respectively, for the no-early-RT and early-RT groups. Of the 20 patients whose disease progressed in the no-early-RT group, 13 received radiation upon progression. Table 1 describes the patient characteristics and treatment details of the no-early-RT and early-RT subgroups.
Five-year and ten-year PFS rates were, respectively, 50% ± 8% and 43% ± 9% for those not receiving early radiation, as compared with 61% ± 7% and 43 ± 7% for the early-RT group. Five-year and ten-year OS rates were, respectively, 97% ± 3% and 92% ± 6% for those receiving no radiation at diagnosis, as compared with 84% ± 5% and 74% ± 6% for those receiving RT at diagnosis.
A multivariate logistic regression was used to confirm predictors for early RT use, and as expected, lesser extent of resection and older age increased the likelihood of patients receiving RT as part of their initial treatment (P < 0.01 for both variables). Indeed, none of the 11 patients who underwent GTRs and only three of 19 children under three years of age received early RT, which substantiates the rationale to exclude these patients in order to limit bias in analyses of potential effects of early RT and to focus on the group of patients for whom the use of RT at time of diagnosis is most controversial. All subsequent analyses comparing the cohorts that did and did not receive early RT excluded patients who underwent GTRs and those younger than three years of age at diagnosis. In using these exclusions, we sought to eliminate subgroups for which a compelling bias existed against early RT administration. After excluding all children with GTRs and those younger than three years of age at diagnosis, 61 children remained in the cohort and were analyzed, 15 in the no-early-RT group and 46 in the early-RT group. Among these 61 patients, the disease of 32 had progressed and 15 had died at the time of this analysis.
For the subgroup of children who did not undergo GTRs and who were older than three years of age, the respective five-year and ten-year PFS rates were 59% ± 13% and 43% ± 14% for those receiving no radiation at diagnosis, as compared with 60% ± 7% and 44 ± 8% for those receiving radiation at diagnosis. The respective five-year and ten-year OS rates were 93% ± 6% and 82% ± 12% for those receiving no radiation at diagnosis, as compared with 84% ± 5% and 77 ± 7% for those receiving RT at diagnosis.
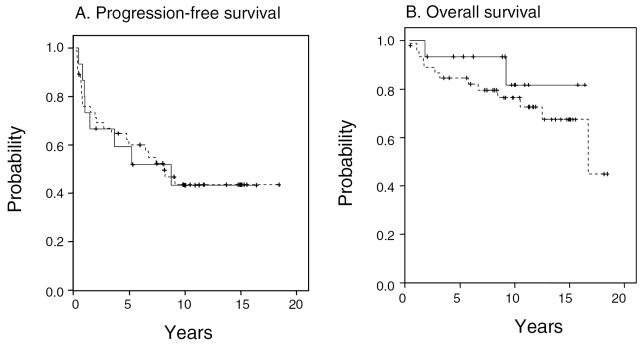
A comparison of the two groups using the log-rank test revealed no statistical difference in PFS or OS between those who did and did not receive radiation at diagnosis (P = 0.98 and P = 0.40, respectively). We also conducted a multivariate analysis to confirm that inclusion of other factors would not change these conclusions. A Cox proportional hazards model was derived after adjusting for potentially confounding variables, including histology, location of primary tumor, patient age, and extent of surgical resection. The PFS and OS hazard ratios (HRs) for the early-RT versus no-early-RT groups were not statistically significant. For PFS, the HR was 0.97 (P = 0.95; 95% CI, 0.41–2.6), and for OS, the hazard ratio was 2.5 (P = 0.33; 95% CI, 0.40–16.4). A hazard ratio of less than 1 would have implied a benefit to early RT. Figure 1 shows the Kaplan-Meier curves for radiation therapy administered or not administered at the time of original diagnosis.
Fig. 1.
Actuarial (A) progression-free survival and (B) overall survival, according to whether radiation was administered (- - -) or not administered (——) at the time of original diagnosis. Analysis excludes patients who are younger than three years old and patients who underwent gross total resections (N = 61; see the text for details).
Influence of Extent of Surgical Resection
A secondary analysis to evaluate the impact of extent of surgical resection was performed including all 90 patients. It was recognized that extent of resection may be a surrogate for other factors such as tumor location, tumor size, or performance status. Included in this analysis were 11 patients with GTRs, 43 with STRs, and 36 with biopsies. As noted earlier, the two patients who underwent biopsies at time of recurrence were included in the biopsy group for analyses involving extent of resection.
Five-year PFS rates were 79% ± 13% for patients treated with GTRs, 60% ± 8% for those with STRs, and 46% ± 8% for those with biopsies alone. Five-year OS rates were 100% for those treated with GTRs, 88% ± 5% for those with STRs, and 89% ± 5% for those with biopsies alone. Ten-year PFS rates were 45% ± 8% for patients with STRs and 30% ± 8% for those with biopsies alone. Ten-year OS rates were 82% ± 6% for patients with STRs and 76% ± 8% for those with biopsies.
Eleven patients in the no-early-RT and no patients in the early-RT group underwent GTRs at initial presentation. Eight of the 11 patients had more than five years of follow-up, and of these, three had more than 10 years of follow-up. All 11 patients were alive without evidence of disease at last follow-up. Of the 11 patients, two experienced recurrences: one at 28 months and one at 36 months. These patients were salvaged at recurrence by repeat GTR and chemotherapy, respectively, and had NED at last follow-up, 48 and 88 months from their original diagnoses.
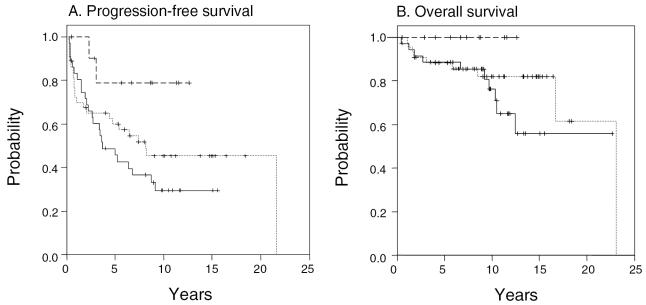
In analyzing the entire cohort of 90 patients and adjusting for location, histology, age, and early radiation, an improved PFS was evident for patients with GTRs as compared to those with biopsy only (P = 0.02; HR = 0.14; 95% CI, 0.02–0.76). Comparison of STRs and biopsy revealed an HR of 0.63, which did not reach statistical significance (P = 0.31; 95% CI, 0.26–1.54). Although none of the patients with a GTR had died, there was no statistically significant difference in overall survival depending on extent of resection (P = 0.2, log-rank test). Figure 2 shows Kaplan-Meier curves according to extent of surgical resection at the time of original diagnosis.
Fig. 2.
Actuarial (A) progression-free survival and (B) overall survival, according to extent of surgical resection. (__ __) gross total resection, (···) subtotal resection, and (——)biopsy only. Analysis includes entire cohort (N = 90).
Discussion
The issue of optimal treatment for these WHO grade II LGGs remains unsettled, largely because of the paucity of prospective randomized trials, coupled with small pediatric populations and limited follow-up in most retrospective studies to date. Although children with LGGs have favorable overall survival rates, controversy persists regarding when to incorporate radiation therapy into the treatment of incompletely resected tumors. Our data, in conjunction with the current literature, do not support a survival benefit for early initiation of postoperative radiation, even in the setting of subtotal resections. Our data do, however, support aggressive, maximal surgical resection as a factor associated with superior PFS, an advantage driven by gross total resections.
This study provides very long follow-up for a large cohort of children with pathological documentation of WHO grade II LGGs. The data reinforce the approach of maximal surgical resection, reserving postoperative radiation for patients with progressive tumors and/or neurological deficits (Campbell and Pollack, 1996; Fisher et al., 1998, 2001; Watson et al., 2001). According to objective radiologic confirmation, 11 patients in our cohort underwent GTRs. Only two of these patients required additional salvage therapy upon disease recurrence, and all 11 had NED at last follow-up. Many studies to date have recognized similarly that extent of resection is a key covariate in survival modeling (Berger, 1996; Bloom et al., 1990; Campbell and Pollack, 1996; Desai et al., 2001; Dhodapkar et al., 1999; Lo et al., 2001; Pencalet et al., 1999; Pollack, 1999; Pollack et al., 1995). However, surgical resectability may be considered a proxy for other prognostic variables, including tumor location. For example, in our database, the vast majority of biopsies (32 of 36) were performed for midline tumors that, relative to nonmidline lesions, have been found in some series to result in worse outcomes.
Despite the successful long-term results of children undergoing GTRs alone, there remains uncertainty regarding the use of adjunctive RT for pediatric patients with incomplete resections. The value of RT in these cases is unclear, given potential adverse effects balanced against a divergence of opinions regarding survival benefit. Previous reports have documented possible short-term and long-term consequences on cognitive development, vascular integrity, endocrine function, and general quality of life (Avizonis et al., 1992; Chadderton et al., 1995; Cohen and Duffner, 1991; Duffner and Cohen, 1991; Ellenberg et al., 1987; Reddy and Packer, 1998). These radiation-induced sequelae, though not particularly studied in our data set, clearly help inform decisions about the appropriate use of RT in the pediatric population.
Compounding the aforementioned potential adverse effects, few available studies have suggested an overall survival advantage for children undergoing postoperative radiation in the setting of incomplete surgical resections (Shaw et al., 1989; Shibamoto et al., 1993; Watson et al., 2001). Serious obstacles are posed by the miscellany of grade I and II lesions analyzed together, small numbers of children, and short follow-up periods that characterize many published studies (Fisher et al., 1998, 2001; Kandil et al., 1999; Karim et al., 1998; Lote et al., 1997; Medbery et al., 1988; Pencalet et al., 1999; Pollack et al., 1995; Sgouros et al., 1995). Numerous contemporary series indicate no significant benefit in OS with early postoperative radiation in pediatric LGGs. Fouladi et al. (2003) analyzed children with LGGs who were enrolled in Children’s Cancer Group high-grade glioma study CCG-945. In accordance with our findings, the authors found no benefit to frontline combined chemoradiation. In a handful of retrospective reviews, benefits of early postoperative radiation for PFS do not translate into OS benefits, likely because salvage therapy is successful in a significant number of progressive cases (Karim et al., 1998; Pollack et al., 1995).
To describe survival effects of early RT in our patient population, we attempted to analyze a more homogeneous group of patients. We included only WHO grade II lesions that generally have a distinct biological course and prognosis as compared with grade I lesions (Fuller and Perry, 2001; Gjerris and Klinken, 1978; Hayostek et al., 1993; Herfarth et al., 2001). Brainstem and spinal cord lesions were also excluded from the current study, given different natural histories and therapeutic options relative to other tumor locations (Desai et al., 2001; Farwell et al., 1977; Merchant et al., 2000; Pencalet et al., 1999; Sgouros et al., 1995). Finally, in the comparison of early radiation versus no early radiation, we excluded patients who had undergone GTRs and those younger than three years of age, because these patients rarely receive radiation at the time of diagnosis unless extremely poor prognostic features exist (Berger, 1996; Jenkin et al., 1998; Sala et al., 1999).
The data from our cases followed for a median of 9.4 years (for surviving patients) corroborate much of the available historical data that describe no benefit in survival with early RT. Controlling for potential confounding sources, early radiation compared with no early radiation does not confer PFS or OS benefit in our cohort. Our study is, however, limited by its retrospective nature. One must consider unidentified sources of selection bias that a retrospective review cannot well control. Those patients referred for radiation likely carried poorer initial prognoses. Hence, the fact that there is no survival difference may indicate that these children benefited from RT, perhaps because RT conferred comparable survival rates despite worse prognostic features. In our multivariate model, we have tried to control for such confounding variables. However, because of sample size, potential missing covariates, and nonrandomized administration of therapies, a benefit from early RT may exist that was missed in this analysis. Although our data show no clear benefit to early radiation, the wide confidence intervals for the hazard ratios reflect the possibility that early RT may be either beneficial or detrimental.
Given the results of this and other recent publications, no statistically significant benefit in overall survival has been firmly established for immediate postoperative radiation following incomplete resections. Following an incomplete resection, the appropriate choice among supportive care, radiation, and chemotherapy must be tailored to a patient’s age, signs, symptoms, and personal preference. Most practitioners would offer early radiation to patients whose tumors or symptoms cannot be controlled medically. Progressive neurological deterioration or radiographic evidence of tumor progression warrants consideration of radiation therapy. Importantly, new developments in neurosurgery, chemotherapy, and radiation treatment may advance survival and quality of life significantly. Refinements potentially include lower radiation doses, conformal treatment plans, and intensity-modulated radiation therapy (Eder et al., 2001; Herfarth et al., 2001; Hodgson et al., 2001; Saran, 2002; Saran et al., 2002; Whittle, 2002).
Our cohort of children with pathologically confirmed WHO grade II LGGs had excellent long-term survival rates consistent with contemporary published literature. We were unable to demonstrate a survival benefit for administering radiation as part of initial management. Lesser extent of surgical resection was associated with a poorer outcome in this patient group. All patients with GTR were alive with NED at last follow-up.
Footnotes
This research was supported in part by NIH grant M01 RR01271, Pediatric Clinical Research Center (D.A.H.-K.), and CA 82103 (K.R.L. and M.S.B.).
This research was presented at the American Society for Therapeutic Radiology and Oncology, New Orleans, October 6–10, 2002.
Abbreviations used are as follows: CI, confidence interval; GTR, gross total resection; HR, hazard ratio; LGG, low-grade glioma; NED, no evidence of disease; OS, overall survival; PFS, progression-free survival; RT, radiation therapy; STR, subtotal resection; UCSF, University of California, San Francisco.
References
- Avizonis VN, Fuller DB, Thomson JW, Walker MJ, Nilsson DE, Menlove RL. Late effects following central nervous system radiation in a pediatric population. Neuropediatrics. 1992;23:228–234. doi: 10.1055/s-2008-1071348. [DOI] [PubMed] [Google Scholar]
- Berger MS. The impact of technical adjuncts in the surgical management of cerebral hemispheric low-grade gliomas of childhood. J Neurooncol. 1996;28:129–155. doi: 10.1007/BF00250195. [DOI] [PubMed] [Google Scholar]
- Bernstein M, Hoffman HJ, Halliday WC, Hendrick EB, Humphreys RP. Thalamic tumors in children. Long-term follow-up and treatment guidelines. J Neurosurg. 1984;61:649–656. doi: 10.3171/jns.1984.61.4.0649. [DOI] [PubMed] [Google Scholar]
- Bloom HJ, Glees J, Bell J, Ashley SE, Gorman C. The treatment and long-term prognosis of children with intracranial tumors: A study of 610 cases, 1950–1981 (erratum in Int. J. Radiat. Oncol. Biol. Phys. [1990] 19, 829). . Int. J. Radiat. Oncol. Biol. Phys. 1990;18:723–745. doi: 10.1016/0360-3016(90)90392-w. [DOI] [PubMed] [Google Scholar]
- Bowers DC, Mulne AF, Weprin B, Bruce DA, Shapiro K, Margraf LR. Prognostic factors in children and adolescents with low-grade oligodendrogliomas. Pediatr Neurosurg. 2002;37:57–63. doi: 10.1159/000065106. [DOI] [PubMed] [Google Scholar]
- Butler D, Jose B, Summe R, Paris K, Bertolone S, Patel CC, Spanos W, Lindberg R. Pediatric astrocytomas. The Louisville experience: 1978–1988. Am J Clin Oncol. 1994;17:475–479. [PubMed] [Google Scholar]
- Campbell JW, Pollack IF. Cerebellar astrocytomas in children. J Neurooncol. 1996;28:223–231. doi: 10.1007/BF00250201. [DOI] [PubMed] [Google Scholar]
- Chadderton RD, West CG, Schuller S, Quirke DC, Gattamaneni R, Taylor R. Radiotherapy in the treatment of low-grade astrocytomas. II The physical and cognitive sequelae (erratum in Childs Nerv Syst [1995] 11, 715) Childs Nerv Syst. 1995;11:443–448. doi: 10.1007/BF00334961. [DOI] [PubMed] [Google Scholar]
- Cohen ME, Duffner PK. Long-term consequences of CNS treatment for childhood cancer, Part I: Pathologic consequences and potential for oncogenesis. Pediatr Neurol. 1991;7:157–163. doi: 10.1016/0887-8994(91)90078-y. [DOI] [PubMed] [Google Scholar]
- Desai KI, Nadkarni TD, Muzumdar DP, Goel A. Prognostic factors for cerebellar astrocytomas in children: A study of 102 cases. Pediatr Neurosurg. 2001;35:311–317. doi: 10.1159/000050443. [DOI] [PubMed] [Google Scholar]
- Deutsch M. Radiotherapy for primary brain tumors in very young children. Cancer. 1982;50:2785–2789. doi: 10.1002/1097-0142(19821215)50:12<2785::aid-cncr2820501216>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Dewit L, Van der Schueren E, Ang KK, Van den Bergh R, Dom R, Brucher JM. Low grade astrocytoma in children treated by surgery and radiation therapy. Acta Radiol Oncol. 1984;23:1–8. doi: 10.3109/02841868409135977. [DOI] [PubMed] [Google Scholar]
- Dhodapkar K, Wisoff JH, Sanford R, Holmes E, Sposto R, Finlay JL. Patterns of relapse and survival for newly-diagnosed childhood low grade astrocytoma: Initial results of CCG 9891/POG 9130. Med Pediatr Oncol. 1999;33:205. (abstract P-35) [Google Scholar]
- Dohrmann GJ, Farwell JR, Flannery JT. Astrocytomas in childhood: A population-based study. Surg Neurol. 1985;23:64–68. doi: 10.1016/0090-3019(85)90162-4. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Cohen ME. Long-term consequences of CNS treatment for childhood cancer, Part II: Clinical consequences. Pediatr Neurol. 1991;7:237–242. doi: 10.1016/0887-8994(91)90038-m. [DOI] [PubMed] [Google Scholar]
- Eder HG, Leber KA, Eustacchio S, Pendl G. The role of gamma knife radiosurgery in children. Childs Nerv Syst. 2001;17:341–346. doi: 10.1007/s003810000435. [DOI] [PubMed] [Google Scholar]
- Ellenberg L, McComb JG, Siegel SE, Stowe S. Factors affecting intellectual outcome in pediatric brain tumor patients. Neurosurgery. 1987;21:638–644. doi: 10.1227/00006123-198711000-00006. [DOI] [PubMed] [Google Scholar]
- Erkal HS, Serin M, Cakmak A. Management of optic pathway and chiasmatic-hypothalamic gliomas in children with radiation therapy. Radiother Oncol. 1997;45:11–15. doi: 10.1016/s0167-8140(97)00102-3. [DOI] [PubMed] [Google Scholar]
- Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977;40:3123–3132. doi: 10.1002/1097-0142(197712)40:6<3123::aid-cncr2820400656>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Fisher BJ, Bauman GS, Leighton CE, Stitt L, Cairncross JG, Macdonald DR. Low-grade gliomas in children: Tumor volume response to radiation. J Neurosurg. 1998;88:969–974. doi: 10.3171/jns.1998.88.6.0969. [DOI] [PubMed] [Google Scholar]
- Fisher BJ, Leighton CC, Vujovic O, Macdonald DR, Stitt L. R esults of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J Radiat Oncol Biol Phys. 2001;51:704–710. doi: 10.1016/s0360-3016(01)01705-9. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Hunt DL, Pollack IF, Dueckers G, Burger PC, Becker LE, Yates AJ, Gilles FH, Davis RL, Boyett JM, Finlay JL. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- Fuller CE, Perry A. Pathology of low- and intermediate-grade gliomas. Semin Radiat Oncol. 2001;11:95–102. doi: 10.1053/srao.2001.21412. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, Langston JW, Muhlbauer M, Boyett JM, Kun LE. Low-grade astrocytoma: A decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- Garcia DM, Fulling KH, Marks JE. The value of radiation therapy in addition to surgery for astrocytomas of the adult cerebrum. Cancer. 1985;55:919–927. doi: 10.1002/1097-0142(19850301)55:5<919::aid-cncr2820550502>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gjerris F, Klinken L. Long-term prognosis in children with benign cerebellar astrocytoma. J Neurosurg. 1978;49:179–184. doi: 10.3171/jns.1978.49.2.0179. [DOI] [PubMed] [Google Scholar]
- Halperin, E.C., Constine, L.S., Tarbell, N.J., and Kun, L.E. (2005) Pediatric Radiation Oncology, 4th ed. Philadelphia: Lippincott Williams & Wilkins.
- Hayostek CJ, Shaw EG, Scheithauer B, O’Fallon JR, Weiland TL, Schomberg PJ, Kelly PJ, Hu TC. Astrocytomas of the cerebellum. A comparative clinicopathologic study of pilocytic and diffuse astrocytomas. Cancer. 1993;72:856–869. doi: 10.1002/1097-0142(19930801)72:3<856::aid-cncr2820720335>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Herfarth KK, Gutwein S, Debus J. Postoperative radiotherapy of astrocytomas. Semin Surg Oncol. 2001;20:13–23. doi: 10.1002/ssu.1012. [DOI] [PubMed] [Google Scholar]
- Hirsch JF, Sainte Rose C, Pierre-Kahn A, Pfister A, Hoppe-Hirsch E. Benign astrocytic and oligodendrocytic tumors of the cerebral hemispheres in children. J Neurosurg. 1989;70:568–572. doi: 10.3171/jns.1989.70.4.0568. [DOI] [PubMed] [Google Scholar]
- Hodgson DC, Goumnerova LC, Loeffler JS, Dutton S, Black PM, Alexander E, 3rd Xu, R. Kooy, H. Silver, B. and, Tarbell NJ. Radiosurgery in the management of pediatric brain tumors. Int J Radiat Oncol Biol Phys. 2001;50:929–935. doi: 10.1016/s0360-3016(01)01518-8. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Soloniuk DS, Humphreys RP, Drake JM, Becker LE, De Lima BO, Piatt JH., Jr Management and outcome of low-grade astrocytomas of the midline in children: A retrospective review. Neurosurgery. 1993;33:964–971. doi: 10.1227/00006123-199312000-00002. [DOI] [PubMed] [Google Scholar]
- Jenkin D, Danjoux C, Greenberg M. Subsequent quality of life for children irradiated for a brain tumor before age four years. Med Pediatr Oncol. 1998;31:506–511. doi: 10.1002/(sici)1096-911x(199812)31:6<506::aid-mpo7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kandil A, Khafaga Y, ElHusseiny G, Allam A, Jamshed A, Schultz H. Low-grade astrocytoma: A retrospective analysis of 102 patients. Acta Oncol. 1999;38:1051–1056. doi: 10.1080/028418699432356. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Karim AB, Cornu P, Bleehan N, Afra D, De Witte O, Schraub S, Darcel F, Brucher JM, Bolla M, Vecht C, Stenning S, Pierart M, Van Glabbeke M. Immediate postoperative radiotherapy in low grade glioma improves progression free survival, but not overall survival: Preliminary results of an EORTC/MRC randomized phase III study. J Neurooncol. 1998;39:101. (abstract O-11) [Google Scholar]
- Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665–673. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- Leibel SA, Sheline GE, Wara WM, Boldrey EB, Nielsen SL. The role of radiation therapy in the treatment of astrocytomas. Cancer. 1975;35:1551–1557. doi: 10.1002/1097-0142(197506)35:6<1551::aid-cncr2820350612>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Levin VA, Lamborn K, Wara W, Davis R, Edwards M, Rabbitt J, Malec M, Prados MD. Phase II study of 6-thioguanine, procarbazine, dibromodulcitol, lomustine, and vincristine chemotherapy with radiotherapy for treating malignant glioma in children. Neuro-Oncology. 2000;2:22–28. doi: 10.1093/neuonc/2.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SS, Cho KH, Hall WA, Hernandez WL, Kossow RJ, Lee CK, Clark HB. Does the extent of surgery have an impact on the survival of patients who receive postoperative radiation therapy for supratentorial low-grade gliomas? Int J Cancer. 2001;96 (suppl.):71–78. doi: 10.1002/ijc.10359. [DOI] [PubMed] [Google Scholar]
- Lote K, Egeland T, Hager B, Stenwig B, Skullerud K, Berg-Johnsen J, Storm-Mathisen I, Hirschberg H. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: A retrospective study in 379 patients. J Clin Oncol. 1997;15:3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- Marsa GW, Probert JC, Rubinstein LJ, Bagshaw MA. Radiation therapy in the treatment of childhood astrocytic gliomas. Cancer. 1973;32:646–655. doi: 10.1002/1097-0142(197309)32:3<646::aid-cncr2820320318>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Medbery CA, 3rd Straus, K.L. Steinberg, S.M. Cotelingam, J.D. and, Fisher WS. Low-grade astrocytomas: Treatment results and prognostic variables. Int J Radiat Oncol Biol Phys. 1988;15:837–841. doi: 10.1016/0360-3016(88)90115-0. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Kiehna EN, Thompson SJ, Heideman R, Sanford RA, Kun LE. Pediatric low-grade and ependymal spinal cord tumors. Pediatr Neurosurg. 2000;32:30–36. doi: 10.1159/000028894. [DOI] [PubMed] [Google Scholar]
- Mercuri S, Russo A, Palma L. Hemispheric supratentorial astrocytomas in children. Long-term results in 29 cases. J Neurosurg. 1981;55:170–173. doi: 10.3171/jns.1981.55.2.0170. [DOI] [PubMed] [Google Scholar]
- Pencalet P, Maixner W, Sainte-Rose C, Lellouch-Tubiana A, Cinalli G, Zerah M, Pierre-Kahn A, Hoppe-Hirsch E, Bourgeois M, Renier D. Benign cerebellar astrocytomas in children. J Neurosurg. 1999;90:265–273. doi: 10.3171/jns.1999.90.2.0265. [DOI] [PubMed] [Google Scholar]
- Pollack IF. The role of surgery in pediatric gliomas. J Neurooncol. 1999;42:271–288. doi: 10.1023/a:1006107227856. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: An analysis of 71 cases. J Neurosurg. 1995;82:536–547. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- Reddy AT, Packer RJ. Pediatric central nervous system tumors. Curr Opin Oncol. 1998;10:186–193. doi: 10.1097/00001622-199805000-00003. [DOI] [PubMed] [Google Scholar]
- Rilliet B, Vernet O. Gliomas in children: A review. Childs Nerv Syst. 2000;16:735–741. doi: 10.1007/s003810000334. [DOI] [PubMed] [Google Scholar]
- Sala F, Colarusso E, Mazza C, Talacchi A, Bricolo A. Brain tumors in children under 3 years of age. Recent experience (1987–1997) in 39 patients. Pediatr Neurosurg. 1999;31:16–26. doi: 10.1159/000028826. [DOI] [PubMed] [Google Scholar]
- Saran FH. Recent advances in paediatric neuro-oncology. Curr Opin Neurol. 2002;15:671–677. doi: 10.1097/01.wco.0000044762.39452.e3. [DOI] [PubMed] [Google Scholar]
- Saran FH, Baumert BG, Khoo VS, Adams EJ, Garre ML, Warrington AP, Brada M. Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys. 2002;53:43–51. doi: 10.1016/s0360-3016(02)02734-7. [DOI] [PubMed] [Google Scholar]
- Scanlon PW, Taylor WF. Radiotherapy of intracranial astrocytomas: Analysis of 417 cases treated from 1960 through 1969. Neurosurgery. 1979;5:301–308. [PubMed] [Google Scholar]
- Schmandt SM, Packer RJ. Treatment of low-grade pediatric gliomas. Curr Opin Oncol. 2000;12:194–198. doi: 10.1097/00001622-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Fineron PW, Hockley AD. Cerebellar astrocytoma of childhood: Long-term follow-up. Childs Nerv Syst. 1995;11:89–96. doi: 10.1007/BF00303812. [DOI] [PubMed] [Google Scholar]
- Shaw EG, Daumas-Duport C, Scheithauer BW, Gilbertson DT, O’Fallon JR, Earle JD, Laws ER, Jr, Okazaki H. Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg. 1989;70:853–861. doi: 10.3171/jns.1989.70.6.0853. [DOI] [PubMed] [Google Scholar]
- Shibamoto Y, Kitakabu Y, Takahashi M, Yamashita J, Oda Y, Kikuchi H, Abe M. Supratentorial low-grade astrocytoma. Correlation of computed tomography findings with effect of radiation therapy and prognostic variables. Cancer. 1993;72:190–195. doi: 10.1002/1097-0142(19930701)72:1<190::aid-cncr2820720134>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Strother, D.R., Pollack, I.F., Fisher, P.G., Hunter, J.V., Woo, S.Y., Pomeroy, S.L., and Rorke, L.B. (2002) Tumors of the central nervous system. In: Pizzo, P.A., and Poplack, D.G. (Eds.), Principles and Practice of Pediatric Oncology, 4th ed. Philadelphia: Lippincott Williams & Wilkins, pp. 751–825.
- Wara WM. Radiation therapy for brain tumors. Cancer. 1985;55 (suppl.):2291–2295. doi: 10.1002/1097-0142(19850501)55:9+<2291::aid-cncr2820551437>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Watson GA, Kadota RP, Wisoff JH. Multidisciplinary management of pediatric low-grade gliomas. Semin Radiat Oncol. 2001;11:152–162. doi: 10.1053/srao.2001.21421. [DOI] [PubMed] [Google Scholar]
- Weir B, Grace M. The relative significance of factors affecting postoperative survival in astrocytomas, grades one and two. Can J Neurol Sci. 1976;3:47–50. doi: 10.1017/s0317167100025993. [DOI] [PubMed] [Google Scholar]
- Whittle IR. Surgery for gliomas. Curr Opin Neurol. 2002;15:663–669. doi: 10.1097/01.wco.0000044761.39452.38. [DOI] [PubMed] [Google Scholar]
- Wisoff, J.H., McGuire-Cullen, P., and Sposto, R. (1996) Treatment of newly diagnosed low-grade astrocytomas: A phase III study (revised). Children’s Cancer Group/Pediatric Oncology Group Protocols. Available to COG members at http://www.childrensoncologygroup.org
- Yeh SA, Lee TC, Chen HJ, Lui CC, Sun LM, Wang CJ, Huang EY. Treatment outcomes and prognostic factors of patients with supratentorial low-grade oligodendroglioma. Int J Radiat Oncol Biol Phys. 2002;54:1405–1409. doi: 10.1016/s0360-3016(02)03053-5. [DOI] [PubMed] [Google Scholar]