Abstract
Gefitinib—a specific inhibitor of epidermal growth factor receptor (EGFR)-associated tyrosine kinase—has demonstrated efficacy in a subgroup of patients with non-small-cell lung carcinoma (NSCLC) who fail conventional chemotherapy. It is also reported to have an antitumor effect in brain metastases from NSCLC. Additionally, EGFR mutations have shown a strong association with gefitinib sensitivity for NSCLC. Here, we assessed the efficacy of gefitinib in brain metastases from NSCLC and evaluated the association of this efficacy with EGFR mutations. We retrospectively reviewed eight cases in which patients were suffering from brain metastases before the initiation of gefitinib treatment. Brain tumor response could be evaluated by MRI in these patients; EGFR gene analyses were also available. We evaluated whether objective tumor response was observed after gefitinib treatment and assessed the efficacy of gefitinib as effective, noneffective, or not assessable in consideration of the influence of previous radiotherapy. Of the eight patients, the efficacy of gefitinib was assessed as effective in three and as noneffective in three. All three patients demonstrating effective efficacy had EGFR mutations in the tyrosine kinase domain (deletion mutation in two patients and point mutation in one patients), whereas none of the three patients demonstrating noneffective efficacy had EGFR mutations. Gefitinib appears to be effective in treating brain metastases in a subgroup of patients. Our data suggested a possible association between the efficacy of gefitinib in the treatment of brain metastases and EGFR mutations.
Keywords: brain metastases, EGFR, gefitinib, mutation
Lung cancer is the most frequent site of origin for brain metastases (Merchut, 1989). Of the patients who are diagnosed with solitary brain metastases, 30% to 70% are confirmed to have lung cancer as the primary lesion (Schuette, 2004). Approximately 40% of all patients with lung cancer suffer from brain metastases in the course of their disease (Rizzi et al., 1990). Although patients with brain metastases are generally treated with corticosteroids and whole-brain radiation therapy (WBRT),2 the prognosis of patients with brain metastases is still disappointing. WBRT extends survival by only 14 to 21 weeks, even when it achieves palliative improvement in neurological symptoms (Borgelt et al., 1981; Shaw et al., 1996); refractory brain metastases cause death in 25% to 50% of these patients (Shaw et al., 1996). Although gamma knife surgery results in tumor reduction, stabilization, or disappearance in approximately 90% of the patients, the survival rate appears to be similar to that with WBRT (Datta et al., 2004). Systemic platinum-based chemotherapy has also been shown to contribute to comparable response rates for brain metastases from lung cancer and may be an option for management of brain metastases. However, the superiority of chemotherapy to radiotherapy remains unclear (Schuette, 2004).
Gefitinib is a tyrosine kinase inhibitor specific for epidermal growth factor receptor (EGFR), which can be administered orally and has already been approved in Japan and the United States for non-small-cell lung carcinoma (NSCLC) (Cohen et al., 2004). Two large phase 2 trials, IDEAL 1 and IDEAL 2 (Iressa Dose Evaluation in Advanced Lung Cancer), showed the efficacy of gefitinib in a subgroup of patients with NSCLC and a response rate of 18.4% and 11.8%, respectively (Fukuoka et al., 2003; Kris et al., 2003). Because only patients with brain metastases who were in stable condition were eligible for these clinical trials, the efficacy of gefitinib in brain metastases was not evaluated. However, subsequent to the initiation of gefitinib treatment worldwide, several researchers have reported that it is also capable of reducing brain metastases from NSCLC, sometimes with a highly dramatic improvement (Cappuzzo et al., 2003a, b; Ceresoli et al., 2004; Haringhuizen et al., 2004; Namba et al., 2004; Poon et al., 2004; Takahashi et al., 2004).
Although clinical trials have revealed that some factors, such as Japanese origin, female gender, adenocarcinoma histology, and nonsmoker status, provided a higher chance of responding to gefitinib (Fukuoka et al., 2003; Kris et al., 2003), no association was observed between EGFR expression and the efficacy of gefitinib in retrospective analyses (Bailey et al., 2003; Cappuzzo et al., 2003b). This result appeared to be contradictory to expectation because the application of gefitinib to the treatment of NSCLC was originally based on the observation that EGFR expression or overexpression is identified in 90% or more patients with lung cancer (Johnson, 2003). Recently, an interesting discovery of the relationship between the efficacy of gefitinib and EGFR gene mutations in the tyrosine kinase domain was reported (Lynch et al., 2004; Paez et al., 2004). EGFR gene mutations are presently expected to be promising predictors of the efficacy of gefitinib. However, these reports did not refer to brain metastases.
In this study, we retrospectively reviewed the cases in which patients received gefitinib therapy for NSCLC. From clinical records, we extracted the cases that coincided with brain metastases during gefitinib treatment, evaluated the efficacy of gefitinib, and examined the association between this efficacy of gefitinib in brain metastases and EGFR mutations.
Patients and Methods
Patients
From July 2002 to July 2004, 75 patients were treated with gefitinib for recurrent NSCLC at the Department of Thoracic Surgery, Aichi Cancer Center Hospital. All of these patients had undergone potentially curative pulmonary resection and had histologically proven NSCLC. Of these 75 patients, 11 were found to have brain metastases before the administration of gefitinib was initiated; in 59 patients, the exons that code for the tyrosine kinase domain of the EGFR gene had been sequenced by using RNAs extracted from primary lung tumors (Kosaka et al., 2004; Mitsudomi et al., 2005). As a result, in nine of 11 patients with brain metastases, information regarding the EGFR gene status was available. In one of these nine patients, the clinical course of brain metastases was not followed by an MRI because it was performed at another hospital. Therefore, a total of eight patients were included in this study.
WBRT or radiosurgery for brain metastases had already been performed once or twice immediately after brain lesions in all eight patients were identified.
Assessment of Efficacy
The efficacy of gefitinib was assessed by the change in tumor size observed by MRI in consideration of the influence of radiotherapy. The determination was made by experienced neurosurgeons without knowledge of the mutational status of the EGFR gene. First, MRIs were reviewed to evaluate whether an objective tumor response was observed after gefitinib treatment in each patient. Objective tumor response referred to either a complete response or a partial response as defined by the RECIST (Response Evaluation Criteria in Solid Tumors) scheme (Therasse et al., 2000), that is, when MRI revealed at least a 30% decrease in tumor diameter. If tumor diameter was not measurable, clinical neurological findings were taken into consideration for this assessment. Then, the efficacy of gefitinib was assessed in light of the influence of radiotherapy before gefitinib treatment. Within the objective response–positive group, the efficacy was judged as effective only when the tumor had exhibited progression or new lesions had appeared even after previous radiotherapy. Efficacy was not assessable if the objective tumor response was continuously observed after radiotherapy and it was thus unclear whether the tumor reduction was attributed purely to gefitinib treatment or whether previous radiotherapy was a major factor leading to tumor reduction. The efficacy was judged as noneffective for the objective response–negative group. Although the imaging studies were not taken at a fixed interval, all patients underwent MRI at least once within three months after starting gefitinib.
EGFR Gene Analysis
The results of EGFR gene analysis that had already been performed for primary resected lung cancer samples were used for this study (Kosaka et al., 2004). The method used for gene analysis is described in our previous report (Mitsudomi et al., 2005). We evaluated the association between the efficacy of gefitinib and EGFR mutations.
Results
Patient Characteristics
Table 1 shows patient characteristics. The median age of the patients was 51.5 years, and six male and two female patients participated in this study. A majority of the patients (six of eight) had adenocarcinoma; two among them had large-cell carcinoma. All patients received limited regimens of chemotherapy before receiving gefitinib treatment. Disease-free survival ranged from 0 to 16 months. All patients except one manifested initial recurrent lesions as brain metastasis. In patient 1, lymph node metastasis in lungs was found initially, and brain metastasis a full 24 months after lung operation.
Table 1.
Patient characteristics, modes of radiotherapy, and tumor response
| Patient | Age | Gender | Histology | Prior CTX | Initial Brain Lesions | Disease-Free Survival (months) | Radiotherapy Before Gefitinib Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 46 | Male | La | Yes | Solitary | 16 | SMART |
| 2 | 40 | Male | Ad | Yes | Multiple | 11 | Gamma knife → WBRT for multiple new lesions → progression |
| 3 | 51 | Male | Ad | Yes | Solitary | 12 | SMART → appearance of new lesions |
| 4 | 61 | Male | Ad | Yes | Multiple | 4 | WBRT → progression |
| 5 | 48 | Male | La | Yes | Multiple | 0 | WBRT + SMART → X knife for multiple new lesions → progression |
| 6 | 69 | Female | Ad | Yes | Multiple | 4 | WBRT → progression |
| 7 | 61 | Male | Ad | Yes | Solitary | 11 | Gamma knife → objective tumor response |
| 8 | 52 | Female | Ad | Yes | Solitary | 8 | SMART → objective tumor response |
Abbreviations: Ad, adenocarcinoma; CTX, chemotherapy; La, large-cell carcinoma; SMART, stereotactic multiple arc radiation therapy; WBRT, whole-brain radiation therapy.
Clinical Course in Brain Metastases Before the Initiation of Gefitinib Treatment
Table 1 also shows initial manifestations of brain metastases and clinical courses in brain lesions from radiotherapy until initiation of gefitinib treatment. All patients underwent radiotherapy immediately after initial identification of brain metastases. Patients 7 and 8 showed objective tumor response to radiotherapy before the initiation of gefitinib treatment. Patients 4 (Fig. 1) and 6 showed tumor progression even after radiotherapy. In three patients (patients 2, 3, and 5), although the lesions for which radiosurgery was performed exhibited improvement, multiple new lesions appeared before the initiation of gefitinib treatment. Of these, patient 2 (Fig. 2) and patient 5 underwent additional radiotherapy, and both showed disease progression before the initiation of gefitinib treatment. In patient 1, gefitinib treatment was started subsequent to the completion of radiotherapy.
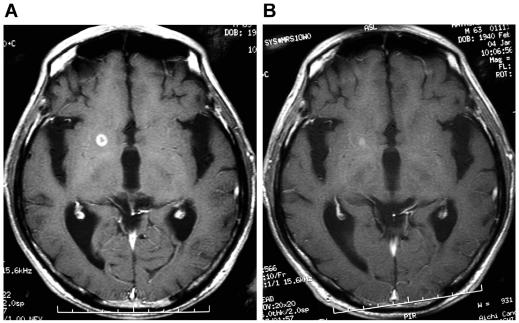
Fig. 1.
Axial gadolinium-enhanced MRI in patient 4. (A) The image taken just before the initiation of gefitinib treatment shows a small, well-enhanced, regrowing mass in the right basal ganglia, which had once exhibited partial remission after whole-brain radiation. (B) The enhanced mass is shown to have nearly disappeared four months after the initiation of gefitinib therapy.
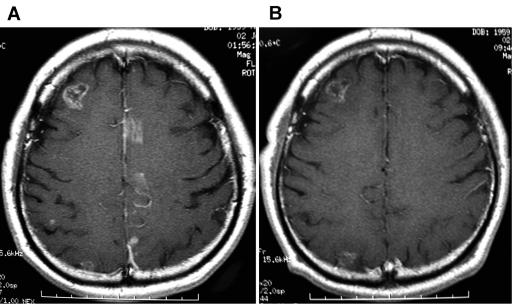
Fig. 2.
Axial gadolinium-enhanced MRI in patient 2. (A) The image taken one month before the initiation of gefitinib therapy shows multiple enhanced areas on both sides of the cerebrum after whole-brain radiation. (B) The enhanced masses on the left side disappeared coincidently with improvement of the right hemiparesis, and the mass on the right side was blurred six months after the initiation of gefitinib therapy. In addition, an enhanced mass in the right frontal lobe became blurred, and a spotty enhanced area in the right parietal lobe disappeared.
Administration of Gefitinib
All patients received a daily dose of 250 mg gefitinib until any progressive lesions were identified or an elevation in serum carcinoembryonic antigen level was observed. Side effects were all within grade 1 or 2 according to the NCI Common Toxicity Criteria and consisted mainly of diarrhea and skin toxicity. Grade 1 diarrhea occurred in four patients (patients 3, 4, 5, and 7), and grade 1 skin rash was observed in four patients (patients 3, 5, 6, and 8). In patient 6, the dose was changed from 250 mg per day to every other day one month after the initiation of gefitinib treatment because of grade 2 nausea. The interval between the completion of the last radiotherapy and initiation of gefitinib treatment and the duration of administration of gefitinib is shown in Table 2.
Table 2.
Treatment with gefitinib, evaluation of the efficacy of gefitinib, and EGFR gene statusa
| Patient | Intervalb(months) | Objective Tumor Response/Months | Efficacy of Gefitinib | Duration of Gefitinib Treatment (months) | Extracranial Lesions/Treatment | Efficacy for Extracranial Lesions | Steroid Therapy/Weeks | Survivalc(months) | EGFRGene Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | No | Noneffective | 9 | Lung LN/RTX, CTX | Noneffective | Yes/10 | >13* | Wild type |
| 2 | 2 | Yes/16 | Effective | 11 | Pleural dissem./CTX | Not assessable | No | 19 | del E746–A750 |
| 3 | 12 | Yes/18 | Effective | 18 | Bone/RTX | Not assessable | No | >18** | del E746–A750 |
| 4 | 12 | Yes/12 | Effective | 12 | Bone/RTX | Not assessable | No | >14* | pm L858R |
| 5 | 3 | No | Noneffective | 2 | — | — | Yes/10 | 2 | Wild type |
| 6 | 7 | No | Noneffective | 2 | Bone/RTX | Noneffective | No | 8 | Wild type |
| 7 | 5 | Yes/10 | Not assessable | 10 | Lung/CTX | Not assessable | Yes/16 | >9* | pm G719C |
| 8 | 4 | Yes/4 | Not assessable | 4 | Liver, bone/CTX | Effective | Yes/8 | 4 | pm L858R |
Abbreviations: CTX, chemotherapy; del, deletion; LN, lymph node; pm, point mutation; RTX, radiotherapy.
Boldface indicates a possible relationship between efficacy of gefitinib and EGFR gene status.
Interval indicates the interval between the completion of last radiotherapy and the initiation of gefitinib.
Survival was calculated from the day of initiating gefitinib treatment. A number with an asterisk means that the patient was still alive at the time of this study, and the number with double asterisks means that the case was not followed up in the course of this study.
Tumor Response and the Assessment of the Efficacy of Gefitinib
Table 2 presents observations of objective tumor response and the assessment of the efficacy of gefitinib. In five of eight patients, an MRI revealed objective tumor response in brain metastases within three months after gefitinib treatment. In three of these five patients (patients 2, 3, and 4), the efficacy was assessed as effective because gefitinib achieved objective tumor response despite the uncontrollable state of the tumor (patients 2 and 4) or the appearance of new lesions (patient 3) after radiotherapy (Figs. 1 and 2). In patient 2, the judgment was partially based on the improvement of his right hemiparesis that coincided with the disappearance of enhanced area in the left frontal lobe. In the remaining two patients with objective tumor response (patients 7 and 8), the efficacy was determined to be not assessable, because objective tumor response was continuously observed from radiotherapy through gefitinib treatment. The duration of objective response ranged from 4 to 18 months. Patient 2 achieved objective tumor response for a longer period than the duration of gefitinib administration, this being a case in which the gefitinib was discontinued because of an elevation in serum carcinoembryonic antigen levels; the patient showed tumor progression in brain metastasis five months after discontinuing the treatment. Follow-up for patient 3 in a progression-free state ceased 14 months after the initiation of gefitinib treatment because he discontinued his visits to the outpatient clinic. Patient 4 showed tumor progression 12 months after gefitinib treatment. Patient 7 was continued on gefitinib treatment and remained in a progression-free state at the time of this study. Patient 8 died of liver dysfunction attributable to liver metastasis four months after the initiation of gefitinib treatment, despite once achieving objective tumor response both in brain and in liver.
In the three patients who never achieved objective tumor response after gefitinib treatment (patients 1, 5, and 6), the efficacy was assessed as noneffective. Tumor progression was apparently observed within three months in patients 5 and 6. In patient 1, tumor progression was noticed five months after starting gefitinib, although during the previous five months, MRI had showed no apparent change in tumor size. The progressive brain tumor was eventually operated on in another hospital, when gefitinib was discontinued.
EGFR Mutations and Their Association with the Efficacy of Gefitinib
EGFR mutations were seen in five patients (Table 2). Patients 2 and 3 had a deletion mutation from codon 746 to 750 in exon 19 (del E746–A750). Patients 4 and 8 had a point mutation, which was a T to G transversion at the second nucleotide of codon 858 in exon 21 (L858R). Patient 7 had a point mutation at codon 719 in exon 18 (G719C). The other three patients had a wild-type EGFR gene. As for an association with the efficacy of gefitinib, all three patients assessed as effective had EGFR mutations. On the contrary, all three patients assessed as noneffective had wild-type EGFR (P = 0.10 by a two-sided Fisher’s exact test). Objective tumor response was achieved by all patients with EGFR mutations but by none of the patients with wild-type EGFR (P = 0.036).
Extracranial Lesions
Extracranial lesions were observed in all patients excluding patient 5. All extracranial lesions were confirmed before gefitinib treatment, and some treatments of either conventional chemotherapy or radiotherapy were performed before gefitinib was started (Table 2). The efficacy of gefitinib for the treatment of extracranial lesions was assessed according to the same definition as for brain metastases, and the results are presented in Table 2. In patient 8, liver metastasis that had never responded to conventional chemotherapy showed remarkable response to gefitinib for two months. However, the liver metastasis recurred immediately afterward, which led to death.
Discussion
It was reported that a prospective trial of gefitinib in 41 patients with brain metastases from NSCLC showed a partial response rate of 10% and an overall disease control rate of 27% (previously irradiated patients, 56%; radionaive patients, 9%) when gefitinib was used for the treatment of brain metastases (Ceresoli et al., 2004). Two retrospective reviews reported objective responses to gefitinib in six of 14 patients (Hotta et al., 2004) and in nine of 15 patients (Namba et al., 2004), all with brain metastases. The patients in these two retrospective studies were all Japanese by origin. Our data, which were also derived from patients of Japanese origin, exhibited a similar response rate. As for the predictive factors for gefitinib sensitivity, previous WBRT and adenocarcinoma histology were confirmed to be of significant prognostic value (Ceresoli et al., 2004). However, cases have also been reported in which dramatic tumor reduction was achieved without prior radiation therapy (Ishida et al., 2004; Poon et al., 2004). Our study did not include radionaive patients and thus could not contribute to the evaluation of how radiation influences the efficacy of gefitinib. Other clinical predictive factors could also not be evaluated because of small sample sizes, and further study is required. The sensitivity of brain metastases to gefitinib has also been reported to be strongly correlated to that of extracranial disease (Hotta et al., 2004). In our study, evaluation of the relationship between these factors was difficult because half of the patients were not assessable for extracranial lesions. However, confined to objective tumor response, the evaluation in brain metastasis and that in extracranial lesions were identical in all patients (Table 2).
The effectiveness of chemotherapy for brain lesions may be influenced by some particular factors in the brain. First, there is poor drug penetration to brain tumors because of the existence of the blood-brain barrier (BBB). Although gefitinib has low molecular weight and excellent cell penetration, it is possible that it may not have free access to the brain, as another small, low-molecular-weight tyrosine kinase inhibitor, imatinib, is shown to have limited brain penetration (Neville et al., 2004). On the other hand, it is possible that the BBB is disrupted and that new blood vessels, which lack normal BBB properties, are developed at the stage when the tumor is recognized on MRI or CT with an intravenous contrast (van den Bent, 2003). Excluding one, all patients in our study received 250 mg gefitinib per day, which is considered to be a sufficient dose for patients with lung cancer (Fukuoka et al., 2003; Kris et al., 2003), and the dosage with which brain metastases were commonly observed to respond to gefitinib in previous studies was 250 mg per day (Cappuzzo et al., 2003a, b; Ceresoli et al., 2004; Poon et al., 2004). The same dosage appears to be appropriate for brain lesions, also. A second factor that may influence chemotherapy is steroid therapy. Corticosteroids are frequently used to reduce brain edema in patients with metastatic brain lesions. It is generally accepted that the beneficial effects of corticosteroids are primarily related to reduction in the permeability of a disrupted BBB, which may occur through various mechanisms (Anderson et al., 1994). Use of corticosteroids may also produce metabolic interaction with gefitinib, a CYP3A4-metabolized agent (Swaisland et al., 2002). We indicate the use of corticosteroid in each case in Table 2. However, the influence of corticosteroid therapy on the efficacy of gefitinib could not be evaluated because of the small sample size of this study.
Recently, mutations in the EGFR gene have been reported to be associated with clinical responsiveness to gefitinib for NSCLC. All the missense and deletion mutations detected in these studies exist within the tyrosine kinase domain of EGFR, which is targeted by gefitinib; moreover, these mutations are limited to the first four of the seven exons that code for the tyrosine kinase domain (Gazdar et al., 2004; Lynch et al., 2004; Mitsudomi et al., 2005; Paez et al., 2004). These mutations are considered to result in the narrowing of the ATP-binding cleft and the increase in both gene activation and tyrosine kinase inhibitor sensitivity by a similar configuration change (Gazdar et al., 2004). At the same time, among the patients with NSCLC, the characteristics of patients with predominant EGFR mutations associate strikingly with those of gefitinib responders: Mutations are more frequently observed in patients with adenocarcinoma, in women, and in Japanese people (Paez et al., 2004). Additionally, these mutations are in particular associated independently with the adenocarcinoma histology and nonsmokers (Kosaka et al., 2004; Pao et al., 2004). All these results suggest that EGFR mutations may predict the responsiveness of NSCLC to gefitinib. However, an association with brain metastases is not referenced in these studies, and we found no other clinical studies that investigated an association between EGFR mutations and the efficacy of gefitinib in brain metastases. Our data in this limited study did not provide a statistically significant result but did exhibit the possibility that a similar relationship between EGFR mutations and the efficacy of gefitinib exists in brain metastases from NSCLC.
At the same time, we should consider the possibility that the actual status of EGFR genes in brain metastases could be different from the status of the sample analyzed. The samples used for EGFR gene analysis in this study were derived from lung tumors, not from brain tumors. All patients underwent radiotherapy before the initiation of gefitinib treatment, and there is a possibility that radiotherapy may change the characteristics of molecules or the expression of genes like tumor necrosis factor alpha in tumors, which tends to contribute to radiosensitivity (Chiang et al., 1997). On the other hand, radiotherapy may induce the release of transforming growth factor alpha (one of the ligands to EGFR), which leads to a radiation-induced tumor (Schmidt-Ullrich et al., 1997). There are also rather interesting studies that show the relationship between the EGFR pathway and radiation sensitivity. EGFR itself enhances cancer cell resistance to radiation (Liang et al., 2003). Furthermore, radioresistance induced by EGFR is indicated to result from the activation of antiapoptotic pathways such as the phosphatidylinositol-3 kinase/AKT pathway (Li et al., 2004), although it was investigated in another EGFR mutation—the type III EGFR variant—which is rarely observed in lung cancer. On the other hand, EGFR mutations found in gefitinib-sensitive patients were also reported to selectively promote the antiapoptotic pathway (Sordella et al., 2004). These facts suggest that radioresistance of the tumors may result from further dependency on the EGFR signaling pathway, particularly on antiapoptotic pathways, and subsequently may become more susceptible to gefitinib. In our opinion, although our study could provide only suggestive data, further prospective clinical trials are warranted to confirm whether EGFR mutation is a predictor of sensitivity of gefitinib for brain metastases. In addition, considering that radiotherapy currently prevails as a standard therapy for brain metastases, we propose the possibility that radiation therapy combined with gefitinib can enhance antitumor activity in brain metastases with EGFR mutations. Actually, preclinical studies have already shown that a cooperative, antiproliferative, and proapoptotic effect was obtained when cancer cells were treated with ionizing radiation followed by gefitinib administration (Bianco et al., 2002). Evaluation of radiotherapy as a sensitizer for gefitinib is also needed in future clinical trials.
In this study, we focused on objective response to brain metastases, but survival benefit could not be evaluated from our data. The striking responders to gefitinib commonly show progressive disease afterward, and the survival benefit of gefitinib is still controversial. However, we recently reported that EGFR mutations were not only a good predictor of tumor response but also factors that prolonged the survival period for the patients with recurrent NSCLC treated with gefitinib (Mitsudomi et al., 2005). Further prospective trials should also determine the association between EGFR gene status and survival benefit to brain metastases from NSCLC.
Footnotes
Abbreviations used are as follows: BBB, blood-brain barrier; EGFR, epidermal growth factor receptor; IDEAL, Iressa Dose Evaluation in Advanced Lung Cancer; NSCLC, non-small-cell lung cancer; WBRT, whole-brain radiation therapy.
References
- Anderson C, Astrup J, Gyldensted C. Quantitation of peritumoural oedema and the effect of steroids using NMR-relaxation time imaging and blood-brain barrier analysis. Acta Neurochir Suppl (Wien) 1994;60:413–415. doi: 10.1007/978-3-7091-9334-1_112. [DOI] [PubMed] [Google Scholar]
- Bailey RL, Kris M, Wolf M, Kay A, Averbuch S, Askaa J, Janas M, Schmidt K. Tumor EGFR membrane staining is not clinically relevant for predicting response in patients receiving gefitinib (‘Iressa’, ZD1839) monotherapy for pretreated advanced non-small-cell lung cancer: IDEAL 1 and 2. Proc Am Assoc Cancer Res. 2003;44:1362. (abstract LB-170) [Google Scholar]
- Bianco C, Tortora G, Bianco R, Caputo R, Veneziani BM, Caputo R, Damiano V, Troiani T, Fontanini G, Raben D, Pepe S, Bianco AR, Ciardiello F. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa) Clin Cancer Res. 2002;8:3250–3258. [PubMed] [Google Scholar]
- Borgelt B, Gelber R, Larson M, Hendrickson F, Griffin T, Roth R. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: Final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7:1633–1638. doi: 10.1016/0360-3016(81)90184-x. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Ardizzoni A, Soto-Parra H, Gridelli C, Maione P, Tiseo M, Calandri C, Bartolini S, Santoro A, Crino L. Epidermal growth factor receptor targeted therapy by ZD 1839 (Iressa) in patients with brain metastases from non-small cell lung cancer (NSCLC) Lung Cancer. 2003a;41:227–231. doi: 10.1016/s0169-5002(03)00189-2. [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Gregorc V, Rossi E, Cancellieri A, Magrini E, Paties CT, Ceresoli G, Lombardo L, Bartolini S, Calandri C, de Rosa M, Villa E, Crino L. Gefitinib in pretreated non-small-cell lung cancer (NSCLC): Analysis of efficacy and correlation with HER2 and epidermal growth factor receptor expression in locally advanced or metastatic NSCLC. J Clin Oncol. 2003b;21:2658–2663. doi: 10.1200/JCO.2003.01.039. [DOI] [PubMed] [Google Scholar]
- Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: A prospective trial. Ann Oncol. 2004;15:1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD, Jr, Morse D, Abraham S, Rahman A, Liang C, Lostritto R, Baird A, Pazdur R. United States Food and Drug Administration Drug Approval summary: Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10:1212–1218. doi: 10.1158/1078-0432.ccr-03-0564. [DOI] [PubMed] [Google Scholar]
- Datta R, Jawahar A, Ampil FL, Shi R, Nanda A, D’Agostino H. Survival in relation to radiotherapeutic modality for brain metastasis: Whole brain irradiation vs. gamma knife radiosurgery. Am J Clin Oncol. 2004;27:420–424. doi: 10.1097/01.coc.0000128863.75360.a5. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial) (erratum in J. Clin. Oncol. [2004] 22, 4811) J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Shigematsu H, Herz J, Minna JD. Mutations and addiction to EGFR: The Achilles ‘heal’ of lung cancers? Trends Mol Med. 2004;10:481–486. doi: 10.1016/j.molmed.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Haringhuizen A, van Tinteren H, Vaessen HF, Baas P, van Zandwijk N. Gefitinib as a last treatment option for non-small-cell lung cancer: Durable disease control in a subset of patients. Ann Oncol. 2004;15:786–792. doi: 10.1093/annonc/mdh177. [DOI] [PubMed] [Google Scholar]
- Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, Okada T, Hisamoto A, Tanimoto M. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–261. doi: 10.1016/j.lungcan.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kanoh K, Nishisaka T, Miyazu Y, Iwamoto Y, Kohno N, Miyazawa T. Gefitinib as a first line of therapy in non-small cell lung cancer with brain metastases. Intern Med. 2004;43:718–720. doi: 10.2169/internalmedicine.43.718. [DOI] [PubMed] [Google Scholar]
- Johnson DH. Targeted therapy in non-small cell lung cancer. Lung Cancer. 2003;41 (suppl. 1):23–28. doi: 10.1016/s0169-5002(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys. 2003;57:246–254. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Merchut MP. Brain metastases from undiagnosed systemic neoplasms. Arch Intern Med. 1989;149:1076–1080. [PubMed] [Google Scholar]
- Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- Namba Y, Kijima T, Yokota S, Niinaka M, Kawamura S, Iwasaki T, Takeda Y, Kimura H, Okada T, Yamaguchi T, Nakagawa M, Okumura Y, Maeda H, Ito M. Gefitinib in patients with brain metastases from non-small-cell lung cancer: Review of 15 clinical cases. Clin Lung Cancer. 2004;6:123–128. doi: 10.3816/CLC.2004.n.026. [DOI] [PubMed] [Google Scholar]
- Neville K, Parise RA, Thompson P, Aleksic A, Egorin MJ, Balis FM, McGuffey L, McCully C, Berg SL, Blaney SM. Plasma and cerebrospinal fluid pharmacokinetics of imatinib after administration to nonhuman primates. Clin Cancer Res. 2004;10:2525–2529. doi: 10.1158/1078-0432.ccr-03-0155. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon AN, Ho SS, Yeo W, Mok TS. Brain metastasis responding to gefitinib alone. Oncology. 2004;67:174–178. doi: 10.1159/000081005. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Tondini M, Rocco G, Rossi G, Robustellini M, Radaelli F, Della Pona C. Lung cancer with a single brain metastasis: Therapeutic options. Tumori. 1990;76:579–581. doi: 10.1177/030089169007600614. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- Schuette W. Treatment of brain metastases from lung cancer: Chemotherapy. Lung Cancer. 2004;45 (suppl. 2):S253–S257. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R, Wharam M, Schultz C, Davey P, Loeffler J, Del Rowe J, Marks L, Fisher B, Shin K. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: Initial report of Radiation Therapy Oncology Group protocol (90–05) Int J Radiat Oncol Biol Phys. 1996;34:647–654. doi: 10.1016/0360-3016(95)02106-x. [DOI] [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- Swaisland H, Smith RP, Farebrother J. The effect of the induction and inhibition of CYP3A4 on the pharmacokinetics of single oral doses of ZD1839 (‘Iressa’), a selective epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), in healthy male volunteers. Proc Am Soc Clin Oncol. 2002;21:83. (abstract 328) [Google Scholar]
- Takahashi H, Ohrui T, Ebihara S, Yamada M, Sasaki H. Effect of gefitinib (ZD1839) on metastatic brain tumour. Lung Cancer. 2004;43:371–372. doi: 10.1016/j.lungcan.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- vanden Bent MJ. The role of chemotherapy in brain metastases. Eur J Cancer. 2003;39:2114–2120. doi: 10.1016/s0959-8049(03)00577-x. [DOI] [PubMed] [Google Scholar]