Abstract
The purpose of this study was to estimate ventricular cerebrospinal fluid (vCSF) topotecan lactone (TPT) exposures in pediatric medulloblastoma patients from plasma concentration–time data by using a pharmaco‐kinetic (PK) model. We studied children with high‐risk medulloblastoma who received pharmacokinetically guided TPT (target plasma area under the concentration–time curve [AUC], 120–160 ng/ml‐h) and obtained serial vCSF samples to assess TPT exposure. Population pharmacokinetic parameters were determined by using linear mixed‐effects modeling via the two‐stage approach. We simulated TPT vCSF exposure duration at plasma TPT AUC values of 120 to 200 ng/ml‐h and determined percentages of studies meeting or exceeding the vCSF exposure duration threshold (EDT) of 1 ng/ ml for 8 h. We then used bootstrap methods to estimate variability in vCSF EDT. Eighteen PK studies were conducted in six patients (median age, 4.8 years). In these patients, seven of nine studies attaining target plasma TPT AUC achieved the vCSF EDT. Given a plasma TPT AUC of 120 ng/ml‐h, the median percentage of results meeting or exceeding EDT was 78% (95% CI, 61%–100%). One patient (four studies) with tumor blockage of CSF flow, which can alter CSF pharmacokinetics, was removed, and the bootstrap analysis was repeated. In this subset, a median 93% (95% CI, 79%–100%) of studies achieved vCSF EDT. Increasing plasma TPT AUC values resulted in increased ability to achieve vCSF EDT. We demonstrated that a plasma PK model could estimate vCSF TPT concentrations. Further, our results indicate that the TPT vCSF EDT can be achieved in more than 80% of studies targeted to a plasma TPT AUC of 120 ng/ml‐h.
Keywords: children, cerebrospinal fluid, pharmacokinetics, topotecan
Medulloblastoma, which accounts for 20% of all CNS tumors in pediatric patients, is treated with a standard combination of maximal surgical tumor resection and craniospinal irradiation (CSI)3 with or without chemotherapy (Ellison et al., 2003). Since medulloblastoma readily disseminates throughout the neuraxis, optimal efficacy with chemotherapy requires therapeutic drug concentrations in the cerebrospinal fluid (CSF). Topotecan, a DNA topoisomerase I inhibitor with activity against medulloblastoma, has been shown to readily penetrate into the CSF of primates and children with CNS malignancies (Baker et al., 1996; Blaney et al., 1993). However, the optimal topotecan CSF exposure required for maximum in vivo effect is currently unknown.
In an in vitro model, topotecan was cytotoxic to medulloblastoma cell lines at a concentration of 1 ng/ml for 8 h (Pawlik et al., 1998), which suggests that a similar CSF exposure would be the minimum required for effective medulloblastoma therapy in patients. To assess whether patients are achieving this threshold CSF topotecan concentration, CSF sampling is necessary by Ommaya reservoir or lumbar tap. However, CSF sampling is associated with complications, such as infection, hemorrhage, or reservoir malfunction (Lishner et al., 1990). Many medulloblastoma patients also lack the CSF access required for such sampling, and creating CSF access specifically for pharmacokinetic sampling can not be clinically justified. In these situations, it would be beneficial to use plasma pharmacokinetic studies to ensure that patients achieve the 1-ng/ml threshold for at least 8 h in the CSF (i.e., exposure duration threshold [EDT]). Therefore, our goal was to determine whether CSF topotecan exposures could be accurately estimated from readily accessible plasma topotecan concentration–time data by using a pharmacokinetic model. Implementing such a model to predict CSF topotecan exposures in pediatric medulloblastoma patients would avoid the need for invasive CSF sampling to ensure that patients achieve an adequate topotecan CSF concentration.
Materials and Methods
Patient Characteristics
Patients with high-risk medulloblastoma, defined as presence of metastatic disease within the neuraxis upon imaging or CSF analysis and/or presence of more than 1.5 cm2 residual disease at the primary site after surgery, were selected to receive an up-front window of pharmacokinetically guided topotecan as part of a multi-institution phase 2 study (Stewart et al., 2004). From this population, patients with preexisting Ommaya reservoirs were identified and served as the cohort for our current pharmacokinetic analyses.
Patients eligible for inclusion into the cohort were between ages 3 and 21 at the time of diagnosis and had no prior chemotherapy or CSI therapy. Additional requirements included normal renal function (serum creatinine ⩽ 1.2 mg/dl or technetium clearance ⩾ 70 ml/ min/m2), normal liver function (serum glutamate pyruvic transaminase < 1.5 times normal and bilirubin < 1.5 mg/dl), normal bone marrow function (hemoglobin > 10 g/dl, WBC count ⩾ 3000/μl, absolute neutrophil count > 1500/μl, and platelets > 100,000/μl), and an Eastern Cooperative Oncology Group performance score of 0 to 3, except in cases of posterior fossa syndrome. Additionally, the St. Jude Children’s Research Hospital Institutional Review Board approved the phase 2 study protocol, and informed consent was obtained from patients, parents, or legal guardians as appropriate.
Drug Formulation and Administration
For intravenous administration, topotecan (Hycamtin; GlaxoSmithKline, Philadelphia, Pa.) was reconstituted with 2 ml of sterile water (U.S. Pharmacopeia, Rockville, Md.), and further dilutions were made in 50 ml of 5% dextrose in water. Each topotecan dose was placed in a syringe-set and attached to an IVAC controller (Alaris Medical Systems, Inc., San Diego, Calif.) set for a volume limit of 50 ml. Topotecan was administered over 30 min through either a central or peripheral venous line daily for five consecutive days. A second course of topotecan was started at day 21, provided the patients had recovered their blood cell counts (e.g., absolute neutrophil count > 1500, platelet count > 75,000, and hemoglobin > 8 g/dl).
Pharmacokinetically Guided Dosing
Since previous data has shown a 7- to 10-fold range in topotecan clearance values in children with cancer (Stewart et al., 2000; Tubergen et al., 1996), we used a pharmacokinetically guided dosing approach to individualize therapy in patients. Patients initially received topotecan 2 mg/m2 per day as a 30-min infusion on day 1 of therapy. After the initial infusion, a pharmacokinetic analysis was performed to ascertain whether patients achieved the target topotecan lactone area under the plasma concentration–time curve (AUC) of 120 to 160 ng/ml-h. Previously conducted pharmacokinetic simulations (Stewart et al., 2004) suggested that such a target AUC had a high likelihood of achieving a CSF concentration of 1 ng/ml for 8 h, that is, the EDT previously found cytotoxic in two medulloblastoma cell lines in vitro (Pawlik et al., 1998).
The topotecan dosage was adjusted on day 2 of topotecan therapy to attain the desired topotecan plasma AUC in patients not reaching target on the initial dosage. Since we have shown topotecan disposition is linear, the following equation was used for dosage adjustments: Adjusted dosage (mg/m2) = [current topotecan dosage (mg/m2)/current AUC] × target AUC. Pharmacokinetic analysis was repeated on day 3 to determine whether the putative target AUC was achieved, and further dosage adjustments were made if necessary.
Pharmacokinetic Sampling Strategy
Plasma samples were collected prior to topotecan infusion and at 0.25, 0.5, 1, 3, and 6 h after the end of the infusion. At each time point, 3 ml of whole blood was collected from an intravenous site contralateral to the topotecan infusion, placed in a heparinized tube, and plasma processed, and the subsequent topotecan lactone (TPT) concentrations were measured by a previously published method (Pratt et al., 1994; Santana et al., 2003; Zamboni et al., 1998a).
Ventricular CSF (vCSF) samples were acquired at 1, 3, and 6 h after the end of topotecan infusion. For each time point, 300 μl of vCSF was collected from the Ommaya reservoir, centrifuged in a microfuge for 2 min at 7000 × g, and then analyzed as described above for plasma. Calibration curves were constructed for the vCSF samples by using pooled human CSF, and the lower limit of quantitation for TPT in vCSF samples was 0.25 ng/ml.
Pharmacokinetic Analysis
Initially, TPT plasma concentrations were fit to a two-compartment model using a maximum a posteriori (MAP) Bayesian algorithm as implemented in ADAPT II (D’Argenio and Schumitzky, 1990). Model parameters that were estimated included the volume of the central compartment (VC ), elimination rate constant (ke), and the intercompartment rate constants (k12 and k21). The prior parameter distributions (mean and variance) for the MAP estimator were determined by using maximum likelihood estimation in a similar group of pediatric cancer patients (n = 67) (Pratt et al., 1994; Tubergen et al., 1996). The prior parameter estimates (mean and coefficient of variation) used for this study were VC, 23.3 liters/m2 (63%); ke, 1.16 h−1 (46%); k12, 1.02 h−1 (68%); and k21, 0.49 h−1 (44%). Standard equations were used to calculate systemic clearance from parameter estimates (Gibaldi and Perrier, 1982). The model parameters for each patient/study were used to simulate the plasma concentration–time profile, from which the area under the plasma concentration–time curve from time zero to infinity (AUC0→∞) was determined by use of the log- linear trapezoidal method.
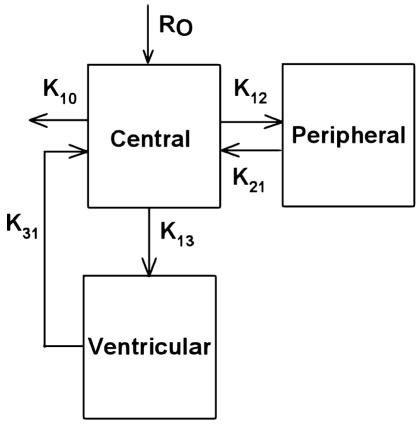
To represent the vCSF disposition of topotecan in the cohort, a third compartment was added to the above model (Fig. 1). This three-compartment model describing topotecan distribution into and from vCSF was derived from a previous model proposed by Baker et al. (1996), which included the intercompartment rate constants (k13 and k31). For each pharmacokinetic study, the plasma topotecan concentration–time profile was modeled by using the aforementioned MAP estimator. Then, with the plasma pharmacokinetic parameters remaining fixed, the parameters describing the topotecan vCSF concentrations were estimated for each study by using the maximum likelihood approach. However, the volume of the vCSF compartment was fixed at 0.132 liter, a value consistent with physiological estimates of CSF volume, because the sparse data did not permit estimation of this volume. Once plasma and vCSF parameters were estimated for each study, the population pharmacokinetic parameters and measures of interindividual and intraindividual variability for both the plasma and vCSF pharmacokinetic parameters were determined via linear, mixed-effects modeling (Steimer et al., 1993).
Fig. 1.
Three-compartment model used for ventricular CSF modeling, in which K is an intercompartment rate constant and Ro is the infusion rate constant.
Estimation of Exposure Duration
We evaluated the topotecan vCSF exposure duration, described as time above the 1-ng/ml threshold, using the derived three-compartment-model-estimated vCSF pharmacokinetic curve in each study. We then used the individual plasma and vCSF pharmacokinetic parameter estimates from each study to simulate the topotecan vCSF concentration–time profile given the dosages used to target plasma AUC values ranging from 120 to 200 ng/ml-h based on the population average plasma pharmacokinetic parameters. Then, for each targeted AUC, the exposure duration and the number of studies that either met or exceeded the vCSF EDT (i.e., ⩾1 ng/ml for at least 8 h) were tabulated. Finally, to obtain an estimate of the variability in the number of studies meeting or exceeding the EDT in our simulations, we applied bootstrap methods (n = 1000) as implemented in Mat-lab V14 (The Mathworks, Natick, Mass.). In particular, for each bootstrap, we randomly chose, with replacement, 18 pharmacokinetic studies. We then added residual variability, based on the observed average residual error of the model parameters (coefficient of variation of 10% for ke, VC, k12, and k21; 15% for k13 and k31), to the pharmacokinetic parameters in each study of each bootstrap. Next, we determined the topotecan vCSF exposure duration for each study in each bootstrap sample. Finally, for each bootstrap, we determined the percentage of studies that met or exceeded the vCSF EDT of 1 ng/ml for at least 8 h. Using the results from the bootstraps, we determined the median, minimum, maximum, quartiles, and 2.5th and 97.5th percentiles (i.e., 95% of the results occur in this interval) of the EDT for each simulated topotecan dosage and AUC.
Results
Six patients from the phase 2 study met the eligibility criteria for inclusion into this analysis, and their demographics are listed in Table 1. The median age was 4.9 years, and only one was female. Five of six were receiving drugs that may increase topotecan systemic clearance secondary to cytochrome P-450 induction (Gajjar et al., 2001; Zamboni et al., 1998b).
Table 1.
Cohort patient demographics
| Patient | Age (years) | Sex | Race | BSA (m2) | Concurrent Therapy | No. of Studies* |
|---|---|---|---|---|---|---|
| 1 | 6.6 | M | H | 0.81 | Dex, DPH | 4 |
| 2 | 8.4 | M | AA | 0.96 | -- | 4 |
| 3 | 3.6 | F | H | 0.67 | Dex | 3 |
| 4 | 4.8 | M | C | 0.97 | Dex | 2 |
| 5 | 3.2 | M | C | 0.61 | Dex | 3 |
| 6 | 4.9 | M | AA | 1.00 | Dex | 2 |
Abbreviations: AA, African American; BSA, body surface area; C, Caucasian; Dex, dexamethasone; DPH, diphenylhydantoin (phenytoin); F, female; H, Hispanic; M, male.
Number of plasma and ventricular cerebrospinal fluid studies.
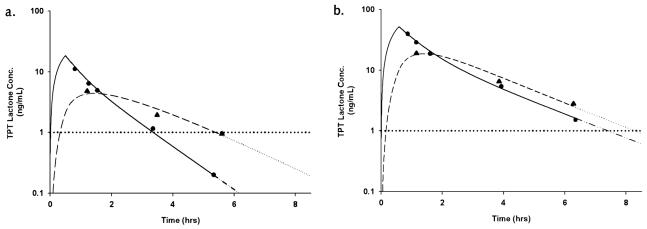
With these six patients, a total of 18 plasma and vCSF pharmacokinetic studies were conducted, and 50 individual vCSF topotecan samples were obtained. Presented in Fig. 2a is a representative topotecan plasma and vCSF concentration–time plot for a patient when below the plasma target and CSF EDT. After a TPT dosage adjustment, the patient achieved the plasma target and vCSF EDT (Fig. 2b). The topotecan plasma and vCSF pharmacokinetic parameter estimates generated from the two-stage approach for all patients are displayed in Table 2. The pharmacokinetic parameters describing vCSF topotecan disposition, k13 and k31, showed the highest variance as compared to all other pharmacokinetic parameters both within and between patients.
Fig. 2.
Topotecan lactone concentration-versus-time plot. a. Before dosage adjustment (AUC = 30 ng/ml-h and vCSF exposure duration [---] ~ 4.8 h). b. After dosage adjustment (AUC = 135 ng/ml-h and vCSF exposure duration [---] > 8 h). Observed plasma (•) and CSF (▴) concentrations are plotted. The solid line represents best-fit plasma curve to last measured data point, and extrapolation is indicated by a dashed and dotted line. The dashed line represents best-fit vCSF curve to last measured data point, with extrapolation indicated by a dotted line. In both cases, extrapolation was achieved by simulation of concentration–time data using the estimated compartmental pharmacokinetic parameters. The bold horizontal dotted line represents the desired vCSF exposure duration of 1 ng/ml.
Table 2.
Cohort population parameter estimates generated from linear mixed-effects modeling
| Pharmacokinetic Parameter | Geometric Mean | Interindividual Variation (%)* | Intraindividual Variation (%)* |
|---|---|---|---|
| Plasma clearance** | 44.03 | 31.9 | 21.8 |
| k10 (h−1 ) | 0.961 | 25.3 | 20.1 |
| k12 (h−1) | 0.403 | 16.9 | 27.5 |
| k21 (h−1 ) | 0.634 | 0.01 | 25.8 |
| k13 (h−1 ) | 0.00208 | 80.3 | 96.8 |
| k31 (h−1 ) | 1.36 | 74.9 | 70.5 |
| VC (liters/m2) | 45.88 | 35.9 | 21.1 |
Abbreviation: k, rate constant; VC, volume of the central compartment (plasma volume of distribution for topotecan lactone).
The interindividual and intraindividual errors are expressed as coefficients of variation.
Plasma clearance of topotecan lactone, in liters per hour per square meter.
Of the 18 plasma pharmacokinetic studies, nine were within the target TPT plasma AUC of 120 to 160 ng/ ml-h. Seven of these nine targeted plasma studies also achieved the EDT in the vCSF (~80%). Of the nine studies that were not within the plasma target range, two were above target at 165 and 212 ng/ml-h. Analysis of vCSF in these two studies showed that the vCSF EDT was achieved in these two instances. Of the remaining seven plasma pharmacokinetic studies not in target, six were conducted after administration of the initial fixed topotecan dosage (2 mg/m2 per day). Neither the target plasma AUC nor the vCSF EDT was achieved at this dosage. Notably, one study was well below the plasma target topotecan AUC, yet still achieved the vCSF EDT for approximately 24 h.
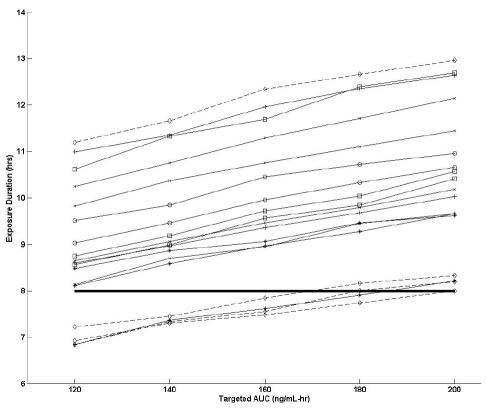
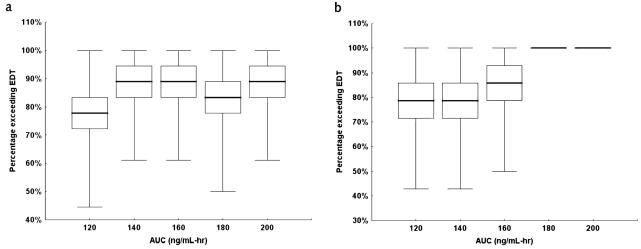
To determine the effect of various target plasma AUC values on the exposure duration, we simulated TPT concentrations in the vCSF by using pharmacokinetic parameter estimates from our population (Fig. 3). From these results, we observed that, at the lowest topotecan plasma target AUC of 120 ng/ml-h, 14 of 18 studies (78%) met or exceeded the EDT, and at the highest AUC of 200 ng/ml-h, all studies met or exceeded the EDT. To estimate the variability in the number of studies that met or exceeded the EDT (for the purpose of determining a confidence interval) the bootstrap approach was used (see Fig. 4a). In particular, at an AUC of 120 ng/ml-h, the 95% CI for the number of studies that met or exceeded the EDT was 10 of 18 (56%) to 17 of 18 (94%).
Fig. 3.
Exposure duration versus targeted area under the concentration–time curve (AUC). Each line represents an individual study, and each symbol represents an individual patient. The dashed lines represent the studies that were removed in the subset analysis (patient 1). The bold horizontal line represents the EDT of 1 ng/ml for 8 h.
Fig. 4.
Percent EDT versus targeted AUC. The bar represents the median, the boxes represent the quartiles, and whiskers represent the minimum and maximum. a. Full data set. b. Subset of data.
Upon closer examination of the clinical condition of each patient, we noted one patient (patient #1 in Table 1) who had MRI evidence of tumor blockage of CSF flow and was receiving dexamethasone and phenytoin. Because these clinical conditions may alter topotecan systemic and CSF pharmacokinetics (Gajjar et al., 2001; Zamboni et al., 1998b), we chose to analyze a subset of the data without the four pharmacokinetic studies that this patient contributed (see Fig. 4b). From these results, we observed that, at the lowest topotecan plasma AUC of 120 ng/ml-h, 13 of 14 studies (93%) in the subset data set met or exceeded the EDT, and at the highest AUC 200 ng/ml-h, all studies met or exceeded the EDT in the subset group. Furthermore, based on the bootstrap approach, the 95% CI for the number of studies that met or exceeded the EDT was 8 of 14 (57%) to 14 of 14 (100%).
Discussion
In this study, we demonstrated that a plasma pharmacokinetic model could be used to estimate vCSF topotecan concentrations in children with medulloblastoma. Furthermore, we showed that as the plasma topotecan plasma AUC increased, so did the likelihood of exceeding the EDT (defined as a topotecan vCSF concentration of 1 ng/ml for 8 h). Whereas previous studies have validated the three-compartment pharmacokinetic model used to evaluate the topotecan plasma and vCSF disposition, this is the first study to evaluate the ability of a plasma pharmacokinetic model to provide an estimate of vCSF exposure that is based solely on plasma topotecan data.
Achieving a therapeutic drug exposure in the CSF is critical for medulloblastoma treatment, since the tumor tends to spread throughout the neuraxis. The optimal CSF topotecan exposure for medulloblastoma treatment has not yet been defined in prospective clinical trials; however, in vitro studies suggest that a topotecan exposure of 1 ng/ml for 8 h is minimally required for efficacy against medulloblastoma. Furthermore, encouraging results were obtained from a recently completed phase 2 clinical trial that used a pharmacokinetically guided approach to attain this topotecan CSF EDT in children with high-risk medulloblastomas (Stewart et al., 2004).
Our results indicate that achieving the target plasma TPT AUC of 120 ng/ml-h will result in attainment of the vCSF EDT in approximately 78% of studies (95% CI, 56%–94%). Our simulations also show that achieving plasma topotecan AUCs greater than this 120-ng/ml-h target (i.e., 140–200 ng/ml-h) increases the likelihood of attaining or exceeding the vCSF EDT (indicated by Fig. 3). The tolerability of such daily topotecan plasma exposures for a five-day duration has been previously demonstrated in children with solid tumors (Tubergen et al., 1996). However, the duration of neutropenia resulting from exposures of 200 ng/ml-h or greater is therapeutically prohibitive, even with current hematopoietic support modalities.
It should be noted that all the studies not achieving the CSF EDT after attainment of target plasma topotecan AUC came from two patients, whose topotecan exposure nevertheless remained above the 1-ng/ml threshold for approximately 7 h. We speculate that both patients’ diagnoses of diffuse leptomeningeal disease and large residual tumor burden may have played a role in their variable topotecan CSF penetration. It is likely that these patients contributed to most of the variation observed in the pharmacokinetic parameters describing topotecan CSF penetration (k13 and k31) in our population analysis. Further, we showed that analyzing the data without one of these two patients who contributed both the highest and lowest observed CSF exposure durations reduced variability in our bootstrap subset. Removal of this one patient also reduced the observed intraindividual variability in k13 and k31 from 96.8% and 70.5% to 71.2% and 50%, respectively, upon reapplication of linear mixed-effects modeling (data not shown). It should be noted that this patient had clinical characteristics that we suspect may have altered systemic and CSF topotecan pharmacokinetics (MRI evidence of disturbances in CSF flow; dexamethasone and phenytoin use). Thus, from these limited data, one may speculate that higher plasma topotecan target AUC values may be necessary to overcome the variation in topotecan CSF penetration observed in patients with these characteristics.
We have shown that the appropriate administration of systemic topotecan therapy (i.e., pharmacokinetically guided dosing to a target systemic exposure) can achieve the topotecan CSF EDT. Direct intrathecal therapy with topotecan also produces effective topotecan concentrations in the vCSF. However, systemic topotecan therapy for medulloblastoma provides therapeutic concentrations in the CSF while also reaching parenchymal tumor distant from ventricular or lumbar CSF via the blood. Moreover, some patients are unable or unwilling to receive intrathecal therapy because of potential complications (i.e., chemical arachnoiditis, CNS infection, and placement of access devices). Thus, pharmacokinetically guided topotecan presents as an effective alternative, or even adjunct, to intrathecal therapy for the treatment of pediatric medulloblastoma.
The use of a pharmacokinetic model and plasma topotecan concentrations to estimate vCSF topotecan exposures eliminates the need for invasive CSF sampling. However, even limited plasma sampling, as well as the need for immediate sample processing for TPT, can be burdensome for patients and families and can be demanding on the health care infrastructure. Therefore, we have begun population pharmacokinetic studies of topotecan to identify patient covariates related to topotecan clearance. These patient-specific covariates (e.g., age, creatinine clearance, and body surface area) will enable the development of a dosing nomogram that will allow the practitioner to estimate a patient’s plasma topotecan exposure and, as shown in the present communication, the topotecan CSF exposure. Thus, using the data from the present study and the dosing nomogram, one could reliably estimate a patient’s topotecan CSF exposure from patient-specific covariates, without the need for invasive CSF sampling or plasma topotecan samples.
In conclusion, we have shown that achieving a plasma TPT AUC of at least 120 ng/ml-h has a very high likelihood of achieving a TPT vCSF exposure of 1 ng/ml for no less than 8 h in children with medulloblastoma. This exposure duration is cytotoxic to medulloblastoma cell lines in vitro. Given the variability in systemic TPT clearance among patients, pharmacokinetically guided topotecan dosing can be used to ensure that patients achieve this desired plasma exposure. Potentially, a dosing nomogram for plasma topotecan exposure, based upon patient-specific characteristics, could be used to adjust a patient’s topotecan dosage to achieve the necessary plasma topotecan exposure and subsequently the vCSF EDT. This proposed method offers an effective, feasible alternative for medulloblastoma patients either unwilling or unable to receive intrathecal topotecan therapy.
Footnotes
This work has been supported by Cancer Center Support (CORE) grant P 30 CA 21765 and grant P01 23099 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations used are as follows: AUC, area under the concentration–time curve; CSF, cerebrospinal fluid; CSI, craniospinal irradiation; EDT, exposure duration threshold; k, rate constant; MAP, maximum a posteriori; TPT, topotecan, lactone form; VC, volume of the central compartment; vCSF, ventricular cerebrospinal fluid.
References
- Baker SD, Heideman RL, Crom WR, Kuttesch JF, Gajjar A, Stewart CF. Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemother Pharmacol. 1996;37:195–202. doi: 10.1007/BF00688317. [DOI] [PubMed] [Google Scholar]
- Blaney SM, Cole DE, Balis FM, Godwin K, Poplack DG. Plasma and cerebrospinal fluid pharmacokinetic study of topotecan in nonhuman primates. Cancer Res. 1993;53:725–727. [PubMed] [Google Scholar]
- D’Argenio, D.Z., and Schumitzky, A. (1990) ADAPT II User’s Guide, 1st ed. Los Angeles: Biomedical Simulations Resource, University of Southern California.
- Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ. What’s new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol. 2003;7:53–66. doi: 10.1016/s1090-3798(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Gajjar AJ, Kirstein MN, Santana VM, Furman WL, Thompson S, Houghton PJ, Stewart CF. Concomitant dexamethasone (DXM) increases topotecan (TPT) clearance in children. Proc Am Assoc Cancer Res. 2001;42:653. (abstract) [Google Scholar]
- Gibaldi, M., and Perrier, D. (1982) Pharmacokinetics, 2nd ed. New York: Marcel Dekker.
- Lishner M, Perrin RG, Feld R, Messner HA, Tuffnell PG, Elhakim T, Matlow A, Curtis JE. Complications associated with Ommaya reservoirs in patients with cancer. The Princess Margaret Hospital experience and a review of the literature. Arch Intern Med. 1990;150:173–176. [PubMed] [Google Scholar]
- Pawlik CA, Houghton PJ, Stewart CF, Cheshire PJ, Richmond LB, Danks MK. Effective schedules of exposure of medulloblastoma and rhabdomyosarcoma xenografts to topotecan correlate with in vitro assays. Clin Cancer Res. 1998;4:1995–2002. [PubMed] [Google Scholar]
- Pratt CB, Stewart CF, Santana VM, Bowman L, Furman W, Ochs J, Marina N, Kuttesch JF, Heideman R, Sandlund JT, Avery L, Meyer WH. Phase I study of topotecan for pediatric patients with malignant solid tumors. J Clin Oncol. 1994;12:539–543. doi: 10.1200/JCO.1994.12.3.539. [DOI] [PubMed] [Google Scholar]
- Santana VM, Zamboni WC, Kirstein MN, Tan M, Liu T, Gajjar A Houghton, P.J. and, Stewart CF. A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clin Cancer Res. 2003;9:633–640. [PubMed] [Google Scholar]
- Steimer J.-L, Ebelin M.-E, Van Bree J. Pharmacokinetic and pharmacodynamic data and models in clinical trials. Eur J Drug Metab Pharmacokinet. 1993;18:61–76. doi: 10.1007/BF03220009. [DOI] [PubMed] [Google Scholar]
- Stewart CF, Liu CY, Zamboni WC, Ma MK, Kirstein MN, Hanna SK, Gajjar AJ, Santana VM, Houghton PJ, Sambol NC. Population pharmacokinetics of topotecan in children and adolescents. Proc Am Soc Clin Oncol. 2000;19:177a. (abstract 687) [Google Scholar]
- Stewart CF, Iacono LC, Chintagumpala M, Kellie SJ, Ashley D, Zamboni WC, Kirstein M, Fouladi M, Seele LG, Wallace D, Houghton PJ, Gajjar A. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. J Clin Oncol. 2004;22:3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- Tubergen DG, Stewart CF, Pratt CB, Zamboni WC, Winick N, Santana VM, Dryer ZA, Kurtzberg J, Bell B, Grier H, Vietti TJ. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1996;18:352–361. doi: 10.1097/00043426-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Stewart CF, Thompson J, Santana VM, Cheshire PJ, Richmond LB, Luo X, Poquette C, Houghton JA, Houghton PJ. Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst. 1998a;90:505–511. doi: 10.1093/jnci/90.7.505. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Gajjar AJ, Heideman RL, Beijnen JH, Rosing H, Houghton PJ, Stewart CF. Phenytoin alters the disposition of topotecan and N-desmethyl topotecan in a patient with medulloblastoma. Clin Cancer Res. 1998b;4:783–789. [PubMed] [Google Scholar]